Answers
Related Questions
- Preparation of NaPO4 solution (So): A solution (So) of sodium phosphate is to be prepared of molar concentration 0.1 mol/L. mL. 100 Given M(Na3PO)=164 g/mol. and a volume 1.1- Calculate the mass of sodium phosphate needed to prepare this solution. Deduce its mass concentration (Cm). 1.2 - Write the materials and glassware needed. 1.3- Write the equation of dissolution of sodium phosphate. 1.4- Determine the molar concentration of Na ions in this solution
Answers
Answer:
Explanation:
1.1 we have to find mass of Na3PO4;
for that we have to Calculate the moles of Na3PO4 needed:
volume is 100mL = 0.1L
Molar concentration = Moles of solute / Volume of solution in L
0.1 mol/L = Moles of Na3PO4 / 0.1 L
Moles of Na3PO4 = 0.1 mol/L * 0.1 L
Moles of Na3PO4 = 0.01 mol
Now, Calculate the mass of Na3PO4 needed:
so, Mass = Moles of Na3PO4 * Molar mass of Na3PO4
Mass = 0.01 mol * 164 g/mol
Mass = 1.64 g of Na3PO4.
1.2 materials and glassware needed:
1.64 g Sodium phosphate (Na3PO4)
100 mL volumetric flask
weighing balance
Distilled water
Glass rod
Pipette and burette
If the pH of a solution is 6.96, then the solution is a
Answers
Answer:
acid
Explanation:
solution with pH less than 7 is acid
those with more that 7 is base
those equal to 7 is neutral
Calculate the energy of a photon emitted when an electron in a hydrogen atom undergoes a transition from n=4 to n=1.energy emitted:
Answers
Answer:
\(\mathbf{\Delta E = 2.042 \times 10^{-19} \ J}\)
Explanation:
Using the Rydberg expression to calculate the change in energy of a photon emitted, we have :
\(\Delta E = R ( \dfrac{1}{n_f^2}-\dfrac{1}{n_i^2})\)
where;
R = Rydberg constant = \(2.178 \times 10 {-18} \ J\)
\(n_f\) = 1 and \(n_i\) = 4
replacing our values into equation, we get:
\(\Delta E = 2.178 \times 10^{-19} (\dfrac{1}{1^2}-\dfrac{1}{4^2})\)
\(\Delta E = 2.178 \times 10^{-19} (\dfrac{1}{1}-\dfrac{1}{16})\)
\(\Delta E = 2.178 \times 10^{-19} (\dfrac{15}{16})\)
\(\mathbf{\Delta E = 2.042 \times 10^{-19} \ J}\)
What charged object moves inside of neutral objects when a charge is induced?NeutronselectronsprotonsQuarks
Answers
We must see what is the structure of matter in its smallest form. Matter is made up of atoms, and these in turn have smaller particles. Neutrons, protons and electrons.
Neutrons have no charge, so we can start to rule out this option.
Protons are positively charged and, like neutrons, are in the center of the atom. The number of protons is always the same and it is the number of these that gives an element its name.
The quarks, are so far the smallest particles in the universe, and are inside the neutrons and protons.
Now, the electrons revolve around the nucleus and are easier to withdraw, move away or approach the center of the atom. So when there is an induced charge, the electrons will be affected.
So, the answer will be: Electrons
Circle the letter of cach sentence that is true about silica.
a. It is formed from oxygen and nitrogen. b. It makes magma thicker, .
C. It is rarcly found in the crust,
d. It produces light-colored lava.
Answers
Answer:
The correct answer choices are B and D.
Explanation:
A volcanic eruption happens when magma reaches the surface of the earth. Depending on the amount of gas and the silica content of the magma, the lava and the type of eruption can vary. Silica, which forms from the elements silicon and oxygen, makes the lava thicker and less runny. When lava is thick, the volcanic eruption tends to be more explosive. The amount of silica also changes the color and density of the lava, which then makes different kinds of igneous rocks. As the gas content of the lava goes up, the eruption becomes more explosive.
how many moles are in 22 grams of argon
Answers
Answer:
0.551 moles
Explanation:
To calculate the number of moles in 22 grams of argon, divide the mass by the molar mass:
Number of moles = Mass / Molar mass
Number of moles = 22 g / 39.95 g/mol
Number of moles ≈ 0.551 moles
Therefore, there are approximately 0.551 moles of argon in 22 grams of argon.
- Lock the volume at 800L. ncrease.
the temperature to around 385K.
What is the Current Pressure?
Answers
Answer: Standard temperature and pressure (STP) refers to the nominal conditions in the atmosphere at sea level. These conditions are 0 degrees Celsius and 1 atmosphere (atm) of pressure. The STP value is important to physicists, chemists, engineers, pilots and navigators, among others.
A constant electric current deposited 365 mg of Ag in 216 minutes from an aqueous Silver trioxonitrate (v). What is the Current?
Answers
The electric current is 0.025 A
Electric current refers back to the go with the flow of energy in an electronic circuit and to the amount of strength flowing through a circuit. it's far measured in amperes (A). the bigger the cost in amperes, the more energy is flowing within the circuit.
Ag+ + e¯ →Ag
1F deposits 107.87 g/mol (molecular mass) of silver
1F = 96500 C
Let, 107.87 g/mol needed = 96500 C
Number of coulombs required to deposit 0.3650 g of silver =(96500/107.87) 0.3650
Q = 326.5 C
According to Faraday’s law, Q = I x t
I = 326.5 C / (216 x 60 s) = 0.025 A
Learn more about electric current here:-https://brainly.com/question/2984202
#SPJ9
Help!!!
Question: What is the correct order of the particles that give texture to soil from smallest to largest?
Options:
A: Clay, sand, silt
B: silt, sand, clay
C: clay, silt, sand
D: sand, silt, clay
Answers
The correct order of the particles that give texture to the soil from smallest to largest is clay, silt, and sand; option C.
What is soil texture?Soil texture refers to a physical classification of the component and types of soils based on their physical texture either as coarse or fine particles.
There are several types of soils and these various types of soils have different textures.
The types of soils and their arrangement based on increasing particle size are as follows:
clay soil - this is the finest particle soil typesilt - this is the next soil type in terms of texturesandy soil - this is the largest of the three soil types in terms of size and texture.Learn more about soil texture at: https://brainly.com/question/8513717
#SPJ1
This is my question in imageIf 16.0 grams of aluminum oxide were actually produced, what is the percent yield of the reaction below given that you start with 10.0 g of Al and 19.0 grams of O2?Reaction: 4Al + 3O2 → 2Al2O3Group of answer choices70%39.6%75.0%100%85.0%

Answers
Answer: the percent yield of this reaction was 84.7% and the best option to answer the question is the last one (letter E, 85.00%)
Explanation:
The question requires us to determine the percent yield of a reaction, given the amount of product obtained, the chemical equation and the amount of reactants used.
The following information was provided by the question:
mass of Al2O3 produced = 16.0 g
mass of Al used = 10.0 g
mass of O2 used = 19.0 g
balanced chemical equation:
\(4Al+3O_2\rightarrow2Al_2O_3\)To solve this problem, we'll need to go through the following steps:
1) Calculate the number of moles used of each reactant;
2) determine the limiting reactant from the stoichiometry of the reaction and the amount of reactants used;
3) calculate the theoretical yield of the reaction, or, in other words, the amount of Al that should be produced, considering the limiting reactant;
4) calculate the percent yield of the reaction.
Next, we'll go through these steps to solve the problem:
1) Calculating the number of moles of each reactant
We can use the following equation to determine the amount of moles of Al and O2 that were used in the reaction:
\(n=\frac{m}{MM}\)where n is the number of moles (in mol), m is the mass of the sample (in grams) and MM is the molar mass of the compound (in g/mol).
Knowing that the molar masses of Al and O2 are 26.98 and 31.98 g/mol, respectively, we can calculate the number of moles of each reactant as:
\(\begin{gathered} n_{Al}=\frac{10.0g}{26.98g/mol}=0.371mol\text{ Al} \\ n_{O_2}=\frac{19.0g}{31.98g/mol}=0.594mol\text{ }O_2 \end{gathered}\)
Therefore, 0.371 and 0.594 moles of Al and O2 were used in the reaction, respectively.
2) Determining the limiting reactant.
From the balanced chemical equation, we can see that 4 moles of Al are necessary to react with 3 moles of O2. Thus, we can determine how many moles of O2 would be necessary to react with 0.371 moles of Al:
4 mol Al ------------------- 3 mol O2
0.371 mol Al ------------- x
Solving for x, we'll have:
\(x=\frac{(3mol\text{ }O_2)\times0.371mol\text{ Al\rparen}}{(4mol\text{ Al\rparen}}=0.278mol\text{ }O_2\)Therefore, 0.278 moles of O2 would be necessary to react with the used amount of Al (0.371 mol). Since the actual amount of O2 used is greater than the necessary amount, we can say that O2 is the excess reactant and Al is the limiting reactant.
3) Calculating the theoretical amount of Al2O3 produced
Now that we know that Al was the limiting reactant in this reaction, we can determine how much Al2O3 should be produced in the reaction.
From the balanced chemical equation, we can see that 4 moles of Al are necessary to produce 2 moles of Al2O3. Thus, we can write:
4 mol Al --------------------- 2 mol Al2O3
0.371 mol Al --------------- y
Solving for y, we'll have:
\(y=\frac{(2mol\text{ A}l_2O_3)\times(0.371mol\text{ Al\rparen}}{(4mol\text{ Al\rparen}}=0.186mol\text{ A}l_2O_3\)Therefore, with the amount of Al used, 0.186 moles of Al2O3 would be produced.
We can convert this amount in mass of Al2O3 using its molar mass (MM = 101.96 g/mol):
\(\begin{gathered} n=\frac{m}{MM}\rightarrow m=n\times MM \\ n_{Al_2O_3}=(0.186mol)\times(101.96g/mol)=18.91g \end{gathered}\)Therefore, 18.9 g of Al2O3 should be obtained from the given mass of Al given.
4) Calculating the percent yield of the reaction
Note that the amount of Al2O3 expected, from the amount of reactants given, was 18.9g, but only 16.0g of the product was obtained. We can calculate the percent yield of a reaction using the following equation:
\(\begin{gathered} percent\text{ yield = }\frac{actual\text{ yield \lparen g\rparen}}{theoretical\text{ yield \lparen g\rparen}}\times100\% \\ \\ \%yield=\frac{16.0g}{18.9g}\times100\%=84.7\% \end{gathered}\)Therefore, the percent yield of this reaction was 84.7% and the best option to answer the question is the last one (letter E, 85.00%).
How is steel made from the raw product of the blast furnace known
as "pig iron"? What are the advantages of using steel?
List references used (if any were used) to answer this question.
Answers
Steel is produced from pig iron through a process known as steelmaking or iron and steel production.
The pig iron obtained from the blast furnace contains high amounts of carbon, impurities, and other elements. To convert pig iron into steel, the carbon content needs to be reduced to desired levels, and impurities must be removed.One common method of steelmaking is the basic oxygen process (BOP). In this process, pig iron is placed in a vessel called a converter, where oxygen is blown through the molten metal. The oxygen reacts with the carbon and impurities, causing them to oxidize and form gases that are released. Alloying elements and desired additives can be added at this stage to achieve specific steel properties. Another method is the electric arc furnace (EAF), where an electric arc is used to heat and melt the pig iron, allowing impurities to be oxidized and removed.The advantages of using steel are numerous. Steel is strong, durable, and versatile, making it suitable for a wide range of applications. It has high tensile strength, which means it can withstand heavy loads and pressures. Steel is also resistant to corrosion, making it ideal for construction, infrastructure, and transportation projects. It is a recyclable material, contributing to sustainability and reducing environmental impact. Additionally, steel can be fabricated into various shapes and sizes, allowing for customization and flexibility in design.References:
A. Ghosh and A. Chatterjee, Ironmaking and Steelmaking: Theory and Practice, PHI Learning, 2008.
R.H. Tupkary and V.R. Tupkary, An Introduction to Modern Iron Making, Khanna Publishers, 2010.
J.R. Davis, ed., ASM Specialty Handbook: Carbon and Alloy Steels, ASM International, 1995.
for such more questions on production
https://brainly.com/question/25597694
#SPJ8
what is the molar concentration of 6.0mol of KCL dissolved in 700.ml of solution
Answers
Answer: 8.57 M KCl
Explanation:
M = moles/liters = 6.0 / 0.7 =8.57 M
Draw the Lewis electron dot
structure for COCI2.
What is the VSEPR shape of this
particle? PLS HELP
Answers
Answer:
Idon't know if this helps but I think it is a linear structure and if I am wrong I am so sorry
True or False
An element is a substance made from the atoms of two or more different substances
Answers
Answer:
True
Explanation:
element are made up of two different of substances
Answer:
true
and element is made out of atoms matter is made of atoms make everything around what we see or what takes up space
As part of an investigation of the population of foxes on Sunday Gill island a scientist graphed the number of foxes presented on the island over a Spam of 15 years as shown below the study began with the earlier 0 and run until the start of year 15 According to the graph during the witch year the event reduced the carrying capacity of the area
Answers
The carrying capacity of the area was reduced in the year 10 according to the graph that shows the number of foxes on the island over a span of 15 years.
The graph shows a population of foxes over a span of 15 years. The y-axis represents the number of foxes on the island, while the x-axis represents time. The study began with the earlier 0 and ran until the start of year 15. According to the graph, the carrying capacity of the area was reduced in the year 10.
In the graph, it is shown that the population of foxes on Sunday Gill island had a significant increase from year 0 to year 3. After year 3, the fox population started to decrease and then remained fairly constant until year 10. After year 10, the population of foxes on the island started to decline more rapidly until the end of the study in year 15
This decline in the population of foxes on the island is most likely due to the reduction in carrying capacity of the area. Carrying capacity refers to the maximum number of individuals that an environment can sustain. When the carrying capacity of an environment is reached, it means that the environment can no longer provide the necessary resources to sustain the population.
There are various factors that can cause a reduction in carrying capacity, such as environmental degradation, competition for resources, or a natural disaster. In this case, it is not clear what caused the reduction in carrying capacity in year 10, but it is likely that it was due to some environmental factor that impacted the availability of resources for the fox population.
For more such information on: graph
https://brainly.com/question/31305548
#SPJ8
Can anyone help please.......
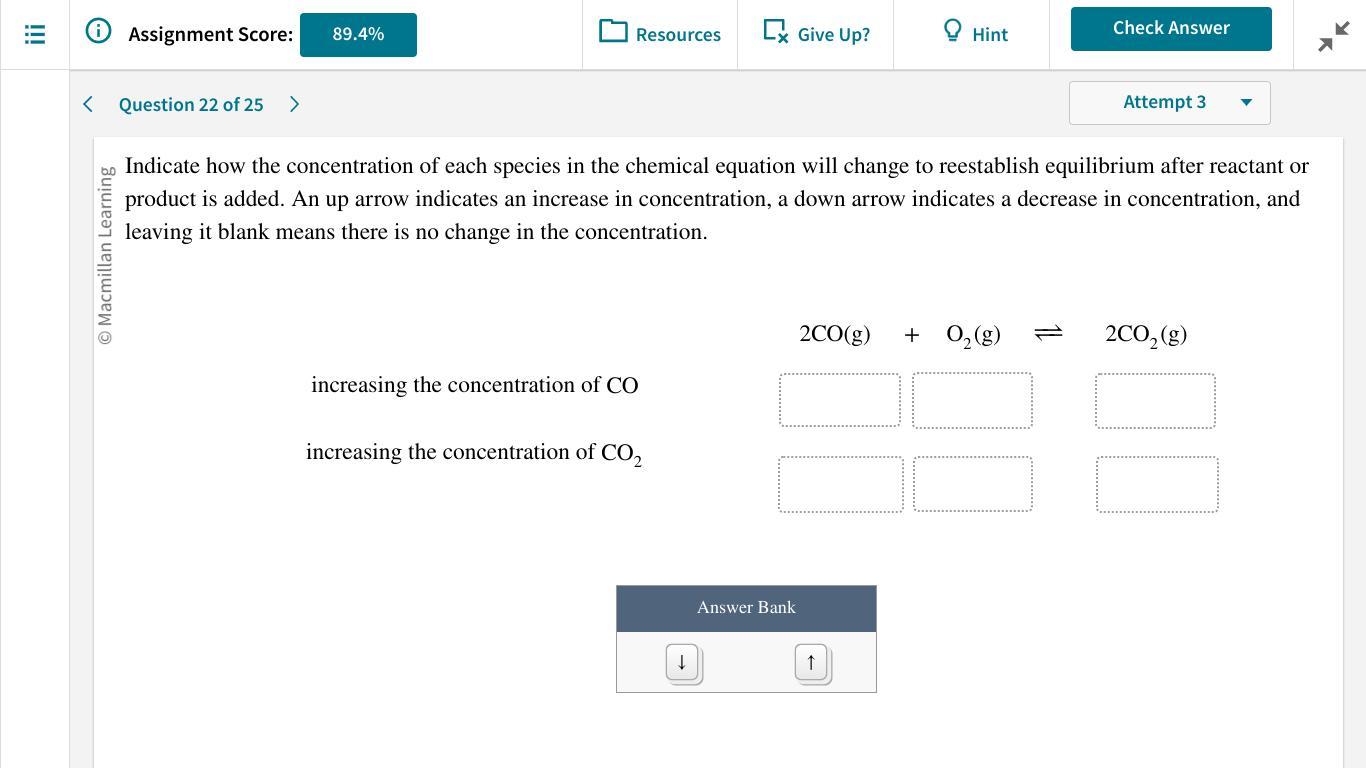
Answers
Increasing the concentration of CO decreases the equilibrium concentration of oxygen and increases the concentration of CO₂, increasing the concentration of CO₂ increases the concentration of CO and O₂.
Chemical equilibrium refers to the state of a system in which the concentration of the reactant and the concentration of the products do not change with time, and the system does not display any further change in properties.
It is the state of a reversible reaction where the rate of the forward reaction equals the rate of the reverse reaction. While a reaction is in equilibrium the concentration of the reactants and products are constant.
Learn more about Equilibrium, here:
https://brainly.com/question/30985040
#SPJ1
A stock solution of HNO3 is prepared and found to contain 14.2 M of HNO3. If 25.0 mL of the stock solution is diluted to a final volume of 0.500 L, the concentration of the diluted solution is ________ M.
Answers
Answer:
First convert volume given into same unit.
Therefore 1000 mL =1L
1000mL=1L
25.0mL=?
(25.0×1)÷1000=0.025L
but using the equation;M1×V1=M2×V2
M1=14.2M
V1=0.025L
M2=?
V2=0.5L
Therefore;. 14.2×0.025=M2×0.5
M2=(14.2×0.025)÷0.5
M2=0.71M.
A stock solution of HNO3 is prepared and found to contain 14.2 M of HNO3. If 25.0 mL of the stock solution is diluted to a final volume of 0.500 L, the concentration of the diluted solution is 0.71M.
What is the stock solution ?To make a stock solution, weigh out the proper amount of a pure solid or measure out the proper amount of a pure liquid, put it in the right flask, and then dilute it to the desired volume. Depending on the intended concentration unit, many methods can be used to measure the reagent.
First we convert volume
Then 1000 mL = 1L
1000mL= 1L
25.0mL = ?
( 25.0 × 1 ) / 1000
= 0.025L
by using the equation;
M1 × V1 = M2 × V2
M1 = 14.2M
V1 = 0.025L
M2 = ?
V2 = 0.5L
14.2 × 0.025 = M2 × 0.5
M2 = (14.2 × 0.025 ) ÷ 0.5
M2 = 0.71M.
Thus, A stock solution of HNO3 is prepared and found to contain 14.2 M of HNO3. If 25.0 mL of the stock solution is diluted to a final volume of 0.500 L, the concentration of the diluted solution is 0.71M.
To learn more about stock solution, follow the link;
https://brainly.com/question/17018950
#SPJ5
A 2.5 M solution of a weak acid is prepared. Using a pH meter, the pH is measured to be 5.1. Calculate the acid ionization constant, \(K_{a}\) , of this weak acid.
Show your work
Answers
Molarity of acid=2.5M
pH=5.1.
ka=?
Now
We need to write an eqn to show the dissociation of the acid
HA + H2O === H3O+ + A-
Writing The Equilibrium(Or Acid dissociation constant) of this reaction
Ka =[H3O+] {A-]/ {HA].
The concept behind this is
concentration of Products divided by those of reactants. Water is not written because its a pure liquid and does not affect the Equilibrium constant.
Now If you have any Idea on ICE tables..
You'd know that the concentration of acid will decrease by 2.5-x
Whilst the products...will increase by x each
Note: This is when the ratio of their Moles are in 1:1
ka= x.x/2.5-x
Since the Moles of A- and H3O+ are in 1:1... Their concentrations at equilibrium will be the same
so
Ka= x²/2.5-x
Now what is x??
x is the Hydrozonium ion concentration.
we can get it from the pH formula
pH= -log (H3O+)
Making H3O+ subject by applying Logarithm Rules
H3O+ = 10^-ph
x=10^-5.1
=7.94x10^-6.
Now back to Ka
Ka= x²/2.5-x
Ka= (7.94x10^-6)²/2.5-(7.94x10^-6)
Ka= (7.94x10^-6)²/2.4999
Ka= 2.52x10^-11.
Was a Fun One
The ionization constant of this weak acid is \(2.52*10^{-11}\). The values can be substituted in dissociation formula.
What information do we have?
Molarity of acid=2.5M
pH=5.1
To find:
ka=?
Calculation of ionization constant:\(HA + H_2O < === > H_3O^+ + A^-\)
The value of dissociation constant will be:
\(Ka =[H_3O^+] [A^-]/ {HA]\)
The Moles of A- and H3O+ are in 1:1.Their concentrations at equilibrium will be the same.
\(Ka= x.x/2.5-x\\\\Ka= x^2/2.5-x\\\\pH= -log (H_3O^+)\\\\H_3O^+ = 10^{-pH}\\\\x=10^{-5.1}\\\\x=7.94*10^{-6}\)
\(Ka= x^2/2.5-x\\\\Ka= (7.94*10^{-6})^2/2.5-(7.94*10^{-6})\\\\Ka= (7.94*10^{-6})^2/2.4999\\\\Ka= 2.52*10^{-11}\\\\\)
Thus, ionization constant of this weak acid is \(2.52*10^{-11}\).
Find more information about ionization constant here:
brainly.com/question/2284518
Find the element that is oxidized and the one that is reduced 2 FeCl3 + SnF2 --> 2 FeF2 + SnCl4
Answers
Explanation
2 FeCl3 + SnF2 => 2 FeF2 + SnCl4
The half-reaction: oxidation
\(\text{ Sn}^{+2}=>\text{ 2e}^{-1}+\text{ Sn}^{+4}\)The half-reaction: reduction
\(2\text{ Fe}^{+3}+\text{ 2e}^{-1}=>\text{ 2Fe}^{+2}\)Answer: Sn is oxidized and Fe is reduced
1. Before starting, make a prediction: If substances B and C are both in the gas phase and are at the same energy level, which of the two substances will need to have more energy transferred out in order to change to the liquid phase? Substance B or substance C? Explain your answer.
Answers
Answer:
Substance C
Explanation:
Substance C would be the answer because Substance C has a lower attraction level. Because of this, it takes more energy to take out of in order to become a liquid.
2.When copper (II) nitrate reacts with sodium hydroxide, copper (11) hydroxide is produced.
How many grams of copper (11) hydroxide can be prepared from 2.7 g of copper (II) nitrate
reacting with sodium hydroxide?
Answers
Answer:
2Cu + S ~~~> Cu2S Copper (C) reacts with sulfur (S) to form copper sulfide as shown in the equation. A scientist adds 12.7 grams of Cu to 3.2 grams of S to start the reaction.
Explanation:
An ideal gas in a sealed container has an initial volume of 2.80 L. At constant pressure, it is cooled to 18.00 °C, where its
final volume is 1.75 L. What was the initial temperature?
Ti =
'c
Answers
Answer:
\(T_1=-91.18\°C\)
Explanation:
Hello there!
In this case, given the T-V variation, we understand it is possible to apply the Charles' law as shown below:
\(\frac{T_1}{V_1}= \frac{T_2}{V_2}\)
Thus, since we are interested in the initial temperature, we can solve for T1, plug in the volumes and use T2 in kelvins:
\(T_1= \frac{T_2V_1}{V_2}\\\\T_1= \frac{(18.00+273.15)K(1.75L)}{(2.80L)}\\\\T_1=182K-273.15\\\\T_1=-91.18\°C\)
Best regards!
If the pH of a solution is 4.5 and the other pH of another solution is 7.9, what are the solutions for pH, pOH, [H+], and [OH-]?
Answers
For the solution with a pH of 7.9:
pH = 7.9
pOH = 14 - pH = 14 - 7.9 = 6.1
[H+] = 10^(-pH) = 10^(-7.9) (in mol/L)
[OH-] = 10^(-pOH) = 10^(-6.1) (in mol/L)
The pH of a solution is a measure of its acidity, while pOH is a measure of its alkalinity. The pH and pOH values are related through the equation pH + pOH = 14.
For the solution with a pH of 4.5:
pH = 4.5
pOH = 14 - pH = 14 - 4.5 = 9.5
[H+] = 10^(-pH) = 10^(-4.5) (in mol/L)
[OH-] = 10^(-pOH) = 10^(-9.5) (in mol/L)
For the solution with a pH of 7.9:
pH = 7.9
pOH = 14 - pH = 14 - 7.9 = 6.1
[H+] = 10^(-pH) = 10^(-7.9) (in mol/L)
[OH-] = 10^(-pOH) = 10^(-6.1) (in mol/L)
Note: The [H+] and [OH-] concentrations can also be calculated using the equation [H+][OH-] = 1 x 10^(-14) at 25°C.
For more questions on alkalinity, click on:
https://brainly.com/question/867708
#SPJ8
State the law of like charges
Answers
Answer:
Like charges repel each other
Explanation:
two objects that carry the same type of electric charge will exert a force of repulsion on each other.
for example, if 2 north poles of a magnet are put together they will repel each other
hope this helps!
Which of the following is least like the others on the list?
a. Amino acid
b. Ribonucleic acid
c. Nucleic acid
d. Nucleotides
Answers
Explanation:
C option i think but I didn't know
Let's do this!
Balance each equation so there are the same number of each type of atom on both sides of the
equation There is a chart above each problem to help you count the atoms
First-Count up the
number of atoms you
currently have Write
that number in the
chart for both sides of
the equation
Second-If the
numbers don't match,
try adjusting the
coefficients one at at
time Make sure to
change the number in
the chart
Remember- you can't
change the formulas!
2
Reactants
Mg
M
Mg
LI
Reactants
H
LO +
Products
Mg
N
L
O
H
но →
_Math
Products
LIOH
You should
do this in
pencil
Answers
The balanced chemical equations of the reactions are given below:
1. Mg (s) + 2 H₂O (l) ----> Mg(OH)₂ (s) + H₂ (g)
2. 2 Li (s) + 2 H₂O (l) ----> LiOH (aq) + H₂ (g)
What is a balanced equation?A balanced chemical equation is an equation in which the number of moles of atoms of elements in a given reaction is equal to the sum of the number of moles of atoms of each element that is produced.
A balanced chemical equation is in accordance with the law of conservation of mass which states that matter can neither be created nor destroyed.
When balancing chemical equations, numerical coefficients are added in front of moles of atoms of an element or moles of a given compound taking part in the reaction.
The balanced chemical equation of the reaction of magnesium and water as well as the reaction of lithium and water is given below:
Magnesium and water:
Mg (s) + 2 H₂O (l) ----> Mg(OH)₂ (s) + H₂ (g)
Lithium and water:
2 Li (s) + 2 H₂O (l) ----> LiOH (aq) + H₂ (g)
Learn more about balanced chemical equations at: https://brainly.com/question/26694427
#SPJ1
Convert 1897cm into km
Answers
Answer:
1.897 km
Explanation:
1897÷1000=1.897km
you would convert it in to 0.019 km
In which type of chemical bond are electrons transferred from 1 atom to another?.
Answers
Ionic bonds are bonds that occur due to the handover of electrons to form positive ions and negative ions whose electron configuration is the same as that of the noble gasses.
Chemical bondsChemical bonds are the forces that hold atoms in elements and compounds together. Chemical bonds can occur with several types of bonds.
Based on the electron configuration that occurs in bond formation, chemical bonds are divided into 4 types:
1. Ionic or electrovalent bonds
This bond occurs because of the electrostatic attraction between positive ions and negative ions in a chemical compound
2. Covalent bonds
Covalent bonds occur when the sharing of electron pairs from each of the bonding atoms.
3. Coordinate covalent bond
Coordinate covalent bond is a bond that uses a shared pair of electrons, but the electrons only come from one of the atoms.
4. Metallic bond
This bond is formed due to the attractive force of the metal atomic nucleus with a sea of electrons.
Learn more about chemical bonds here: https://brainly.com/question/819068#SPJ4
what is the concentration of a nitric acid solution if 10.0 ml of the solution is neutralized by 3.6 ml of 0.2 m naoh?
Answers
Answer:
The concentration of the nitric acid (HNO3) solution is 72 M.
Explanation:
To determine the concentration of the nitric acid solution, we can use the concept of stoichiometry and the equation of the neutralization reaction between nitric acid (HNO3) and sodium hydroxide (NaOH):
HNO3 + NaOH → NaNO3 + H2O
The balanced equation shows that the molar ratio between HNO3 and NaOH is 1:1. This means that 1 mole of HNO3 reacts with 1 mole of NaOH.
Given:
Volume of HNO3 solution = 10.0 ml
Volume of NaOH solution = 3.6 ml
Molarity of NaOH solution = 0.2 M
To find the concentration of the HNO3 solution, we need to calculate the number of moles of NaOH used in the neutralization reaction:
moles of NaOH = volume of NaOH solution * molarity of NaOH solution
= 3.6 ml * 0.2 M
= 0.72 mmol (millimoles)
Since the molar ratio between HNO3 and NaOH is 1:1, the number of moles of HNO3 in the solution is also 0.72 mmol.
Now, we can calculate the concentration of the HNO3 solution using the formula:
concentration (in M) = moles of solute / volume of solution (in L)
concentration = 0.72 mmol / 0.010 L
= 72 mmol/L
= 72 M
Therefore, the concentration of the nitric acid (HNO3) solution is 72 M.
Suppose certain sample takes 100 min for 750 mL water to percolate into the
soil. Calculate the rate of percolation of water.
Answers
The rate of percolation of water into the soil will be 0.125 mL per second
Percolation rateThe rate of percolation of water into the soil is determined by the volume of water that enters the soil per unit of time under specific conditions.
In this case, 750 mL of water percolated in 100 minutes.
100 minutes = 100 x 60 = 6,000 seconds
Percolation rate = 750/6000 = 0.125 mL per second
More on percolation rate can be found here: https://brainly.com/question/16882186
#SPJ1
