how are minerals classified? question 3 options: a) based on their chemical structure b) based on solubility c) based on amount needed per day d) based on food source
Answers
Based on their solubility, minerals are divided into categories.
Depending on the quantity required by the body, minerals are either categorised as significant minerals or trace minerals. Based on their solubility, minerals are divided into categories. Major minerals are all those that must be consumed in dietary quantities more than 100 mg daily. These include calcium, phosphorus, magnesium, sodium, potassium, chloride, and sulfur. While vitamins are composed of the thirteen organic micronutrients that the body need for regular functioning, minerals are inorganic substances that are crucial to human nutrition. A mineral is an inorganic element and compound that occurs in nature and has a recognizable chemical composition, crystal structure, and physical characteristics. Quartz, granite, mica, actinolite, olivine, and calcite are examples of common minerals. Heiskanen defined classification as a process for dividing mineral combinations into two or more categories.
Learn more about minerals
https://brainly.com/question/8831889
#SPJ4
Related Questions
Acetone (nail polish remover) has a density of 0.7857 g >cm3.
a. What is the mass in g of 28.56 mL of acetone?
b. What is the volume in mL of 6.54 g of acetone?
Answers
If acetone has a density of 0.7857 \(\frac{g}{cm^{3} }\) the mass in grams of point A is 22.4 g and the volume at point B is 8.32 mL.
What is acetone?Acetone is known as a chemical substance that is usually found in the environment but can also be produced artificially. Acetone is a polar organic product that interacts very well with water molecules, generating dipole-dipole relationships.It is colorless with a distinctive smell and taste, we find it in products known as cleaning and personal care products, but we can also use it as a solvent for substances.
Also in the environment in plants, trees and in volcano emissions or in forest fires, it does not become toxic in low doses but if it is exposed to an individual in high doses it can become fatal.
In the statement we can find that acetone has a density of 0.7857 \(\frac{g}{cm^{3} }\).
Therefore, we can confirm that if acetone has a density of 0.7857 \(\frac{g}{cm^{3} }\) the mass in grams of point A is 22.4 g and the volume at point B is 8.32 mL.
To learn more about acetone visit: https://brainly.com/question/13334667?referrer=searchResults
#SPJ1
Tow it's time to put all of the digestive anatomy and physiology rogether to get a "big picture" view of the digestive system. In this exercise you will trace the pathway that three different nutrients take from their ingestion at the mouth to their arrival at the heart. You will trace a cookie (primarily carbohydrates), an egg (primarily protein), and MATERIALS greasy fried food (primarily lipids). Laminated outline of the human body Water-soluble marking pens Along the way, detail the following for each 1. The anatomical pathway that each takes, from ingestion, through in passage through the alimentary canal, to its absorption into the blood, and finally to its passage through the blood until it reaches the heart. 2. The physical and chemical processes that break down each substance, including enzyme-catalyzed chemical Some hints: Don't forget that carbohydrates and amino acids travel through the hepatic portal system before they enter the general circulation. Remember that digestion and absorption are quite different for lipids. For example, fats are not absorbed into the intestinal blood capillaries. Use the text in Exercise 24-3 (p. 649) and your list of enzymes that you completed in Pre-Lab Exercise 24-3 (p. 628) for reference. Refer to the tracing exercises from Unit 18 (p. 486) and Unit 21 (p. 553) to review the pathway of blood and lymph flow through the body. You may find it helpful to physically trace the pathway on a laminated outline of the human body to better visualize the processes.
Answers
Tow it's time to put all of the digestive anatomy and physiology together to get a "big picture" view of the digestive system. The pathway that three different nutrients take from their ingestion at the mouth to their arrival at the heart: a cookie (primarily carbohydrates), an egg (primarily protein), and greasy fried food (primarily lipids).
Anatomical pathway:
a) Cookie (carbohydrates):
The cookie is broken down mechanically in the mouth through chewing and mixed with saliva containing salivary amylase, initiating the digestion of carbohydrates. The food bolus then travels down the esophagus through peristaltic contractions and enters the stomach.
In the stomach, gastric acid and enzymes continue to break down the carbohydrates. The partially digested food, called chyme, moves into the small intestine. In the small intestine, pancreatic amylase and brush border enzymes further break down the carbohydrates into simple sugars.
The final step is the absorption of these sugars through the intestinal epithelium into the bloodstream. From there, they are transported to the liver via the hepatic portal system and eventually reach the heart.
b) Egg (protein):
The egg is broken down mechanically and chemically in the stomach. The stomach secretes gastric acid and the enzyme pepsinogen, which is converted to pepsin, initiating protein digestion.
The partially digested proteins form chyme, which enters the small intestine. In the small intestine, pancreatic enzymes and brush border enzymes break down proteins into amino acids. These amino acids are absorbed through the intestinal epithelium into the bloodstream. They also travel through the hepatic portal system to the liver and then to the heart.
c) Greasy fried food (lipids):
The greasy fried food is mechanically broken down in the mouth and mixed with saliva. In the stomach, some emulsification of lipids occurs due to the agitation caused by gastric contractions.
However, the majority of lipid digestion occurs in the small intestine. Bile salts, produced by the liver and stored in the gallbladder, emulsify the lipids, increasing their surface area for digestion by pancreatic lipase. Pancreatic lipase breaks down the triglycerides into fatty acids and monoglycerides.
These products, along with bile salts, form micelles that allow for absorption through the intestinal epithelium. Once absorbed, the fatty acids and monoglycerides are reassembled into triglycerides, packaged into chylomicrons, and transported through the lymphatic system. Eventually, they reach the bloodstream, travel through the systemic circulation, and reach the heart.
Physical and chemical processes:
a) Carbohydrates:
The physical process of chewing breaks down the cookie into smaller particles, increasing its surface area. The chemical process involves the action of salivary amylase, gastric acid, pancreatic amylase, and brush border enzymes, which hydrolyze the complex carbohydrates into simpler sugars.
b) Proteins:
The physical process of chewing helps break down the egg into smaller pieces. The chemical process involves the action of gastric acid, pepsinogen, and pancreatic and brush border enzymes. These enzymes break down the proteins into peptides and amino acids.
c) Lipids:
The physical process of chewing and the mechanical mixing of lipids with saliva aid in breaking down the greasy fried food. The chemical process involves the emulsification of lipids by bile salts, the action of pancreatic lipase, and the formation of micelles. Pancreatic lipase hydrolyzes triglycerides into fatty acids and monoglycerides, which are then absorbed and processed into chylomicrons.
In conclusion, the digestive system is a complex and coordinated system
Learn more about digestive system here https://brainly.com/question/28978933
#SPJ11
Recycling of aluminum beverage cans is an example of the fact that Group of answer choices decreasing entropy is a spontaneous process. increasing entropy is a spontaneous process. decreasing entropy is a nonspontaneous process. increasing entropy is a nonspontaneous process.
Answers
Answer:
The correct option is;
decreasing entropy is a nonspontaneous
Explanation:
Recycling of aluminium beverage cans involves the process of remelting scrapped beverage after the cans were first produced and used initially
1) The cans are cut into pieces so as to make them more compact and accessible for cleaning
2) The cans are then blocked and melted by heating and the impurities and hydrogen gas are removed
3) The composition of the molten aluminium alloy is altered by the addition of the alloy composition to get the right specification of the alloy
4) The molten alloy can then be sent for usage where it will be processed into solid aluminium objects without the requirement for much energy addition
By the second law of thermodynamics, processes 1, and 2 which involve the addition of energy from the surrounding to decrease the randomness of the scrapped beer cans such that the entropy of the surrounding decreases are not spontaneous processes, while process 4 where the molten aluminium will be cooled down to form solid aluminium does not require energy input but adds energy to the surrounding in the surrounding's entropy, is therefore a spontaneous process.
Therefore, decreasing the entropy of the universe by heating aluminium to melt it is a non spontaneous process
Realice una historieta que resuma su comprensión acerca de la teoría atómica y los diferentes modelos atomicos que se
han propuesto a lo largo de la historia.
Answers
Respuesta:
Los modelo atómicos han permitido representar el modo de funcionamiento de los átomos. A lo largo de la historia han surgido un numero de modelos atómicos diferentes incluyendo los modelos de Bohr, Thomson, Rutherford, Sommerfeld, Dalton y Schrödinger.
Explicación:
El modelo atómico propuesto por John Dalton (1808) demostró que las sustancias químicas reaccionan en proporciones fijas y cómo mediante su combinación se producen elementos diferentes. Dalton fue el primero en postular la existencia de elementos indivisibles llamados átomos. A continuación, Thomson (1904) desarrolló un modelo en el cual el átomo estaba compuesto por protones con carga positiva y electrones con carga negativa los cuales se incrustaban uniformemente dentro de este átomo, asemejándose a las pasas de uva de un budín. En 1911, Ernest Rutherford desarrolló un nuevo modelo donde la masa principal del átomo tenía carga positiva y se localizaban en el núcleo, mientras que los electrones con carga negativa se posicionaban en la región externa del átomo. Subsecuentemente, Niels Bohr (1913) represento el funcionamiento del átomo de hidrógeno mediante un protón inmóvil en el núcleo atómico y un electrón girando a su alrededor. El modelo atómico de Sommerfeld permitió generalizar el diagrama de Bohr a otros tipos de átomos mas allá del Hidrógeno, incluyendo diferentes niveles energéticos para cada átomo particular. El modelo de Schrödinger (1926) permitió corregir aquellas discordancias surgidas del modelo atómico de Bohr. Schrödinger incluyó diferentes niveles y subniveles de energía a los electrones e incorporó órbitas elípticas a su movimiento, con lo cual permitiendo predecir los efectos relativos de los campos magnético y eléctrico sobre el movimiento de los electrones.
How many moles of Ar gas are
present in a container with a
volume of 78.4 L at STP?
Answers
1 mole of a gas at STP occupies 22.4 L volume
Now the volume is given =78.4 therefore,
No. of moles of gas = 78.4 ÷ 22.4 = 3.5 moles
I hope it helps you~
explain how you would find the number of moles that are rpresented by a certaib nunver if reoresnetatuce oartuckes
Answers
To find the number of moles represented by a certain number of particles, you can use Avogadro's number and the concept of molar mass.
Avogadro's number (symbolized as N<sub>A</sub>) is a fundamental constant that represents the number of particles (atoms, molecules, ions, etc.) in one mole of a substance. It is approximately equal to 6.022 × 10²³ particles/mole.
Molar mass is the mass of one mole of a substance and is expressed in grams/mole. It represents the sum of the atomic masses or molecular masses of the constituent particles in a substance.
To calculate the number of moles, you can follow these steps;
Determine the number of particles you have (atoms, molecules, ions, etc.).
Identify the molar mass of the substance or the average molar mass if it's a mixture.
Divide the number of particles by Avogadro's number to convert them into moles. The formula is:
Moles = Number of Particles / Avogadro's number
Moles = Number of Particles / (6.022 ×10²³ particles/mole)
The result will give you the number of moles represented by the given number of particles.
To know more about Avogadro's number here
https://brainly.com/question/24175158
#SPJ4
--The given question is incorrect, the correct question is
"Explain how you would find the number of moles that are represented by a certain number of representative particles."--
you make a 100 ml solution by dissolving 5g of a solid solute in water. the molar mass of the solute is 110 g/mol.What is the molarity?
Answers
The molarity to make a 100 ml solution by dissolving 5g of a solid solute is 0.45 M. It can be calculated by using molarity formula.
Molarity is the concentration of a solution in terms of moles of solute per liter of solution. It is denoted by the unit "M" (moles per liter).
The molarity of a solution can be calculated as the number of moles of solute divided by the volume of the solution in liters.
First, we need to determine the number of moles of solute:
5g / 110 g/mol = 0.045 moles
Next, we need to convert the volume of the solution from milliliters to liters:
100 ml * (1 L / 1000 ml) = 0.1 L
Finally, we can calculate the molarity:
0.045 moles / 0.1 L = 0.45 M
Here you can learn more about Molarity
https://brainly.com/question/8732513
#SPJ4
What is the concentration of hydrogen ions in a solution with pH = 4. 282?
1. 92 x 10-10 M.
4. 28 M
1. 66 x 104 M
5. 22 x 10-5 M
Answers
The correct answer is option 4: 1.66 x 10^(-5) M.Concentration of hydrogen ions in a solution with pH = 4. 282 is 4: 1.66 x 10^(-5) M.
The concentration of hydrogen ions in a solution with pH = 4.282 can be calculated using the formula:
[H+] = 10^(-pH)
where [H+] represents the concentration of hydrogen ions.
Plugging in the given pH value:
[H+] = 10^(-4.282)
Using a calculator, we find:
[H+] ≈ 1.66 x 10^(-5) M
Therefore, the correct answer is option 4: 1.66 x 10^(-5) M.
Concentration of hydrogen ions in a solution with pH = 4. 282 is 4: 1.66 x 10^(-5) M.
To know more about solution visit:
https://brainly.com/question/25326161
#SPJ11
What are two thermal properties of water that make it unique?
Answers
The fact that water has a high melting and boiling point (0°C/32°F for melting and 100°C/212°F for boiling) makes it unique.
Where do melting and boiling points lie?When a substance's solid and liquid phases are in equilibrium, that point is known as its melting point. When a substance's vapour pressure matches the outside pressure, that's when it reaches its boiling point.
What is another name for boiling point?Saturation temperature is another name for boiling point. The pressure at when the measurement was made can occasionally be used to define boiling point. The standard boiling point is the temperature at which water begins to boil at one bar of pressure, according to the International Union on Pure an Applied Chemistry (IUPAC) definition from 1982.
To know more about boiling point visit:
https://brainly.com/question/2153588
#SPJ1
Balance the following equations
a.H2+O2 -> H2O
b.ZnS + O2 -> ZnO + SO2

Answers
Answer:
a. 2H2 + O2--> 2H2O
b. 2ZnS +3O2-->2ZnO +2SO2
Explanation:
a.
note:
(when you put coefficients before the elements or the compounds you should multiply it by each element and the number that's on its right...
so if it's 2H2O
that is 2xH2 which is 4 hydrogen atoms
and 2 x O which is 2 oxygen atoms )
......
on the left side of the equation
there are two atoms of oxygen
but on the right side there's only one atom
so in order to balance them you have to put 2 as a coefficient for H2O
H2+O2 ->2H2O
so now you have 2 hydrogen atoms on the left side and 4 on the right side
you put 2 as a coefficient for H2
and now
there's 4 hydrogen atoms on both sides
and 2 oxygen atoms on both sides
b.
you do the same here
ZnS + O2 -> ZnO + SO2
2 oxygen atoms ---> 3 oxygen atoms
ZnS + 2O2 -> ZnO + SO2
4 oxygen atoms ---> 3 oxygen atoms
ZnS + 2O2 -> 2ZnO + SO2
4 oxygen atoms ---> 4 oxygen atoms
but
1 Zn ---> 2Zn
2ZnS + 2O2 -> 2ZnO + SO2
2S --> 1S
2ZnS + 2O2 -> 2ZnO + 2SO2
2S--> 2S
but
4O---> 6O
2ZnS + 3O2 -> 2ZnO + 2SO2
Howie wants to know how many apples he could pick from his grandpa's orchard. There are 9 rows of trees and he can pick 925 apples from each row. Approximately how many apples can he pick in total? Round the larger factor to the nearest hundred to estimate.
Answers
Answer:
8,300
Explanation:
925*9 is 8325.
After you round to the nearest hundred, its 8,300.
Answer:
Explanation:
eggggggggggg
The volume of a sample of oxygen has increases from 88 ml to 130 ml. if the initial pressure was 1200 mmHg, what is the final pressure?
Answers
Answer:
To solve this problem, we can use Boyle's Law, which states that the pressure and volume of a gas are inversely proportional when temperature is held constant. This can be expressed as:
P1V1 = P2V2
where P1 and V1 are the initial pressure and volume, and P2 and V2 are the final pressure and volume.
We can plug in the given values and solve for P2:
P1 = 1200 mmHg
V1 = 88 ml
V2 = 130 ml
P1V1 = P2V2
1200 mmHg * 88 ml = P2 * 130 ml
105600 mmHgml = 130P2 mlmmHg
105600 / 130 = P2
P2 = 811.08 mmHg
Therefore, the final pressure is approximately 811.08 mmHg.
How many molecules are there in 4.00 moles of glucose, C6H1206?
Answers
Molecules in 4 moles of glucose are 24.088x10²³.
We need to find the number of molecules by applying the concept of moles
number of moles(n)= Number of Molecules(N)/Avogadro's Number(Nₐ)
n=N/Nₐ
4=N/6.022x10²³
N=24.088x10²³
Therefore, the number of molecules in 4 moles of Glucose is 24.088x10²³.
To know more about moles, click on https://brainly.com/question/15356425
On all aerosol cans you see a warning that tells you to keep the can
away from heat because of the danger of explosion. What is the
potential volume of the gas contained in a 500.0 mL can at 25°C if it
were heated to 54°C. In other words if the can could expand to allow
the gas to take up a greater volume, what would be the new volume of
the gas when heated as previously described?
Answers
Gas laws show the relationship between tempertaure, pressure, volume, volume and number of moles. The new volume of the gas is 549 mL.
What is Charles law?Charles law states that, the volume of a given mass of gas is directly proportional to its temperature at constant pressure .
Given that;
V1 = 500.0 mL
T1 = 25°C or 298 K
V2 = ?
T2 = 54°C + 273 = 327 K
Since;
V1/T1 = V2/T2
V1T2 = V2T1
V2 = V1T2/T1
V2= 500.0 mL * 327 K/298 K
V2 = 549 mL
Learn more about Charles law: https://brainly.com/question/14842720?
Which statement describes a way in which a mole is useful

Answers
Answer:
A mole in chemistry is defined as the number or quantity of a chemical substance that consists of as many fundamental entities such as atoms, molecules, and ions. Therefore, the statement that best describes a mole is that, it is used for directly comparing the amounts of substances.
Explanation:
I found this hope it helps!! (read and try your best)
Th statement which describes a way in which moles is useful is that it can be used to keep track of chemical amounts.
What is mole?Mole is unit to calculate the amount of chemical substances and it is represented as:
n = W/M, where
W = given mass
M = molar mass
It can be useful in chemical reactions to calculate the relative amount of species present before and after the reaction. In one mole of substance 6.022 × 10²³ atoms of that substance is present.
Hence, option (A) is correct.
To know more about Mole, visit the below link:
https://brainly.com/question/1464305
Which three terms name a domain of life
Answers
Answer:
Bacteria, Archaea and Eukarya are the three domains of life.
which is the best colored indicator to use in the titration of 0.1 m ch3cooh(aq) with naoh(aq)? why? look up ka values in the appendix. indicator pka bromocresol green 4.8 bromothymol blue 6.8 phenolphthalein 9.2 group of answer choices
Answers
Answer:
Complete the sentence.
During a chemical reaction, __________.
Complete the sentence.
During a chemical reaction, __________.
Complete the sentence.
During a chemical reaction, __________.
Complete the sentence.
During a chemical reaction, __________.
Complete the sentence.
During a chemical reaction, __________.
Complete the sentence.
During a chemical reaction, __________.
Complete the sentence.
During a chemical reaction, __________.
Complete the sentence.
During a chemical reaction, __________.
Complete the sentence.
During a chemical reaction, __________.
Complete the sentence.
During a chemical reaction, __________.
Complete the sentence.
During a chemical reaction, __________.
Complete the sentence.
During a chemical reaction, __________.
Complete the sentence.
During a chemical reaction, __________.
Complete the sentence.
During a chemical reaction, __________.
Complete the sentence.
During a chemical reaction, __________.
Explanation:
Complete the sentence.
During a chemical reaction, __________.
Complete the sentence.
During a chemical reaction, __________.
Complete the sentence.
During a chemical reaction, __________.
Complete the sentence.
During a chemical reaction, __________.
Complete the sentence.
During a chemical reaction, __________.
Complete the sentence.
During a chemical reaction, __________.
Complete the sentence.
During a chemical reaction, __________.
Complete the sentence.
During a chemical reaction, __________.
Complete the sentence.
During a chemical reaction, __________.
Complete the sentence.
During a chemical reaction, __________.
Complete the sentence.
During a chemical reaction, __________.
1. what type of polymer would you obtain if sorbital (a sugar alcohol found in sugar free gum) was used as a plasticizer addictive?
2a. Starch-borate and starch-glycerol polymers have been used for encapsulation of pharmaceutical drugs or pesticides. Explain what effect ths might have and why it would be beneficial.
2b. Are these polymers considered to be biodegradable? why or why not?
Answers
1. The type of polymer that would you obtain if sorbitol is a polyol.
2a. The use of starch-borate and starch-glycerol polymers for the encapsulation of pharmaceutical drugs or pesticides would have a number of beneficial effects.
2b. Yes, these polymers are considered to be biodegradable.
1. The type of polymer that would you obtain if sorbitol (a sugar alcohol found in sugar-free gum) was used as a plasticizer additive is a polyol. This is because sorbitol is a polyol, which is a substance used to modify the properties of polymers. The process of polymer modification involves adding polyols to the polymer matrix, which helps to reduce the glass transition temperature of the polymer. Sorbitol can be used as a plasticizer addictive because it is a natural and non-toxic compound that is biodegradable.
2a. The use of starch-borate and starch-glycerol polymers for the encapsulation of pharmaceutical drugs or pesticides would have a number of beneficial effects. These polymers are natural and non-toxic, and they are biodegradable, which means that they do not pose a risk to the environment. Additionally, they can be used to modify the properties of the drugs or pesticides, making them more effective and reducing their toxicity.
2b. Yes, these polymers are considered to be biodegradable. This is because they are made from natural materials that can be broken down by biological processes. Starch-borate and starch-glycerol polymers are particularly attractive for use in biodegradable materials because they are non-toxic and biocompatible. They can be used in a variety of applications, including packaging materials, agricultural films, and medical devices, where their biodegradability is an important factor.
To know more about polyol visit:
https://brainly.com/question/32067095
#SPJ11
If you could repeat the lab and make it better, what would you do differently and why?
There are always ways that labs can be improved. Now that you are a veteran of this lab and have experience with the procedure, offer some advice to the next scientist about what you suggest and why. Your answer should be at least two to three sentences in length.
need help with lab!- earth and space science 1
Answers
In order to obtain more accurate results as well as to improve the efficiency of the laboratory procedure, the following recommendations are given:
There should be accurate calibration of instrumentsThe samples should be properly labeledRepeated measurements should be takenHow can improvements be done to a lab to obtain better results?Improving laboratory results can be achieved through several strategies aimed at enhancing experimental conditions, equipment, procedures, and data analysis.
Some possible methods for improving laboratory performance:
Regularly calibrate and maintain laboratory instruments and equipment to ensure accuracy and reliability. Implement robust quality control measures by using appropriate standards, controls, and reference materials. Develop and follow standardized operating procedures for all experiments and tests.Proper labeling, preservation, and storage at appropriate temperatures.Learn more about lab procedures at: https://brainly.com/question/13517732
#SPJ1
can someone give me facts about Venus, it's for a project
it would help a lot!!
I'm looking for at least 4
Answers
Answer:
A day on Venus is longer than a year. ...
Venus is hotter than Mercury – despite being further away from the Sun. ...
Unlike the other planets in our solar system, Venus spins clockwise on its axis. ...
Venus is the second brightest natural object in the night sky after the Moon.
Venus has a hostile environment. ...
Venus is hellishly hot. ...
Venus has volcanic features. ...
Venus has year-long days. ...
Venus has two sunrises in a year. ...
Venus spins in reverse gear. ...
Venus is showing mysterious life signals.
Explanation:
hope it helps
Please rate and mark as brainliest
Thank you and have a good day
A day on Venus is longer than a year. ...
Venus is hotter than Mercury – despite being further away from the Sun. ...
Unlike the other planets in our solar system, Venus spins clockwise on its axis. ...
Venus is the second brightest natural object in the night sky after the Moon.
Venus has a hostile environment
How many significant figures are in each underlined measurement?
Answers
Answer:
Give the number of significant figures in each measurement. By rule 1, all nonzero digits are significant, so this measurement has three significant figures.
if the temperature of this equilibrium was increased, what would happen to the equilibrium yield of chlorine?
Answers
Answer:
the temperature of the system decreases
Which ion is a cation? A. Ca2+ B. Cl2 C. S2- D. Br-
Answers
Answer:
Ca2+ is cation
Explanation:
Because cation contain positive charge.
If a molecule has bond angles of 120° between the atoms, what type of hybrid orbitals are on the central atom in the molecule?
Answers
Answer:
Solution
verified
Verified by Toppr
Correct option is B)
In sp
3
d type of hybridisation, shape of molecule is trigonal bipyramidal and bond angle will be of 120
o
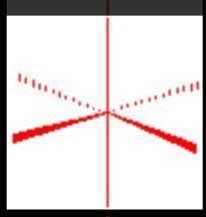
An dipole occurs when one molecule with a permanent dipole repels another molecules electrons causing the electrons to be more concentrated on one end of the molecule than another
Answers
The statement is true. An dipole occurs when one molecule with a permanent dipole repels another molecules electrons causing the electrons to be more concentrated on one end of the molecule than another.
Dipole-dipole attraction:
Dipole-dipole forces are attractive forces between the positive end of one polar molecule and the negative end of another polar molecule.
For molecules of similar size and mass, the strength of these forces increases with increasing polarity. Polar molecules can also induce dipoles in nonpolar molecules, resulting in dipole-induced dipole forces.
A molecule with a permanent dipole moment is called a polar molecule. A permanent dipole occurs when two atoms in a molecule have substantially different electronegativity.
Therefore a dipole occurs when one molecule with a permanent dipole repels the electrons of another molecule to be more concentrated on one end of the molecule than another is a true statement.
To know more about the dipole-dipole attraction refer to the link given below:
https://brainly.com/question/9929584
#SPJ4
Một hỗn hợp X gồm CH3OH; C2H5OH; phenol có khối lượng 28,9 gam phản ứng vừa hết với 100ml dung dịch NaOH 2M. Phần trăm theo khối lượng phenol là ? Biết C = 12; O = 16; H = 1; Na = 23. *
Answers
Answer:
nNaOH = 0,2 mol
Trong hỗn hợp các chất đề bài cho chỉ có phenol tác dụng với NaOH
C6H5OH + NaOH → C6H5ONa + H2O
0,2 ← 0,2 (mol)
⟹ mphenol = 0,2.94 = 18,8 gam
⟹
%mC6H5OH=18,8/28,9 x100%= 65,05%
Explanation:
Anyone help me answer this please anyone

Answers
Answer:
\(\huge\boxed{\sf Q = 5880\ Joules}\)
Explanation:
Given:
m = mass = 40 g = 0.04 kg
c = specific heat constant of water = 4200 J/Kg°C
ΔT = change in temperature = T2-T1 = 60 °C - 25 °C = 35°C
Required:
Q = change in heat energy = ?
Formula:
Q = mcΔT
Solution:
Q = (0.04)(4200)(35)
Q = 5880 Joules
\(\rule[225]{225}{2}\)
A heterozygous dominant green pea plant is crossed with a homozygous recessive yellow pea plant.
Which of the following Punnett squares correctly represents the possible offspring resulting from this cross?
Answers
The combination of the genotypes of heterozygous dominant green pea plant and a homzygous recessive yellow pea plant that will be in the punnet square is Gg × gg.
What is a punnet square?A punnet is a graphical representation used to determine the probability of an offspring expressing a particular genotype.
According to this question, a heterozygous dominant green pea plant is crossed with a homzygous recessive yellow pea plant.
The heterozygous dominant green pea plant has a genotype of Gg while the homzygous recessive yellow pea plant has a genotype of gg.
Therefore, the combination of the genotypes of heterozygous dominant green pea plant and a homzygous recessive yellow pea plant that will be in the punnet square is Gg × gg.
Learn more about punnet square at: https://brainly.com/question/20260008
#SPJ1
dentify the type of reaction and balance the following equation
1. Br2 + KI ----> KBr + I2
2. P4 + O2 ----> P4O10
3. PBr3 + H2O ----> H3PO3 + HBr
4. C2H6 + O2 ----> CO2 + H2O
Answers
Answer:
its rthhhh
Explanation:
At 303 K, the volume of a gas is 30.0 ml. At constant pressure, what is the new
volume of the gas if the temperature is decreased to 283K?
Answers
Answer:
V₂ = 28 mL
Explanation:
Given data:
Initial temperature = 303 K
Initial volume = 30.0 mL
Final temperature = 283 K
Final volume = ?
Solution:
The given problem will be solve through the Charles Law.
According to this law, The volume of given amount of a gas is directly proportional to its temperature at constant number of moles and pressure.
Mathematical expression:
V₁/T₁ = V₂/T₂
V₁ = Initial volume
T₁ = Initial temperature
V₂ = Final volume
T₂ = Final temperature
Now we will put the values in formula.
V₁/T₁ = V₂/T₂
V₂ = V₁T₂/T₁
V₂ = 30.0 mL × 283 K /303 K
V₂ = 8490 mL.K / 303 K
V₂ = 28 mL