High-temperature superconducting oxides hold great promise in the utility, transportation, and computer industries.
(b) Another common superconducting oxide is made by heating a mixture of barium carbonate, copper(II) oxide, and yttrium(III) oxide, followed by further heating in O₂:
4BaCO₃(s) + 6CuO(s) + Y₂O₃(s) → 2YBa₂Cu₃O₆.₅ (s) + 4CO₂ (g)
2YBa₂Cu₃O₆.₅ (s) + 1/2 O₂ (g) → 2YBa₂Cu₃O₇(s)
When equal masses of the three reactants are heated, which reactant is limiting?
Answers
The main class of high-temperature superconductors are in the class of copper oxides (only some particular copper oxides) especially the Rare-earth barium copper oxides (REBCOs) such as Yttrium barium copper oxide (YBCO).
What superconducting material works with the highest temperature?As of 2020, the material with the highest accepted superconducting temperature is an extremely pressurized carbonaceous sulfur hydride with a critical transition temperature of +15°C at 267 GPa.
How do high-temperature superconductors work?High-temperature superconductivity, the ability of certain materials to conduct electricity with zero electrical resistance at temperatures above the boiling point of liquid nitrogen, was unexpectedly discovered in copper oxide (cuprate) materials in 1987.
Learn more about high temperature superconductors here:
https://brainly.com/question/1657823#SPJ4Related Questions
Consider the following half-reaction balanced for an acidic solution: 2H2O + SeO2 → SeO42- + 4H+ + 2e-. What is the balanced half-reaction for a basic solution?
Answers
Answer
\(SeO_2+4OH^-\rightarrow SeO^{2-}_4+2H_2O+2e^-\)Explanation
The given balanced half-reaction for an acidic solution:
\(2H_2O+SeO_2\rightarrow SeO^{2-}_4+4H^++2e^-\)What to find:
Tha balanced half-reaction for a basic solution.
Step-by-step-solution:
To balance the half-reaction for a basic solution;
1. Add OH⁻ ions to BOTH SIDES to neutralize any H⁺
\(2H_2O+SeO_2+4OH^-\rightarrow SeO^{2-}_4+4H^++4OH^-+2e^-\)2. Combine H+ and OH- to make H2O.
\(2H_2O+SeO_2+4OH^-\rightarrow SeO^{2-}_4+4H_2O+2e^-\)3. Simplify by canceling out excess H2O
\(SeO_2+4OH^-\rightarrow SeO^{2-}_4+2H_2O+2e^-\)4. Balance the charges by adding e-
\(SeO_2+4OH^-\rightarrow SeO^{2-}_4+2H_2O+2e^-\)2. The mass of a neutron is approximately equal to the mass of a proton.
a. True
b. False
Answers
____________ In a group all on their own. No neutrons.
____________ The groups containing the transition metals.
____________ The Lewis Dot Diagram for Bromine.
____________ The Bohr Diagram for Argon.
____________ 7 valence electrons, halogen family, 74 neutrons.
____________ 16 protons, neutrons, and electrons.
____________ Creator of the periodic table.
____________ Bohr diagram for oxygen.
____________ Period 4, transition metal, 26 electrons.
____________ Noble Gases.
____________ Lewis Dot Diagram for Phosphorus.
____________ 12 protons, neutrons, and electrons.
____________ Bohr diagram for Sulfur.
____________ Full outer shell, mass is less than 10, noble gas.
____________ Lewis dot diagram for Tin.
____________ The group of the Alkali Metals.
____________ Bohr diagram for Carbon.
____________ Alkaline Earth Metal, period 4.
____________ 13 protons, 13 electrons, 14 neutrons.
____________ Same atomic number, different atomic mass.
____________ Lewis dot diagram for Barium.
____________ 8 valence electrons, period 6.
____________ Bohr Diagram for Sodium.
____________ Lewis Diagram for Sodium.
____________ The rows of the periodic table.
____________ The columns of the periodic table.
i really need help these are the only ones i really need we have 10 min plss help
Answers
Explain why I2 is a solid, Br2 is a liquid but Cl2and F2 are gases even though they are all Halogens
Answers
I₂ is a solid, Br₂ is a liquid, while Cl₂ and F₂ are gases because of their increasing molecular size and decreasing strength of their intermolecular forces.
The main factor influencing the physical states of halogens is the strength of the intermolecular forces (Van der Waals forces) between their molecules.
As you move down Group 17 in the periodic table (from F₂ to I₂), the size and mass of the halogen molecules increase. Larger molecules have a greater number of electrons, leading to stronger dispersion forces (a type of Van der Waals forces) between molecules.
For I₂, these forces are strong enough to hold the molecules together in a solid form. For Br₂, the forces are slightly weaker but still strong enough to form a liquid. However, in Cl₂ and F₂, the forces are weaker, allowing the molecules to be in a gaseous state at room temperature.
In summary, the physical states of the halogens depend on the strength of their intermolecular forces, which is influenced by the size and mass of the molecules.
To know more about intermolecular forces click on below link:
https://brainly.com/question/9007693#
#SPJ11
At what point would a chemical bond form between two atoms.
During attractive interactions
During Balanced interactions
During repulsive interactions
Answers
Answer:
A
Explanation:
Chemical bonds form when the valence electrons of one atom interact with the valence electrons of another atom.
which metal is the most easily oxidized?
A. highly active metal
B. moderately active metal
C.slightly active metal
D.an inactive metal
Answers
Answer:
B. Moderately active metal
Explanation:
An equilibrium mixture of N2, O2and NO gases at 1500K is determined to consist of 6.4 x 10-3Mof N2, 1.7 x 10-3M O2, and 1.1 x 10-5M NO. Write the equilibrium expression and solve for the equilibrium constant (Keq) for the systemat this temperature?
Answers
Answer
Explanation:O2 = 1.7×10-3M;
N2 = 6.4×10-3M;
NO = 1.1 10-5M.
Arrange the electrons in the sulfur atom in the orbital diagram. Use the periodic table to determine the number of electrons for sulfur. Drag each image to the correct location on the diagram. Each image can be used more than once.

Answers
Answer: 1s= 11
2s=11 2p= 11,11,11
3s=11 3p=11,1,1
Explanation:
The statement, that describes the electrons in the sulfur atom in the orbital diagram is "\(1s^{2}2s^{2}2p^{6}3s^{2}3p^{4}\)"
What is electrons?Electron can be referred as subatomic particle containing negative charge on it.
What is an orbital diagram?Orbital diagrams are graphical representations of electrons in an atom. Three rules can be used to create orbital diagrams. Each electron, according to the Aufbau Principle, occupies the lowest energy orbital. According to the Pauli Exclusion Principle, only two electrons can fit into a single orbital.
According to Hund's' rule, electrons enter distinct orbitals within the same sub-level before doubling up inside orbitals. A Sulphur atom is a neutral atom with the atomic number of 16, implying that it possesses a total of 16 electrons. According to the Aufbau rule, electrons will be put into the 1s orbital first, then the 2s, then the 2p, and so on. Because the s subshell can only accommodate two electrons, the first two electrons in the Sulfur electron configuration will go in the 1s orbital.The next two electrons will enter the 2s orbital, followed by the following six electrons, which will enter the 2p orbital because the p subshell can store up to six electrons. The following two electrons will enter the 3s orbital, and the final four electrons will enter the 3p orbital. As an outcome, the Sulphur electron configuration is \(1s^{2}2s^{2}2p^{6}3s^{2}3p^{4}\)
Hence the correct answer is \(1s^{2}2s^{2}2p^{6}3s^{2}3p^{4}\).
Learn more about orbital diagram and electron here
https://brainly.com/question/14487703
#SPJ2
20) When ice melts, what happens to the water molecules?
Answers
Answer:
start moving faster
Explanation:
i would guess because they slow down our freeze when they get cold
Which do you think has the greater total kinetic energy?
a. The molecules of the water in the teacup
b. The molecules of the water in the bathtub
Answers
Answer:
a The molecules of the water in the teacup
hope it helps
30 POINTS PLEASE HELP ME I NEED YALL PLEASE

Answers
6CO2 + 6H2O → C6H12O6 + 6O2
glucose produced = 1/6 x 23.6 = 3.93 moles
Answer:59/150
Explanation:
How can you figure out the properties of a star?
Answers
Answer:
Measuring distances to stars through stellar parallax. Measuring distances to stars through the moving cluster method. Astronomers measure the temperature of a star by looking at the star's color and its spectrum. The apparent brightness of a star tells how bright it seems to us
Explanation:
Answer:
Analyze the Characteristics of a Star
Explanation:
Surface Temperature
First, you want to determine the color of the star - this can determine the heat and age of the star.
Second, you want to measure the spectrum and get the spectral type.
Chemical Composition
Determine which lines are present in the spectrum.
Luminosity
Measure the apparent brightness and compensate for distance
Radical Velocity
Measure the Doppler shift in the spectrum.
Rotation
Measure the width of spectral lines.
Mass
Measure the period and radial velocity curves of spectroscopic binary stars.
Diameter
1. Measure the way a star’s light is blocked by the Moon.
2. Measure the light curves and Doppler shifts for eclipsing binary stars.
What is the percent composition of Phosphorus in Li3PO3
Answers
Answer:
you will get 17.983 g of lithium for every 100 g of lithium phosphate.
Explanation:
69.7% is the percent by mass of Lithium in Li\(_3\)PO\(_3\). A percent is obtained by multiplying the result by 100.
One approach to show the concentration for an element within a compound or component in a combination is as a mass percentage. The mass percentage is computed by dividing the total weight of the combination by the mass of each component and multiplying the result by 100%. The mass percent is calculated by dividing the mass that contains the compound and solute by the mass for the element or solute.
99.795g/mol = molar mass of Li\(_3\)PO\(_3\)
6.94g/mol = molar mass of Li
mass percentage of Li=(molar mass of Li/molar mass of Li\(_3\)PO\(_3\))× 100
=(6.94/99.7)× 100
=69.7%
To know more about mass percentage, here:
brainly.com/question/32197511
#SPJ6
What is the pH of a 1.0L solution of 0.445 M hydrocyanic acid, HCN? (Ka = 4.0 x 10-10)
a. 3.516 b. 4.250 c. 4.602 d. 4.699 e. 4.875
Answers
To find the pH of the solution, we need to first calculate the concentration of H+ ions in the solution using the dissociation constant (Ka) of hydrocyanic acid. Therefore, the correct option is (d) 4.699.
The dissociation reaction of HCN is: HCN ⇌ H+ + CN-
The expression for the Ka is: Ka = [H+][CN-]/[HCN]
We are given the Ka value as 4.0 x 10^-10, the concentration of HCN as 0.445 M, and we assume the concentration of [CN-] is negligible compared to [HCN].
Therefore, we can write: Ka = [H+][CN-]/[HCN] ≈ [H+]^2/[HCN]
Solving for [H+], we get: [H+] = sqrt(Ka x [HCN]) = sqrt(4.0 x 10^-10 x 0.445) = 1.881 x 10^-6 M
Now, we can calculate the pH using the equation: pH = -log[H+]
Substituting the value of [H+], we get: pH = -log(1.881 x 10^-6) ≈ 4.699
Therefore, the correct answer is (d) 4.699.
To know more about hydrocyanic acid click here:
https://brainly.com/question/3849827
#SPJ11
|x-8|=3x
whats the solution for x
Answers
Match each type of heat flow to how it is transmitted.
Radiation
Conduction
Convection
Direct contact
Waves
Liquid or gas

Answers
Heat flow refers to the transfer of thermal energy between two objects with different temperatures.
Heat flows from a hotter object to a cooler object until they reach thermal equilibrium and have the same temperature. This transfer can occur through conduction, convection, or radiation. Heat flow plays a fundamental role in many areas of science and technology, including thermodynamics, heat transfer, and climate science.
The correct match of the heat flow and its transmission is:
Radiation: Waves
Conduction: Direct contact
Convection: Liquid or gas
Learn more about heat flow, here:
https://brainly.com/question/9132604
#SPJ1
Which of the following is a property of an ionic compound? low boiling point soft malleable high melting point
Answers
Answer:
High melting point.
Explanation:
An example of a high melting point would be: 4215 K (3942 °C, 7128 °F).
Answer:
High melting point.
Explanation:
Use the drop-down menus to rank the boiling points of the following hydrocarbons. Use a "1" to indicate
the compound with the lowest boiling point.

Answers
Answer:
1. 4
2. 2
3. 3
4. 1
Explanation: just did it on edge 2021
Answer:
one.) 4
two.) 2
three.) 3
four.) 1
Read the article and answer the question. Aspirin Which property does aspirin have that salicylic acid does not have? ability to dull pain lower solubility lower acidity
Answers
The lower acidity is property aspirin have that salicylic acid does not have. So, option (d) is right one.
Aspirin is insoluble in water, so aspirin precipitates when water is added. Some other compounds, acetic anhydride and acetic acid, are soluble in water, but salicylic acid is sparingly soluble in cold water. For this reason, it is also called 2-hydroxybenzoic acid.
The lower solubility of salicylic acid in water compared to aspirin is due to the intramolecular hydrogen bonding in S.A. molecules around many heavy molecules (water) and dissolve each SA molecule, so that more molecules of solvent (water) is required to surround and solvate each S.A. molecule. Hence, salicylic acid is less acidic than aspirin.
For more information about salicylic acid , visit :
https://brainly.com/question/14711636
#SPJ4
Complete question:
Read the article and answer the question. Aspirin Which property does aspirin have that salicylic acid does not have?
a) ability to dull pain
b) lower solubility
c) lower acidity
Based on your answer to the previous question, would you expect meta-hydroxyacetophenone to be more or less acidic than para-hydroxyacetophenone? explain your answer.
Answers
Based on the structure of meta-hydroxyacetophenone and para-hydroxyacetophenone, we can make an assessment of their relative acidity. In both compounds, the hydroxyl group (OH) is attached to the phenyl ring. The position of the hydroxyl group relative to the acetophenone moiety is what distinguishes the two isomers.
In meta-hydroxyacetophenone, the hydroxyl group is attached to the meta position, which means it is three carbons away from the carbonyl group (C=O). In para-hydroxyacetophenone, the hydroxyl group is attached to the para position, meaning it is directly opposite the carbonyl group.The acidity of a phenolic compound is influenced by the stability of the phenoxide ion formed when the hydroxyl group loses a proton (H+). The stability of the phenoxide ion is affected by the electron density and resonance stabilization in the phenyl ring.In the case of para-hydroxyacetophenone, the para position allows for greater electron delocalization and resonance stabilization within the phenyl ring. This increased stability of the phenoxide ion makes para-hydroxyacetophenone more acidic than meta-hydroxyacetophenone.
Therefore, we would expect para-hydroxyacetophenone to be more acidic than meta-hydroxyacetophenone due to the enhanced resonance stabilization of the phenoxide ion in the para position.
To learn more about hydroxyacetophenone;
https://brainly.com/question/32196547
#SPJ11
(q001) in this exercise, you will indicate what physical property you think would make a mineral appropriate for the use indicated, and name a mineral from your set that could be used for this purpose. in some instances, more than one mineral will meet the requirements and more than one property is required. part 1.a. what physical property would make a mineral appropriate to use as an abrasive (e.g., sandpaper)?
Answers
The physical property that would make a mineral appropriate to use as an abrasive is hardness.
A mineral with a high level of hardness is able to resist being scratched or abraded, which makes it ideal for use in abrasive applications such as sandpaper. Some minerals that are commonly used as abrasives include diamond, corundum, and garnet. These minerals have a high level of hardness and are able to effectively remove material from a surface through the process of scratching or cutting. The choice of which mineral to use will depend on factors such as the type of material being worked on and the level of abrasiveness required.
To know more about abrasiveness, here
brainly.com/question/9624379
#SPJ4
50 POINTS
a 6.7g piece of rock boiled to 100.0 degrees celsius is placed in 100.0 mL of water with an initial temperature of 23 degrees celsius. the equilibrium temperature when the rock is added is 45 degrees celsius. what is the specific heat of the rock?
Answers
q = m * c * ΔT
where q is the heat absorbed or released, m is the mass of the substance, c is the specific heat of the substance, and ΔT is the change in temperature.
In this case, the heat released by the rock is equal to the heat absorbed by the water, so we can write:
q_rock = -q_water
where q_rock is the heat released by the rock and q_water is the heat absorbed by the water.
The heat released by the rock can be calculated as:
q_rock = m_rock * c_rock * ΔT
where m_rock is the mass of the rock and c_rock is the specific heat of the rock. We know that the mass of the rock is 6.7 g and the ΔT is 45 - 100 = -55 degrees Celsius (because the rock is losing heat to the water).
The heat absorbed by the water can be calculated as:
q_water = m_water * c_water * ΔT
where m_water is the mass of the water and c_water is the specific heat of water. We know that the mass of the water is 100.0 g (which is equivalent to 100.0 mL) and the ΔT is 45 - 23 = 22 degrees Celsius (because the water is gaining heat from the rock).
Since q_rock = -q_water, we can set the two equations equal to each other and solve for c_rock:
m_rock * c_rock * ΔT = -m_water * c_water * ΔT
c_rock = -m_water * c_water * ΔT / (m_rock * ΔT)
Plugging in the values, we get:
c_rock = -(100.0 g) * (4.184 J/g°C) * (22°C) / [(6.7 g) * (-55°C)]
c_rock = 0.811 J/g°C
Therefore, the specific heat of the rock is 0.811 J/g°C.
Answer:
To calculate the specific heat of the rock, you can use the formula for heat transfer: Q = mcΔT, where Q is the heat transferred, m is the mass of the substance, c is the specific heat capacity and ΔT is the change in temperature.
In this case, we can assume that the heat lost by the rock is equal to the heat gained by the water. Therefore:
Q(rock) = Q(water)
m(rock)c(rock)(T(final) - T(initial, rock)) = m(water)c(water)(T(final) - T(initial, water))
where m(rock) = 6.7 g, T(initial, rock) = 100.0°C, T(final) = 45°C, m(water) = 100.0 g (assuming the density of water is 1 g/mL), c(water) = 4.18 J/g°C (specific heat capacity of water), and T(initial, water) = 23°C.
Substituting these values into the equation above and solving for c(rock), we get:
c(rock) = (m(water)c(water)(T(final) - T(initial, water))) / (m(rock)(T(final) - T(initial, rock)))
c(rock) = (100.0 g * 4.18 J/g°C * (45°C - 23°C)) / (6.7 g * (45°C - 100.0°C))
c(rock) ≈ 1.26 J/g°C
So the specific heat of the rock is approximately 1.26 J/g°C.
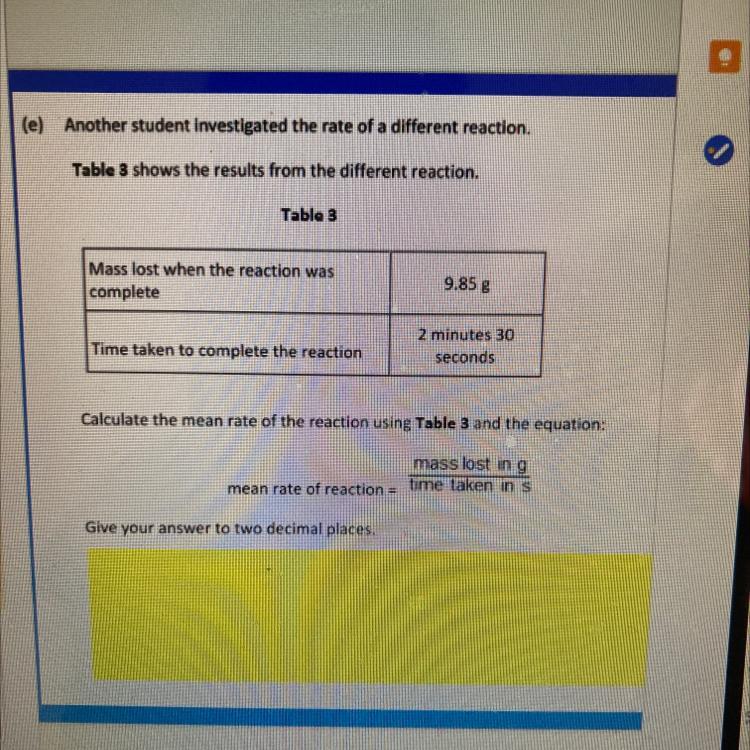
(e) Another student investigated the rate of a different reaction
Table 3 shows the results from the different reaction
Headings that you a
will appear here.
Table 3
Mass lost when the reaction was
complete
9.85 g
Time taken to complete the reaction
2 minutes 30
seconds
Calculate the mean rate of the reaction using Table 3 and the equation:
mass lost in g
mean rate of reaction time taken in s
Give your answer to two decimal places.
Answers
Answer:
0.07 g/s.
Explanation:
From the question given above, the following data were obtained:
Mass lost = 9.85 g
Time taken = 2 min 30 s
Mean rate =?
Next, we shall convert 2 min 30 s to seconds (s). This can be obtained as follow:
1 min = 60 s
Thus,
2 min = 2 × 60 = 120 s
Therefore,
2 min 30 s = 120 s + 30 s = 150 s
Finally, we shall determine the mean rate of the reaction. This can be obtained as illustrated below:
Mass lost = 9.85 g
Time taken = 150 s
Mean rate =?
Mean rate = mass lost / time taken
Mean rate = 9.85 / 150
Mean rate = 0.07 g/s
Therefore, the mean rate of the reaction is 0.07 g/s
How many moles are in 20 grams of O₂ gas?
Answers
4) When solid sodium nitrate is heated, solid sodium nitrite and oxygen gas
(02) are produced. Write the balanced chemical equation for this chemical
reaction using the correct chemical formulas and include conditions (s, l, g, or
aq). Put your answer in the box below.
Write the balanced chemical equation and state what type of chemical reaction this is.
Answers
Answer: The balanced chemical equation is \(2NaNO_{3}(s) + Heat \rightarrow 2NaNO_{2}(s) + O_{2}(g)\) and it is a decomposition chemical reaction.
Explanation:
A chemical reaction in which single compound is decomposed into two or more species is called a decomposition reaction.
For example, \(NaNO_{3}(s) + Heat \rightarrow NaNO_{2}(s) + O_{2}(g)\)
As the compound \(NaNO_{3}\) is decomposing to given two different substances. So, it is a decomposition reaction.
Here, the number of atoms on reactant side are as follows.
Na = 1N = 1O = 3The number of atoms on product side are as follows.
Na = 1N = 1O = 4To balance this equation, multiply \(NaNO_{3}\) by 2 on reactant side and multiply \(NaNO_{2}\) by 2 on product side. Therefore, the equation will be rewritten as follows.
\(2NaNO_{3}(s) + Heat \rightarrow 2NaNO_{2}(s) + O_{2}(g)\)
Here, number of atoms on reactant side are as follows.
Na = 2N = 2O = 6The number of atoms on product side are as follows.
Na = 2N = 2O = 6Hence, this equation is balanced.
Thus, we can conclude that the balanced chemical equation is \(2NaNO_{3}(s) + Heat \rightarrow 2NaNO_{2}(s) + O_{2}(g)\) and it is a decomposition chemical reaction.
How do isotopes affect the average atomic mass of an element?
Answers
Answer:
The versions of an element with different neutrons have different masses and are called isotopes. The average atomic mass for an element is calculated by summing the masses of the element's isotopes, each multiplied by its natural abundance on Earth.
Explanation:
a piece of oak wood has a density of 0.921 g/cm3 and a mass of 7.31 g. what is the volume of this piece of wood? enter just the number - not the unit and round to 3 significant digits
Answers
The volume of this piece of oak wood is 7.93 cubic centimeters.
Density is defined as the mass per unit volume of a substance. The mass of the piece of oak wood is given as 7.31 g and the density is given as 0.921 g/cm³.
We can use the formula for density to find the volume of the piece of oak wood, which is given as: [Density = frac{Mass}{Volume}]
Rearranging the formula, we get:
[Volume = frac{Mass}{Density}]Substituting the given values, we get:
[Volume = frac{7.31 g}{0.921 g/cm³}]
Volume = 7.9271772058 cm³
Rounding to 3 significant digits, we get the volume of the piece of oak wood as:
Volume = 7.93 cm³
Learn more about volume from:
https://brainly.com/question/14197390
#SPJ11
Write the correct abbreviation for each metric unit.
1) Kilogram __ 4) Milliliter __ 7) Kilometer __ 2) Meter 5) Millimeter __
8) Centimeter __ 3) Gram __ 6) Liter __ 9) Milligram __
Answers
The correct abbreviation for each metric unit is:
Kilogram - kg, Milliliter - ml, Kilometer- Km, Meter- m, Millimeter - mm, Centimeter - cm, Gram - g, Liter - L, and Milligram - mg.
What is the metric system?The metric system can be described as a system of measurement that succeeded the decimalized system based on the meter. Each of the fundamental dimensions can be expressed by a single base unit of measure.
For quantities derived from the base units of the system, units derived from the base units are used such as the square meter being the derived unit for the area, a quantity derived from length.
Metric units can be described as units based on the meter, gram, or second and decimal multiples or sub-multiples of these. The units of the International System of Units (SI). By extension, they involve units of electromagnetism from the CGS units and SI units systems.
Learn more about Metric units, here:
https://brainly.com/question/19483018
#SPJ1
HELP PLEASE
Which of the following BEST describes
the size of asteroids?
A. All of them are smaller than a kilometer in diameter.
B. All of them are larger than 100 kilometers in diameter.
C. They range from smaller than 1 km to about 300 km in
diameter.
D. They range from 1 km to about 100 km in diameter.
I put chemistry because there’s no astronomy
Answers
Answer:
I think B is the answer so hope
Answer:
It's C
Explanation:
A bottle of shampoo is shaped like a right cylinder. The cylinder is six inches high with a radius
of 0.9 inches. One cubic inch is the same as 16.4 milliliters. What is the capacity of the bottle in
milliliters? Round to the nearest whole number. Use the following formula to help you decide:
Vegy = 17p• where Vcy is the volume of a right cylinder, ne is about 3.14, ris the radius
of the base, and h is the height of the cylinder
A 250 ml
B 200 ml
C 150 ml
D 100 ml
Answers
Answer:
A) 250 ml
Explanation:
Vcyl= π • r2 • h -----> Vcyl= π· 0.9^2 · 6= 15.2681402964464 x 16.4 millimetres = 250.3975008617209 rounded off to 250 millimetres :)