Holt Chemistry Textbook Pg. 014: #01-03
1. Convert each of the following masses to the units requested.
a. 0.765 g to kilograms
b. 1.34 g to milligrams
c. 34.2 mg to grams
d. 23 745 kg to milligrams (Hint: Use two conversion factors.)
2. Convert each of the following lengths to the units requested.
a. 17.3 m to centimeters
b. 2.56 m to kilometers
c. 567 dm to meters
d. 5.13 m to millimeters
3. Which of the following lengths is the shortest, and which is the longest:
1583 cm, 0.0128 km, 17 931 mm, and 14 m?
Answers
The given unit conversions for the mass and length are as follows:
a. 0.765 g = 0.000765 kg
b. 1.34 g = 1340
c. 34.2 mg = 0.0342 g
d. 23 745 kg = 2.3745 * 10⁹ mg
2. a. 17.3 m = 17300 cm
b. 2.56 m = 0.00256 km
c. 567 dm = 56.7 m
d. 5.13 m = 5130 m
3. The shortest length is 0.0128 km and the longest length is 1583 cm
What is the mass of an object?The mass of an object is the amount of matter present in the substance.
The mass of objects is measured using a chemical balance, the unit of measurement is kilograms.
The various units can be interconverted.
To convert each of the following masses to the units requested, the conversion factor is used;
a. 0.765 g to kilograms
0.765 g = 0.765/ 1000
0.765 g = 0.000765 kg
b. 1.34 g to milligrams
1.34 g = 1.34 * 1000
1.34 g = 1340
c. 34.2 mg to grams
34.2 mg = 34.2 / 1000
34.2 mg = 0.0342 g
d. 23 745 kg to milligrams
23 745 kg = 23 745 * 10⁶
23 745 kg = 2.3745 * 10⁹ mg
2. To convert each of the following lengths to the units requested, the steps are as follows:
a. 17.3 m to centimeters
17.3 * 100 cm
17.3 m = 17300 cm
b. 2.56 m to kilometers
2.56 m = 2.56/ 1000
2.56 m = 0.00256 km
c. 567 dm to meters
567 dm = 567 / 10
567 dm = 56.7 m
d. 5.13 m to millimeters
5.13 m = 5.13 * 1000
5.13 m = 5130 m
3. Converting the following lengths to meters:
1583 cm = 15.83 m
0.0128 km = 12.8 m
17 931 mm = 17.931 m
14 m
The shortest length is 0.0128 km and the longest length is 1583 cm
Learn more about length and mass at: https://brainly.com/question/18270472
#SPJ1
Related Questions
Using the following chemical equation, answer each question.
C(s) + O₂(g) → CO₂(g)
(a) What is the theoretical yield of CO₂ from 5.03 mol of charcoal?
(b) What is the percent yield if the reaction gives 135.6 g of CO₂?
Answers
The theoretical yield of CO₂ from 5.03 mol of charcoal is 221.32 g.
The percent yield if the reaction gives 135.6 g of CO₂ is 61.2 %.
The following chemical equation
C(s) + O₂(g) → CO₂(g)
moles of carbon = 5.03 mol
1 mol of carbon produces 1 mol of CO₂
5.03 mol of carbon form5.03 mol of CO₂
mass of CO₂ = moles × molar mass
= 5.03 × 44
= 221.32 g
theoretical yield of CO₂ = 221 g
The percent yield = (actual yield / theoretical yield ) × 100 %
= (135.6 / 221.32 ) × 100 %
= 61.2 %
Thus, The theoretical yield of CO₂ from 5.03 mol of charcoal is 221.32 g.
The percent yield if the reaction gives 135.6 g of CO₂ is 61.2 %.
To learn more about theoretical yield here
https://brainly.com/question/15577251
#SPJ1
How would a small bar magnet be oriented when placed at position x?

Answers
Answer:
d
Explanation:
Answer:C is the answer
Explanation:
When ADP accumulates, what is the effect on the rate of metabolic chemical reactions?
Speeds them up
Slows them down
Has no effect
Answers
The accumulation of ADP (adenosine diphosphate) affects the rate of metabolic chemical reactions by slowing them down.
What is chemical reaction?
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. It involves the breaking and forming of chemical bonds, resulting in the formation of new substances with different properties. Chemical reactions are essential for many processes in nature, including respiration, digestion, and photosynthesis.
ADP is a by-product of the energy-producing reactions that take place in cells. This by-product can build up and inhibit the activity of enzymes involved in these metabolic reactions. Therefore, an accumulation of ADP can slow down the rate of metabolic chemical reactions.
However, the rate of metabolic chemical reactions can also be sped up by the presence of ADP. This occurs when the cellular metabolic processes become inhibited by the presence of ADP and require a boost to be activated again. In this case, the presence of ADP can activate enzymes and thus speed up the rate of metabolic chemical reactions.
In sum, the accumulation of ADP can either slow down or speed up the rate of metabolic chemical reactions, depending on the current state of the metabolic process.
To know more about chemical reaction click-
https://brainly.com/question/26487468
#SPJ4
P4+O2=P2O3
What’s the balance of this equation?

Answers
Explanation:
P₄+O₂=P₂O₃; non-balanced
P₄+O₂=2P₂O₃; to add '2' on the right side; non-balanced
P₄+3O₂=2P₂O₃. to add '3' before O₂ in the left side; balanced
a cube of iron pyrite is 0.31 cm on each side and has a mass of 0.040g. what is the density of the sample?
Answers
The density of the iron pyrite cube is 1.343 g/cm³.
Given,
Side of iron pyrite cube = 0.31 cm
Mass of iron pyrite = 0.040 g
The volume of iron pyrite cube = s³ cm³
Or, volume = 0.029791 cm³
We have to find the density of the sample.
Density is defined as the mass per unit volume. Or, it is the ratio of mass to the volume of the substance.
Using the formula for density, we get,
Density = mass/volume
Or, density = 0.40/0.029791
Or, density = 1.343 g/cm³
Hence, the density of the iron pyrite cube is 1.343 g/cm³.
To learn more about density, visit: https://brainly.com/question/15164682
#SPJ9
Put in order how to process film: Below is a sequence of events. Place them in the order they should occur, number 1 being the first item. Select the step number from the drop down next to each item. Items to order: 1 Wetting agent 2. Presoak 3. Stop bath 4. Fixer 5. Final wash 6. First wash 7. Fixer removal 8. Developer Wetting agent 1 Presoak 1 Stop bath 1 Fixer 1 Final wash 1 First wash 1 Fixer removal 1 Developer
Answers
The sequence of events used to process film in film photography s:
Stop bathWetting agentPresoakFixerFirst washFixer removalDeveloperFinal washHow Does Film Photography Work?The practice of shooting pictures on the thin, clear plastic strips we refer to as film is known as film photography. The contrast and resolution of a photograph are determined by the gelatin emulsion that is coated on one side of the film strip. This emulsion contains tiny silver halide crystals.
Learn more about film photography at: https://brainly.com/question/4130836
#SPJ1
Answer:
Processing film starts with you loading a film reel and putting it in a light tight tank.
You will then do these steps in order for set times: water presoak, developer, stop bath, fixer, first wash, fixer remover, final wash, wetting agent, and then finally drying your film.
Explanation:
Took the test.
True or false: You must make observations and conduct research prior to creating a hypothesis.
Answers
Answer:
true
Explanation:
...................
Please help, its due today! I'll also make you brainiest (put them in an order that's simple, look at the picture and you'll see what I mean) Thank you and God bless! <33
On beaches there are often areas of grassy dunes where people are prohibited from walking. How do these protected areas preserve ecosystem services? Use the graphic organizer to categorize the following as either examples of land reclamation of protecting biodiversity.

Answers
Answer:
Preventing erosion – Land Reclamation
Protecting nesting areas – Protecting Biodiversity
Preventing littering – Land Reclamation
Preventing habitat disruption – Protecting Biodiversity
Protecting native species – Protecting Biodiversity
Preventing contamination of soil – Land Reclamation
Explanation:
I really hope I'm right! I tried my hardest, please give me brainliest :)
have a good day!
A 1.90 g sample of elemental sodium, Na(s) is reacted with water, yielding sodium
hydroxide, NaOH, and hydrogen. 2Na(s) + 2H2O(l)→ 2NaOH(aq) + H2(g); The H2(g) is collected
over water at 18 oC. What are the partial pressures of the two gases (hydrogen and water
vapor) when contained in a 1.00 l container at 25
oC? What is the total pressure?
Answers
The partial pressure of hydrogen is 0.98 atm while the partial pressure of water is 0.02 atm. The total pressure of the system is 1 atm.
What is the pressure?We have seen the reaction as it has been shown in the equation that is attached to the question above. Now, we must have to find the number of moles of the hydrogen that was obtained.
Number of moles of sodium = 1.90 g/23 g/mol = 0.08 moles
2 moles of sodium produces 1 mole of hydrogen
0.08 moles of sodium would produce 0.08 moles * 1 mole/ 2 moles
= 0.04 moles
Given that;
P = pressure
V = volume
n = Number of moles
R = gas constant
T = temperature
PV = nRT
P = nRT/V
P = 0.04 * 0.082 * 298/1
P = 0.98 atm
The partial pressure of the hydrogen = 0.98 atm
The partial pressure of the water = SVP of water at 18°C = 0.02 atm
Total pressure of the system = 0.98 atm + 0.02 atm = 1 atm
The system has a total pressure of 1 atm.
Learn more about partial pressure:https://brainly.com/question/15075781
#SPJ1
Which of the following relationships between
the pressure P, the volume-V and the
e temperature T, represents an ideal gas
behaviour?
A.paVT
B.p TaT/V
C. pTaVet
D.pval/T
E. paV/T
Answers
Out of the options given, the expression that represents the ideal gas behavior is:
E. P × V / TWhat is ideal gas equation?The ideal gas law is expressed by the formula PV = nRT,
where
P is the pressure,
V is the volume,
n is the number of moles of gas,
R is the gas constant, and
T is the temperature.
By rearranging this equation, we can derive different expressions for the ideal gas behavior in terms of the pressure, volume, and temperature.
This can be obtained by rearranging the ideal gas law equation as follows:
PV = nRT
Dividing both sides by nT, we get:
P × V / (nT) = R
Since R is a constant for a given gas, the left-hand side of the equation must also be constant for an ideal gas. Thus, the expression P × V / T represents the ideal gas behavior.
Learn more about ideal gas at
https://brainly.com/question/27870704
#SPJ1
Why is the half-life of a radioisotope important for radioactive dating?
A. The half-life forms a type of clock used to calculate time passed.
B. The half-life of a radioisotope tells how long ago the element was
formed.
C. The age of an object is equal to the radioisotope with the longest
half-life in it.
D. The half-life gives the period over which the radioisotope doesn't
change.

Answers
Answer:Dating Using Radioactive Decay
The best-known techniques for radioactive dating are radiocarbon dating, potassium-argon dating and uranium-lead dating.
After one half-life has elapsed, one half of the atoms of the nuclide in question will have decayed into a “daughter” nuclide.
Explanation:d
Which combination of atoms is most likely to produce a compound
with ionic bonds?
Answers
Answer: In an ionic bond, one atom donates an electron to another atom. This stabilizes both atoms. Because one atom essentially gains an electron and the other loses it, an ionic bond is polar. In other words, one atom in the bond has a positive charge, while the other has a negative charge. Often, these atoms dissociate into their ions in water. Atoms that participate in ionic bonding have different electronegativity values from each other. If you look at a table of electronegativity values, it is apparent ionic bonding occurs between metals and nonmetals. Examples of compounds with ionic bonds include salt, such as table salt (NaCl).
Four different methods are described for validating the results of a particular analysis. Indicate for each whether the method primarily checks the accuracy of the analysis or the precision of the analysis. Method 1: Five aliquots of the same sample are injected for a gas-chromatographic analysis by one person on the same day. a. precision.b. accuracy.Method 2: A known amount of analyte is added to an aliquot of the sample and analyzed with the sample. a. precision.b. accuracy. Method 3: Aliquots from a blood sample are sent to three separate laboratories for analysis using the same method. a. precision.b. accuracy. Method 4: Identical standards are analyzed by two different methods. a. accuracy.b. precision.
Answers
Answer:
Method 1 - precision
Method 2 - accuracy
Method 3 - precision
Method 4 - accuracy
Explanation:
The accuracy of a method refers to how close the experimental result is to the accepted value. Accuracy could be checked by carrying out the required test on the standard and not on the sample or by adding a known amount of analyte to the sample so that the results obtained can be carefully compared.
Precision on the other hand, refers to how close the results of a replicate analysis are to each other. In testing for precision, the analysis must be carried out several times, in order to check how close the results are to each other.
What are the coefficients when the following reaction is properly balanced?
Si4C3 +o2 -> si2o3+ c
Answers
The balanced equation for the given reaction is:
4 Si4C3 + 15 O2 → 8 Si2O3 + 3 C
What is Balanced Chemical Equation?
The coefficients in a balanced chemical equation represent the stoichiometric relationship between the reactants and products. They show the relative amounts of each substance that are involved in the reaction. In the given chemical equation, Si4C3 + O2 -> Si2O3 + C, the coefficients can be determined by balancing the number of atoms of each element on both sides of the equation.
Starting with Si, there are 4 Si atoms on the left and 2 Si atoms on the right, so a coefficient of 2 is needed in front of Si2O3 to balance the number of Si atoms.
Moving on to C, there are 3 C atoms on the left and 1 C atom on the right, so a coefficient of 3 is needed in front of C to balance the number of C atoms.
Finally, for O, there are 2x3=6 O atoms on the left and 2x2=4 O atoms on the right, so a coefficient of 3 is needed in front of O2 to balance the number of O atoms.
The balanced equation is thus: Si4C3 + 3O2 -> 2Si2O3 + 3C, with coefficients of 1, 3, 2, and 3 for Si4C3, O2, Si2O3, and C, respectively.
Learn more about Balanced Chemical Equation from given link
https://brainly.com/question/29367108
#SPJ1
I NEED HELP! Match the terms with their definitions
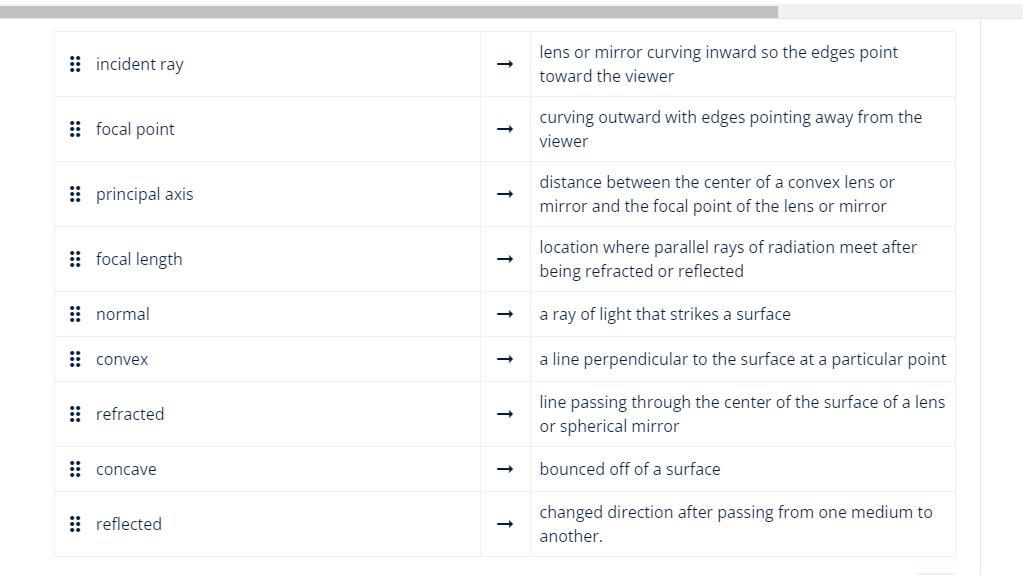
Answers
Incident Ray: A ray of light that propagates toward a surface or interface where it can be reflected, refracted, or absorbed.Focal point: The point where all parallel rays of light passing through a convex lens or concave mirror converge or appear to converge after refraction or reflection.
Principal axis: An imaginary line passing through the center of curvature and the optical center of a curved mirror or lens.Focal length: The distance between the center of a convex lens or concave mirror and their focal point.
Normal: A line perpendicular to the surface of a mirror or lens at the point of incidence of a ray of light.Convex: A lens or mirror that curves outward with the edges facing away from the viewer.
Refraction: The change in direction of a ray of light as it passes through a medium with a different index of refraction, such as air to glass or water to air.Concave: A lens or mirror that curves inward so that the edges face the viewer.
Reflected: The change in direction of a light beam as it strikes a surface and is reflected back.A lens or mirror curved inward so that the edges face the viewer: Concave is Curved outwards with edges pointing away from the viewer: Convex.
Learn more about convex mirror at :
https://brainly.com/question/31234954
#SPJ1
J.J. Thomson discovered the electron in 1897. In 1904, he proposed a model of an atom, describing that there was an equal distribution of negative and positive charges throughout the atom. In 1909, Ernest Rutherford tested J.J. Thomson’s model by shooting positive particles at gold foil. Based on Thomson's model, it was predicted that the particles would fly through the foil with a small amount deflected back. Analyzing the results, Rutherford discovered that more of the particles bounced back than expected. Which of the following best explains how the results of Rutherford’s experiment affected Thomson’s widely-accepted atomic model?
A. Rutherford’s results were invalidated and discarded because Thomson’s model was correct.
B. Rutherford’s results supported parts of Thomson's model, but also provided new data and interpretations.
C. Rutherford’s results suggested that the model proposed by Thomson was based on false research and required a change in his hypothesis.
D. Rutherford’s results supported Thomson’s model that there was a negative core surrounded by positive charges and caused a modification in the overall atomic theory.
Answers
Rutherford's results declined the results of Thomson's model, the correct option is C.
What are Atomic models?Atomic Models are the scientific theories proposed to determine the structure of an atom.
There are mainly 5 theories proposed for atomic models.
1. John Dalton's Atomic Model: An atom is the basic building block of all physical entities in the universe.
2. Plum Pudding Model, created by J.J. Thomson, uses the comparison of plum pudding, where the positive charge is uniformly dispersed throughout and the negative charge is randomly sprinkled on top, to explain how subatomic particles are structured.
3. Rutherford's model: proved the presence of a nucleus.
4.Niel Bohr's model: Arrangement of electrons in shells.
5. Erwin Schrodinger's model is also called as Quantum Model.
In J.J. Thomson's model, the equal distribution of positive and negative charges is proposed while,
Rutherford's theory declines this arrangement and proposes that the positively charged nucleus occupies a very small part and there is empty space in the atom.
So, Rutherford’s results suggested that the model proposed by Thomson was based on false research and required.
To know more about Atomic Models
https://brainly.com/question/9145431
#SPJ6
calculate number of water molecules and number of oxygen and hydrogen atom in a drop of water containing 0.05 mole of water
Answers
Answer: \(0.301\times 10^{23}\) molecules of water
\(0.6023\times 10^{23}\) atoms of hydrogen
\(0.301\times 10^{23}\) atoms of oxygen
Explanation:
According to avogadro's law, 1 mole of every substance occupies 22.4 L at STP and contains avogadro's number \(6.023\times 10^{23}\) of particles.
1 mole of water \((H_2O)\) contains = \(6.023\times 10^{23}\) molecules of water
Thus 0.05 moles of water \((H_2O)\) contain = \(\frac{6.023\times 10^{23}}{1}\times 0.05=0.301\times 10^{23}\) molecules of water
Now , 1 molecule of water contains= 2 atoms of hydrogen
\(0.301\times 10^{23}\) molecules of water contain = \(\frac{2}{1}\times 0.301\times 10^{23}=0.6023\times 10^{23}\) atoms of hydrogen
Now , 1 molecule of water contains = 1 atom of oxygen
\(0.301\times 10^{23}\) molecules of water contain = \(\frac{1}{1}\times 0.301\times 10^{23}=0.301\times 10^{23}\) atoms of oxygen
A voltaic cell is constructed with an Ag/Ag half-cell and a Pb/Pb2 half-cell. The silver electrode is positive. Write the balanced half-reactions and the overall reaction. Include the phases of each reactant and product.
Answers
Answer:
Following are the chemical equation to the given question:
Explanation:
The Electrode is a silver film that is covered with such a thin coating of silver chloride, either by dipping its wire directly into silver-molten chloride, plating the wire using hydrogen peroxide, or oxidation silver in a chloride. In the given silver electrode, this anode acts as a cathode and thus reduces.
Half of the response reduction: \(Ag^+(aq)+e^-\rightarrow Ag(s)\)
Half-effect oxidation: \(Pb(s)\rightarrow Pb^{2+}(aq)+2e^-\)
Complete reaction: \(Pb(s)+2Ag^+(aq) \rightarrow Pb^{2+}(aq)+2Ag(s)\)
According to Charles's Law, ________ and ___________ are directly proportional, with ______________ being the constant.
Answers
Answer: A) Volume of a gas B) Number of molecules C) temperature &pressure
Explanation: The law also states that the Kelvin temperature and the volume will be in direct proportion when the pressure exerted on a sample of a dry gas is held constant.
HELP ME PLLSS (with both)
2. What is the ratio of moles of CuSO4 to moles of NaOH?

Answers
Answer: 1. 1 : 2 2. NaOH is the limiting reactant
Explanation: Hope this helps you
Answer:
2. Ratio 1CuSO4 : 2NaOH
Explanation:
CuSO4 + 2NaOH ⟶ Cu(OH)2 + Na2SO4
so 1 mole of CuSO4 will react with 2 moles of NaOH
therefore the ratio is 1:2
Hope this helps
How many grams of CaCl2 are needed to make 923.3 g of a solution that is 32.5
% (m/m) CaCl2 in water? Note that mass is not technically the same thing as weight, but % (m/m) has the same meaning as % (w/w).
grams CaCl2:
How many grams of water are needed to make this solution?
grams H2O:
Answers
623.0 g of water are needed to make 923.3 g of a 32.5% (m/m) solution of CaCl2 in water.
We can start by using the definition of percent by mass to calculate the mass of CaCl2 in the solution:
% mass of CaCl2 = (mass of CaCl2 / mass of solution) x 100
Rearranging this equation to solve for the mass of CaCl2:
mass of CaCl2 = (% mass of CaCl2 / 100) x mass of solution
Substituting the given values:
mass of CaCl2 = (32.5 / 100) x 923.3 g = 300.3 g
Therefore, 300.3 g of CaCl2 are needed to make 923.3 g of a 32.5% (m/m) solution of CaCl2 in water.
To find the mass of water needed, we can subtract the mass of CaCl2 from the total mass of the solution:
mass of water = mass of solution - mass of CaCl2
mass of water = 923.3 g - 300.3 g = 623.0 g
For more question on water click on
https://brainly.com/question/24304533
#SPJ11
1.. A saturatedsolutionA.contains more solute thansolvent.B.contains more solvent thansolute.C.contains equal moles of solute andsolvent.D.contains the maximum amount of solute that will dissolve in that solvent at that temperature.E.contains a solvent with only sigma bonds and no pi bonds (i.e. only single bonds, with no doubleor triplebonds).
Answers
Answer:
D. contains the maximum amount of solute that will dissolve in that solvent at that temperature.
Explanation:
Solubility is a term used to describe how readily a substance can be dissolved in a solvent to form a solution. Thus, a substance is said to be soluble if it dissolves completely in a solvent and insoluble if it doesn't dissolve or only dissolves partially.
For example, sodium chloride (NaCl) when mixed with water dissociates into sodium and chloride ions. Thus, salt (sodium chloride) is said to be soluble because it dissolves completely in water.
Hence, a compound that dissolves in water to produce an aqueous solution is said to be soluble in water. Also, the solute of an aqueous solution that conducts electricity is called an electrolyte.
In Chemistry, dissolution can be defined as the process of dissolving or dissociating a solute in solid, liquid or gaseous phase into fragmented particles by a solvent in order to form a solution. For dissolution to occur in solids, the crystalline structure of the substance must be broken up so as to release ions, atoms or molecules to produce a solution. For liquid and gases, the substance to be dissolved must form a non-covalent bond with the solvent to produce a solution.
Generally, a saturated solution contains the maximum amount of solute that will dissolve in that solvent at that temperature.
A natural gas (CH4) furnace leaks into the room which is at one atmospheric
pressure and 18°C. The flammability limits of methane are approximately 0.5 to
1.6. When this room is closed and leak tight, it would take between 38 – 56 hours
to reach flammability range. What would you predict happens when the same
scenario occurs in a home in the mountains of New Mexico where the atmospheric
pressure is 0.89 atm? Explain in terms of combustible range and relative ratios.
b) Where do you expect to find methane “residues” in a room?
Answers
The flammability of natural gas ranges from 5 to 15 percent. This indicates that no combustion would take place in any mixture with a natural gas to air ratio of less than 5% or greater than 15%.
50% LEL methane – what is it?The lowest concentration of a gas at which combustion can occur is known as the LEL. A reading in%LEL measures the percentage of that LEL value. The LEL, for instance, is 5% by volume for methane. Half of that amount, or 2.5% by volume, is 50% LEL.
How do you determine methane's LEL?Divide the unknown concentration by the LEL specified in the NFPA Handbook to determine the LEL of any gas in air.
To know more about flammability visit:-
brainly.com/question/13323225
#SPJ1
PLS ANSWER
if a gas is initially at 400L and 600K, then the temperature drops to 295K, what is the new volume?
A) 215 L
B) 305 L
C) 197 L
D) 150 L
Answers
The new volume is approximately 197 L. The correct option is C
How to determine the new volume ?
We can use the combined gas law, which relates the pressure (P), volume (V), and temperature (T) of a gas. The combined gas law states that:
(P1 x V1) / T1 = (P2 x V2) / T2
Where
P1, V1, and T1 are the initial pressure, volume and temperature P2, V2, and T2 are the final pressure, volume and temperatureIn this case, we know that the initial volume (V1) is 400 L and the initial temperature (T1) is 600 K. We want to find the final volume (V2) when the temperature (T2) drops to 295 K. We can assume that the pressure is constant.
Substituting these values into the combined gas law, we get:
(P1 x V1) / T1 = (P2 x V2) / T2
P1 and P2 are the same, so we can cancel them out:
V1 / T1 = V2 / T2
Substituting the values we know:
400 L / 600 K = V2 / 295 K
Solving for V2, we get:
V2 = (400 L / 600 K) x 295 K
V2 = 196.67 L
So, the new volume is approximately 197 L
Therefore the correct option is C
Learn more about combined gas law here : brainly.com/question/29568314
#SPJ1
Draw the Lewis structure of sulfanilamide. Include all lone pairs
Answers
Sulfanilamide (NH2-SO2-NH-C6H4-NH2) can be represented by the following Lewis structure.
What is the Lewis structure?
The Lewis structure for a molecule is a type of structural formula that shows how the atoms are bonded together in a molecule. It uses symbols to represent the elements and to show the number of valence electrons. It also shows the number of covalent bonds between the atoms and any lone pairs of electrons.
N-H | S=O | N-C=C-C=C-C=C-N
H | | H
H | | H
H | | H
The Lewis structure of sulfanilamide (NH2-SO2-NH-C6H4-NH2) consists of two nitrogen atoms, two oxygen atoms, and six hydrogen atoms. The two nitrogen atoms are bonded to each other by a double bond, and each nitrogen is also bonded to two hydrogen atoms. The two oxygen atoms are both single bonded to the two nitrogen atoms, and each oxygen is also bonded to one hydrogen atom. The six hydrogen atoms are all single bonded to the other atoms in the structure.
To know more about the Lewis structure,
https://brainly.com/question/29606276
#SPJ1

The specific heat of aluminum is 0.900 J/g*°C, the specific heat of air is 1.01 J/g*°C and the specific heat of iron is 0.450 J/g*°C.
Identify the correct statement.
Air requires the most energy to increase its temperature followed by iron and then aluminum.
Iron requires the most energy to increase its temperature followed by aluminum and then air.
Air requires the most energy to increase its temperature followed by aluminum and then iron.
Iron requires the most energy to increase its temperature followed by air and then aluminum.
Answers
The correct statement regarding the specific heat capacity of the substance is as follows: air requires the most energy to increase its temperature followed by aluminum and then iron.
What is specific heat capacity?Specific heat capacity of a substance refers to the amount of thermal energy required to raise the temperature of a system by one temperature unit (1°C or 1K) without any change of phase.
According to this question, the specific heat of different elements are as follows:
Aluminum = 0.900 J/g°CAir = 1.01 J/g°CIron = 0.450 J/g°CTherefore, it can be said that air requires the most energy to increase its temperature followed by aluminum and then iron.
Learn more about specific heat at: https://brainly.com/question/13145357
#SPJ1
23.780 g of acetaldehyde (CH3CHO) reacts completely with oxygen to produce acetic acid (HC2H3O2) using manganese (II) acaetate catalyst according to the equation shown below: 2CH3CHO + O2 → 2HC2H3O2 What mass of oxygen reacted with acetaldehyde ? (Atomic mass: C-12.011 g/mol; 0-15.999 g/mol; H- 1.008 g/mol). Show answer choices 34.558 g 34.494 g 8.623 g 17.247 g
Answers
The mass of oxygen, O₂ required to react with the 23.780 g of acetaldehyde, CH₃CHO is 8.636 g
How do i determine the mass of oxygen required?The mass of oxygen, O₂ required to react with the 23.780 g of acetaldehyde, CH₃CHO can be obtain as follow:
2CH₃CHO + O₂ → 2HC₂H₃O₂
Molar mass of CH₃CHO = 44.053 g/molMass of CH₃CHO from the balanced equation = 2 × 44.053 = 88.106 gMolar mass of O₂ = 31.998 g/molMass of O₂ from the balanced equation = 1 × 32 = 32 gFrom the balanced equation above,
88.106 g of CH₃CHO reacted with 32 g of O₂
Therefore,
23.780 g of CH₃CHO will react with = (23.780 × 31.998) / 88.106 = 8.636 g of O₂
Thus, the mass of oxygen required for the reaction is 8.636 g. None of the options are correct
Learn more about mass needed:
https://brainly.com/question/29263739
#SPJ1
What are the charges of the ions in an ionic compound containing cobalt(III) and fluoride ions?
Write the formula for the compound.
Answers
The charge on the ions in an ionic compound containing cobalt(III) and fluoride ions is Co³⁺ and F⁻¹ and the formula of the compound is CoF₃.
Ionic compounds are a type of chemical compound where the oppositely-charged ions of a metal and a nonmetal are attracted to each other to form an ionic bond.
The compound formed from the bonded ions will have very different properties from the elements that make up the compound.
While atoms are neutral because they have an equal number of protons and electrons, ions have a net charge and result when an atom loses or gains electrons.
Learn more about Ionic compound, here:
https://brainly.com/question/3222171
#SPJ1
4. Calculate the molarity of a solution made by dissolving 23.0 g NaCl in enough water to make 0.040 L
of solution?
Answers
Answer:
The molarity (M) of a solution is the number of moles of solute dissolved in one liter of solution. To calculate the molarity of a solution, you divide the moles of solute by the volume of the solution expressed in liters.
Explanation:
The rotational spectrum of 79BrºF shows a series of equidistant lines spaced 0-714 33 cm - apart. Calculate the rotational constant B, and hence the moment of inertia and bond length of the molecule. Determine the wavenumber of the J = 9+= 10 transition, and find which transition gives rise to the most intense spectral line at room temperature (say 300 K).
and calculate the number of revolutions per second which the Brf molecule undergoes when in (a) the J = 0 state, (b) the J = 1 state, and (c) the J = 10 state. Hint: Use E = {lwin conjunction with Eqs (2.10) and (2.13), but remember that here w is in radians per second.[its Q season 2 from fundamentals of molcular spectruscopy . banwell.c.n]
Answers
In the J = 0 state, the BrF molecule does not undergo any revolutions per second. In the J = 1 state, it undergoes approximately 0.498 revolutions per second, and in the J = 10 state, it undergoes approximately 15.71 revolutions per second.
To calculate the rotational constant B, we can use the formula:
B = 1 / (2 * π * Δν)
Where:
B = rotational constant
Δν = spacing between consecutive lines in the rotational spectrum
Given that the spacing between consecutive lines is 0.71433 cm^(-1), we can substitute this value into the formula:
B = 1 / (2 * π * 0.71433 cm^(-1))
B ≈ 0.079 cm^(-1)
The moment of inertia (I) of the molecule can be calculated using the formula:
I = h / (8 * π^2 * B)
Where:
h = Planck's constant
Given that the value of Planck's constant (h) is approximately 6.626 x 10^(-34) J·s, we can substitute the values into the formula:
I = (6.626 x 10^(-34) J·s) / (8 * π^2 * 0.079 cm^(-1))
I ≈ 2.11 x 10^(-46) kg·m^2
The bond length (r) of the molecule can be determined using the formula:
r = sqrt((h / (4 * π^2 * μ * B)) - r_e^2)
Where:
μ = reduced mass of the molecule
r_e = equilibrium bond length
To calculate the wavenumber (ν) of the J = 9+ to J = 10 transition, we can use the formula:
ν = 2 * B * (J + 1)
Substituting J = 9 into the formula, we get:
ν = 2 * 0.079 cm^(-1) * (9 + 1)
ν ≈ 1.58 cm^(-1)
To determine the most intense spectral line at room temperature (300 K), we can use the Boltzmann distribution law. The intensity (I) of a spectral line is proportional to the population of the corresponding rotational level:
I ∝ exp(-E / (k * T))
Where:
E = energy difference between the levels
k = Boltzmann constant
T = temperature in Kelvin
At room temperature (300 K), the population distribution decreases rapidly with increasing energy difference. Therefore, the transition with the lowest energy difference will have the most intense spectral line. In this case, the transition from J = 0 to J = 1 will have the most intense spectral line.
To calculate the number of revolutions per second, we can use the formula:
ω = 2 * π * B * J
Where:
ω = angular frequency (in radians per second)
J = rotational quantum number
For J = 0:
ω = 2 * π * 0.079 cm^(-1) * 0 = 0 rad/s
For J = 1:
ω = 2 * π * 0.079 cm^(-1) * 1 ≈ 0.498 rad/s
For J = 10:
ω = 2 * π * 0.079 cm^(-1) * 10 ≈ 15.71 rad/s
For more such questiosn on BrF molecule visit;
https://brainly.com/question/30624940
#SPJ8