Answers
Answer:
Explanation:
1060 grams / 1.0 ml = 1060 g/ml ≅ 1100 g/ml (final answer has 2 sig. figs.
Answer:
Final answer => 1100 (2 sig. figs.)
Explanation:
1060 grams / 1.0 ml = 1060 g/ml ≅ 1100 g/ml (final answer).
The rule is, the final answer of the calculation should have answer rounded to the data value having the least number of significant figures of the data used in the calculation. That is to the data value having the least number of significant figures.
Counting Significant Figures (a method of determining no. of sig. figs.).
For data values without decimal in data value, count left to right until reaching 1st nonzero digit. Begin count, include all zeros after 1st zero as enclosed zeros as significant figures.
Present final results with the least number of sig. figs. as defined by data of computations.
Examples: data values without decimal. Count right to left.
12000 => 2 sig. figs. (trailing zeros are not sig. figs)
2051000 => 4 sig. figs.
300 => 1 sig. fig.
93000000 => 2 sig. fig.
Examples: data values containing decimal. Count left to right.
0.0012 => 4 sig. figs. (leading zeros are sig. figs.)
12.0035 => 6 sig. figs. (enclosed zeros are sig. figs.)
0.000001 => 6 sig. figs. (leading zeros are sig. figs.)
13.010 => 5 sig. figs. (enclosed zeros are sig. figs.)
--------------------------------------------------------------------------------------------
=> Go to Y o u T u b e => search 'Counting Significant Figures'
Related Questions
1.Draw the born-Haber lattice energy cycle for sodium chloride. Explain the concept of resonance using the nitrate ion structure.
Answers
Answer:
1. Born Haber cycle is used to calculate enthalpy of formation of an ionic solid
2. Resonance structures are used to represent the bonding in some chemical species.
Explanation:
The Born–Haber cycle is a method popularly known in chemistry used in computing enthalpy. The enthalpy of formation of an ionic solid cannot be measured directly. The lattice enthalpy refers to the enthalpy change involved in the formation of an ionic compound from gaseous ions the process is exothermic process. A Born–Haber cycle works on the principle of Hess's law. It can be used to calculate the lattice enthalpy by comparing the standard enthalpy change of formation of the ionic compound from the elements to the enthalpy required to make gaseous ions from the elements.
Resonance is an idea introduced by Linus Pauling to explain chemical bonding from the valence bond perspective. The idea of resonance affords us the opportunity to describe the bonding in certain molecules by combining several structures called chemical or canonical structures. The real structure of the specie lie somewhere between the structures indicated by the resonance structures. The resonance structures of the nitrate ion are shown in the image attached.


EASY CHEM, WILL GIVE BRAINLIEST!!

Answers
Hi there!
\(\large\boxed{4.04g}\)
To solve, we can use dimensional analysis to convert from molecules to grams.
We must use Avogadro's number (6.02 × 10²³) in converting from molecules to moles.
After converting, multiply by the atomic mass, or grams per mole.
\(1.204 * 10^{24} moles *\frac{1 mol}{6.02*10^{23}moles}* \frac{2.02g}{1mol }= 4.04g\)
What is the C - O bond order in carbon dioxide?
Answers
Answer:
The bond order between carbon and oxygen in carbon dioxide (CO2) is two.
The Lewis structure of CO2 shows that each of the two double bonds (C=O) consists of one sigma bond and one pi bond. The bond order is the number of electron pairs being shared by a pair of atoms in a molecule, divided by the number of chemical bonds between the same pair of atoms. In CO2, there are two double bonds between carbon and oxygen, and each double bond has a bond order of 2. Therefore, the overall bond order between carbon and oxygen in CO2 is 2.
At 52°C, the pressure inside a gas-filled container is 467 kPa.
What is the absolute temperature of the gas?
O 163 K
O 325 K
O 335 K
O 651 K
Answers
The absolute temperature of the gas is 325 K.
To determine the absolute temperature of the gas, we need to convert the given temperature from Celsius to Kelvin. The Kelvin scale is an absolute temperature scale where 0 K represents absolute zero, which is the lowest possible temperature.
To convert from Celsius to Kelvin, add 273.15 to the Celsius value. In this case, the given temperature is 52°C. So, to convert it to Kelvin, we perform the following calculation:
Temperature in Kelvin = 52°C + 273.15 = 325.15 K
Therefore, the absolute temperature of the gas is 325.15 K.
It's important to note that the Kelvin scale is used in scientific calculations involving temperature because it directly corresponds to the average kinetic energy of particles in a substance. Absolute zero on the Kelvin scale (0 K) represents the complete absence of molecular motion. The Kelvin scale is particularly useful in gas laws and other thermodynamic calculations as it provides a meaningful reference point.
know more about temperature here:
https://brainly.com/question/2339046
#SPJ8
How many grams of Aluminum Sulfate do you have if you have 2.837x10^26 atoms of Sulfur?
Apparently, the right answer is 5.373x10^4, but I do not know how to get there, please help.
Answers
The mass of Aluminum Sulfate is 5.373 grams if you have \(2.837*10^{26\) atoms of Sulfur .
The molecular formula of Aluminum Sulfate is \(Al_2(SO_4)_3.\) In one molecule of aluminum sulfate, there are 3 sulfur atoms. To calculate the mass of aluminum sulfate, follow the steps below:
Step 1: Calculate the molar mass of aluminum sulfate using the periodic table.Al = 27.0 g/molS = 32.1 g/molO = 16.0 g/mol
(2 × Al) + (3 × S) + (12 × O) = molar mass of \(Al_2(SO_4)_3.\) = 342.2 g/mol
Step 2: Find the number of moles of sulfur in the given number of atoms of sulfur.2\(2.837*10^{26\) atoms of sulfur × 1 mol S/\(6.022 * 10^{23\)atoms S = 0.0470 mol S
Step 3: Use the molar ratio of sulfur to aluminum sulfate to calculate the number of moles of aluminum sulfate.1 mol \(Al_2(SO_4)_3.\) / 3 mol S = 0.333 mol\(Al_2(SO_4)_3.\) per mol S0.0470 mol S × 0.333 mol \(Al_2(SO_4)_3.\)/mol S = 0.0157 mol \(Al_2(SO_4)_3.\)
Step 4: Calculate the mass of aluminum sulfate.0.0157 mol \(Al_2(SO_4)_3.\) × 342.2 g/mol\(Al_2(SO_4)_3.\)= 5.373 g\(Al_2(SO_4)_3.\)
Therefore, the mass of Aluminum Sulfate is 5.373 grams if you have \(2.837*10^{26\) atoms of Sulfur.
Know more about aluminum sulfate here:
https://brainly.com/question/28299913
#SPJ8
Look at the structure of ethane below and answer the following questions:
A. Calculate the electronegativity difference between the C and H atoms using the table below.
B. Copy the molecule and show where you think the partial + and partial - charges would be.
C. Is the ethane molecule more or less polar than water? Why or why not?
D. If the oceans were filled with ethane rather than water how might they be different? (Hint: think about hydrogen bonding)
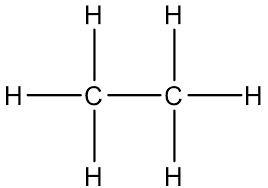
Answers
Answer:
Hydrogen + carbon - hydrochloride
A 25 ml solution of 0.5 M NaOH is titrated until neutralized into a 50 ml sample of HCl?
Answers
The concentration of the acid is \(0.25 M\).
Titration is a laboratory technique used to determine the concentration of a substance in a solution by reacting it with a standardized solution of known concentration.
The titration formula can be given by,
(Volume of the Base) \(\times\) (Normality of the Base) = (Volume of the Acid) \(\times\) (Normality of the Acid)
\(\Rightarrow V_1N_1=V_2N_2\)
Given, the volume of the base (\(NaOH\)), \(V_1 =25 ml\).
The concentration of the base (\(NaOH\)), \(M_1=0.5 M\).
The equivalence of the base (\(NaOH\)) is \(1\).
Hence, the normality of the base (\(NaOH\)), \(N_1=\frac{0.5}{1}N=0.5N\).
Given, the volume of the acid (\(HCl\)), \(V_2 =50 ml\).
Let us assume that the normality of the acid (\(HCl\)) \(N_2\).
Substitute the values in the given formula of titration.
\((25\times0.5)=(50 \times N_2)\\\Rightarrow 12.5=50N_2\\\Rightarrow N_2=\frac{12.5}{50} N\\\Rightarrow N_2=0.25 N\)
Hence, the normality of the acid (\(HCl\)), \(N_2=0.25 N\).
The equivalence of the acid (\(HCl\)) is \(1\).
Therefore, the concentration of the acid, \(M_1=\frac{0.25}{1}=0.25 M\).
Learn more about titration here: brainly.com/question/186765
You have a solution which is 45.6% by mass isopropyl alcohol (MM = 60.11 g/mol) in water. The density of this solution is 0.905 g/mL.
What is the molality of the isopropyl alcohol in the solution?
Answers
The molality of the isopropyl alcohol in the solution is 13.95 m
AssumptionLet the mass of the solution be 100 g. 45.6% by mass isopropyl alcohol = 45.6 gMass of solvent = 100 – 45.6 = 54.4 gDetermination of the mole Mass of isopropyl alcohol = 45.6 gMolar mass of isopropyl alcohol = 60.11 g/molMole of isopropyl alcohol =?
Mole = mass / molar mass
Mole of isopropyl alcohol = 45.6 / 60.11
Mole of isopropyl alcohol = 0.759 mole
How to determine molality Mole of isopropyl alcohol = 0.759 moleMass of solvent = 54.4 g = 54.4 / 1000 = 0.0544 KgMolality =?Molality = mole / mass of solvent
Molality = 0.759 / 0.0544
Molality = 13.95 m
Learn more about Molality:
https://brainly.com/question/4251997
12. What is the chemical name for the compound
CH3CH₂CH₂CH3?
(1) butane
(3) decane
(2) butene
(4) decene
Answers
The chemical name for the compound CH3CH₂CH₂CH3 is butane.
What is alkane?Alkanes are any acyclic saturated hydrocarbon with a carbon to carbon single bond e.g. methane, ethane etc.
Alkanes have a general molecular formula of CnH2n+2. The number of carbon atoms determines the name of the alkane member.
According to this question, a chemical compound with the molecular formula; CH3CH₂CH₂CH3 is given. This compound posseses 4 carbon atoms and 10 hydrogen atoms, hence, is butane.
Learn more about alkane at: https://brainly.com/question/31386716
#SPJ1
Which of the following reactions is balanced and shows incomplete combustion
Answers
The equation that can be said to show incomplete combustion is option A
What is incomplete combustion?
Unburned carbon particles and carbon monoxide (CO), as well as water vapor and other byproducts, are examples of incomplete combustion products that are produced when a fuel does not completely react with the oxygen that is present during the combustion process.
Complete combustion occurs when a fuel combines with oxygen in the presence of enough heat to produce the primary byproducts of carbon dioxide, water, and heat.
Learn more about incomplete combustion:https://brainly.com/question/14335621
#SPJ1
Carla applied a force of 35 N to a wheelbarrow full of bricks and moved it 2.5 m. Which of the following can be determined from this information?
Answers
Answer:
The answer is 87.5
how do one get this solution
-log10 (2* 10^-2)
Answers
The result of the computation when you follow the steps is 1.699.
A logarithm is a mathematical function that represents the exponent or power to which a specific base must be raised to obtain a given number. In simpler terms, it answers the question: "To what power must we raise a base number to obtain a certain value?"
What you should do is that on your calculator, you could press the logarithm key and then put in the value that has been shown and then the result would be displayed on your calculator.
Learn more about logarithm:https://brainly.com/question/30226560
#SPJ1
The energy stored in an object is called potential energy
True or false
Answers
its true
Potential energy is the stored or latent energy in an object at rest. It’s fundamental to many physics-related concepts because its laws hold true on any level, from the planetary to the atomic level. The potential energy of an object is measurable.
why does glass containing an iced beverage feel cold
Answers
Answer:
Molecules in the skin are moving faster than molecules in the glass.
Explanation:
Put it in your own words.
Answer:
The energy from the ice transferred to the glass.
describe another way in which human activities that benefit society can also cause environmental risk.
Answers
A 488.3 gram sample of an unknown substance (MM = 92.41 g/mol) is heated from -23.1 °C to 51.8 °C. (heat capacity of solid = 2.96 J/g・°C; heat capacity of liquid = 1.75 J/g・°C; ∆Hfus = 8.04 kJ/mol; Tfinal = 17.6 °C)
a) How much energy (in kJ) is absorbed/released to heat the solid?
b)How much energy (in kJ) is absorbed/released to melt the solid?
c)How much energy (in kJ) is absorbed/released to heat the liquid?
d) What is the total amount of energy that must be absorbed/released for the entire process?
Answers
Answer:
a) Q₁ = 58.82 KJ
b) Q₂ = 42.48 KJ
c) Q₃ = 29.22 KJ
d) Q = 130.52 KJ
Explanation:
a)
In order to find the energy absorbed to heat the solid, we will use:
\(Q_{1} = mC_{1}\Delta T_{1}\)
where,
Q₁ = Heat absorbed for heating solid = ?
m = mass of solid = 488.3 g = 0.4883 kg
C₁ = Specific Heat Capacity of Solid = 2.96 J/g °C
ΔT₁ = Change in temperature of Solid = Melting Temperature - Initial Temp.
ΔT₁ = 17.6°C - (-23.1°C) = 40.7°C
Therefore,
\(Q_{1} = (488.3\ g)(2.96\ J/g\ ^{0}C)(40.7\ ^{0}C)\)
Q₁ = 58.82 KJ
b)
In order to find the absorbed to melt the solid at 17.6°C, we will use:
\(Q_{2} = nH_{fus}\)
where,
Q₂ = Heat absorbed for melting solid = ?
H_fus = Heat of Fusion = 8.04 KJ/mol
n = no. of moles = \(\frac{m}{MM} = \frac{488.3\ g}{92.41\ g/mol} = 5.28 mol\)
Therefore,
\(Q_{2} = (5.28\ mol)(8.04\ KJ/mol)\)
Q₂ = 42.48 KJ
c)
In order to find the energy absorbed to heat the liquid, we will use:
\(Q_{3} = m C_{3}\Delta T_{3}\)
where,
Q₃ = Heat absorbed for heating Liquid = ?
m = mass of solid = 488.3 g = 0.4883 kg
C₃ = Specific Heat Capacity of Liquid = 1.75 J/g °C
ΔT₃ = Change in temperature of Liquid = Final Temp. - Melting Temp.
ΔT₃ = 51.8°C - 17.6°C = 34.2°C
Therefore,
\(Q_{3} = (488.3\ g)(1.75\ J/g\ ^{0}C)(34.2\ ^{0}C)\)
Q₃ = 29.22 KJ
d)
Total amount of energy absorbed during entire process is:
\(Q = Q_{1} + Q_{2} + Q_{3}\)
\(Q = 58.82\ KJ + 42.48\ KJ + 29.22\ KJ\)
Q = 130.52 KJ
In order to heat a 488.3 g solid, 58.8 kJ are required. To melt the solid, 42.5 kJ are required. To heat the liquid, 29.2 kJ are required. The total amount of energy absorbed is 130.5 kJ.
Initially, a 488.3 g solid at -23.1 °C is heated up to 17.6 °C (melting point). We can calculate the heat required (Q₁) using the following expression.
\(Q_1 = c \times m \times \Delta T = \frac{2.96J}{g.\° C } \times 488.3g \times (17.6\° C-(-23.1\° C)) \times \frac{1kJ}{1000J} = 58.8 kJ\)
where,
c: heat capacity of the solidm: massΔT: change in the temperatureAt 17.6 °C, we can calculate the heat (Q₂) required to melt the solid using the following expression.
\(Q_2 = \Delta H_{fus} \times \frac{m}{MM} = 8.04 kJ/mol \times \frac{488.3 g}{92.41g/mol} = 42.5kJ\)
where,
∆Hfus: enthalpy of fusionm: massMM: molar massThe liquid is heated from 17.6 °C to 51.8 °C. We can calculate the heat required (Q₃) using the following expression.
\(Q_3 = c \times m \times \Delta T = \frac{1.75J}{g.\° C } \times 488.3g \times (51.8\° C-17.6\° C)) \times \frac{1kJ}{1000J} = 29.2 kJ\)
c: heat capacity of the liquidm: massΔT: change in the temperatureThe total amount of energy absorbed (Q) is the sum of the energy absorbed in each step.
\(Q = Q_1 + Q_2 + Q_3 = 58.8kJ+42.5kJ+29.2kJ= 130.5kJ\)
In order to heat a 488.3 g solid, 58.8 kJ are required. To melt the solid, 42.5 kJ are required. To heat the liquid, 29.2 kJ are required. The total amount of energy absorbed is 130.5 kJ.
Learn more: https://brainly.com/question/10481356

Question 1 of 32
Which of the following tends to increase the rate of a chemical reaction?
A. Decreasing the concentration of the reactants
B. Increasing the amount of solvent used
C. Using larger particles of solid reactants
D. Increasing the temperature of the reaction mixture
Answers
Fractional distillation
Answers
Miscible liquids are separated via a kind of distillation called fractional distillation. he mixture is typically divided into component parts after a series of distillations and condensations.
Miscible liquids are separated via a kind of distillation called fractional distillation. The mixture is typically divided into component parts after a series of distillations and condensations. When the combination is heated to a specific temperature where some of the mixture begins to vaporise, the separation takes place.
To know more about Fractional distillation, here:
https://brainly.com/question/31829958
#SPJ1
How much water has to be evaporated from 250 mL of 1 M Ca(OH)2 to make it 3 M?
Answers
Approximately 166.67 mL of water needs to be evaporated from 250 mL of 1 M Ca(OH)2 to make it 3 M.
To find the amount of water that needs to be evaporatedThe relationship between the initial and final concentrations and volumes must be taken into account.
Given: Initial concentration \((C^1) = 1 M Initial volume (V^1) = 250 mL\)
\((C^2) = 3 M final concentration\)
We can use the equation:
\(C^1 * V^1 = C^2 * V^2\)
Where:
\(V^2\)is the final volume of the solution
Rearranging the equation to solve for V2:
\(V^2 = (C^1 * V^1) / C^2\)
Substituting the given values:
\(V^2 = (1 M * 250 mL) / 3 M\)
\(V^2 = 250 mL / 3\)
\(V^2\) ≈ \(83.33 mL\)
To find the amount of water that needs to be evaporated, we subtract the final volume from the initial volume:
Amount of water to be evaporated = \(V^1 - V^2\)
Amount of water to be evaporated = 250 mL - 83.33 mL
Amount of water to be evaporated ≈ 166.67 mL
Therefore, approximately 166.67 mL of water needs to be evaporated from 250 mL of 1 M Ca(OH)2 to make it 3 M.
Learn more about Initial concentration here: brainly.com/question/30720317
#SPJ1
Imagine that you have a 7.00 L gas tank and a 3.00 L gas tank. You need to fill one tank with oxygen and the other with acetylene to use in conjunction with your welding torch. If you fill the larger tank with oxygen to a pressure of 115 atm , to what pressure should you fill the acetylene tank to ensure that you run out of each gas at the same time? Assume ideal behavior for all gases.
Answers
According to Boyle's law, the pressure in the acetylene tank is 268.33 atmospheres.
What is Boyle's law?Boyle's law is an experimental gas law which describes how the pressure of the gas decreases as the volume increases. It's statement can be stated as, the absolute pressure which is exerted by a given mass of an ideal gas is inversely proportional to its volume provided temperature and amount of gas remains unchanged.
Mathematically, it can be stated as,
P∝1/V or PV=K. The equation states that the product of of pressure and volume is constant for a given mass of gas and the equation holds true as long as temperature is maintained constant.
In the given problem , by substituting values in formula P₁V₁=P₂V₂, P₂=115×7/3=268.33 atmospheres.
Hence, the pressure in acetylene tank is 268.33 atmospheres.
Learn more about Boyle's law ,here:
https://brainly.com/question/1437490
#SPJ1
express the number 0.000114 in scientific notation.
Answers
1.14 x 10^-4
its correct
1. What do you call the continuous change in position of a body relative to a pon
reference point, as measured by a particular observer in a particular frame
of reference?
Answers
Answer:
In physics, motion means a continuous change in the position of a body relative to a reference point, as measured by a particular observer in a particular frame of reference.
Explanation:
The pOH of a solution is 6.0. Which statement is correct?
Use pOH = -log[OH-] and PH+pOH = 14.
The pH of the solution is 20.0.
O The concentration of OH ions is 1.0 x 108 M.
The concentration of OH ions is 1.0 x 106 M.
O The pH of the solution is 8.0.
A
Answers
At pOH value of 6.0 the pH value of the following solution is 8.0 and the concentration of [\(H^{+}\) ] ion is \(10^{-8}\)
In this question we will apply the formula
pH +pOH = 14 . . . . . . . . . . . . .(1)
where pH = concentration of [\(H^{+}\) ] ion
pOH = concentration of [\(OH^{-}\) ] ion
As per the question
pOH =6.0
Putting the value of pOH in equation (1) we get the value of pH
pH + 6.0 =14
pH = 14 -6.0
pH = 8.0
The value of pH if the pOH value is 6.0 is 8.0
To find the concentration of \(H^{+}\) ion we will use the following formula
This is calculated by the formula
[\(H^{+}\)} = \(10^{-pH}\)
where we will write the values of pH
Hence the concentration of [\(H^{+}\)} ion is \(10^{-8}\)
Therefore at pOH of 6.0 the pH value of the following solution is 8.0 and the concentration of [\(H^{+}\) ] ion is \(10^{-8}\)
Read more about pH
https://brainly.com/question/11300720
The complete question is -
What is the pH value and concentration of [\(H^{+}\) ] ion of the following if the pOH value of the solution is 6.0 ?
zn zn2+ + 2e oxidation or reduction; what are the redox reactions that take place?; zn(s) + 2hcl(aq) → zncl2(aq) + h2(g); oxidation can also be defined as the loss of oxygen atoms and/or the gain of hydrogen atoms; the first step when balancing redox reaction involves:; hcl reduction half reaction; cu half reaction; acetone redox reaction
Answers
Zn (s) + 2 HCl (aq) --→ ZnCl2 (aq) + H2 (g) Zn being oxidized during the reaction: Correct answer is 1. oxidized. Zn's oxidation status is shifting from 0 to +2.
A chemical process called oxidation occurs. As a result of atoms or groups of atoms losing electrons, it is described as a process. The addition or loss of oxygen or hydrogen in a chemical species is another method to define oxidation. An oxidation reaction takes place when oxygen interacts with a substance or an element. Another way to think of oxidation is as the process of removing hydrogen from the species of reactants. Molecule, atom, or ion oxidation is the process of losing electrons. Initially, reactions where an element interacts with oxygen were referred to as oxidations. For instance, the chemical interaction between magnesium metal and oxygen to produce magnesium oxide is the oxidation of magnesium.
To learn more about oxidation here
https://brainly.com/question/27239694
#SPJ4
Asteroids in the main asteroid belt at certain distances from the Sun are in an orbital resonance with Jupiter, creating gaps in the belt called Kirkwood gaps Jupiter orbits the Sun with a period of 11.9 years What is the period of a belt asteroid in an 11:5 orbital resonance with Jupiter
Answers
Asteroids in the main asteroid belt at certain distances from the Sun are in an orbital resonance with Jupiter, the period of a belt asteroid in an 11:5 orbital resonance with Jupiter is mathematically given as
TA=5.4090years
What is the period of a belt asteroid in an 11:5 orbital resonance with Jupiter?Generally, the equation for the ratio is mathematically given as
asteroid in an 11:5 orbital resonance= 11/5
Therefore, with Jupiter at
TJ=11.9years
Hence
11/4=TJ/TA
TA=5/11)*11.9
TA=5.4090years
Read more about Time
https://brainly.com/question/4931057
#SPJ1
Determine the name or formula for each polyatomic ion.
formula: PO3−4
name:
name: sulfite ion formula:
name: sulfate ion formula:

Answers
Answer:
See explanation
Explanation:
PO4{3-} is phosphate
Sulfite's formula is SO3{2-}
Sulfate is SO4{2-}
OH- is hydroxide
Note: {x±} signifies the charge of the entire molecule
The polyatomic ions in question are phosphite ion, sulfite ion, and sulfate ion.
Explanation:The formula PO3−4 represents the polyatomic ion called phosphite ion. It is composed of one phosphorus atom bonded to three oxygen atoms. The name of the sulfite ion is SO3−2, and it consists of one sulfur atom bonded to three oxygen atoms. Lastly, the sulfate ion has the formula SO4−2, and it is composed of one sulfur atom bonded to four oxygen atoms.
Learn more about Polyatomic ions here:https://brainly.com/question/35456287
#SPJ6
the basicity of tetraoxosulphate vi acid is 2 explain this statement with an equation showing the reaction between the acid and potassium hydroxide
Answers
The balanced chemical equation for the reaction is: H2SO4 + 2KOH → K2SO4 + 2H2O. It can be concluded that the basicity of tetraoxosulphate VI acid is 2.
The basicity of tetraoxosulphate VI acid is 2. The term "basicity" is used to describe the number of replaceable hydrogen atoms in an acid molecule. Tetraoxosulphate VI acid, also known as sulfuric acid (H2SO4), contains two hydrogen atoms that can be replaced by a metal or base, hence its basicity of 2. This means that two molecules of a base or metal hydroxide are required to neutralize one molecule of sulfuric acid. This can be represented by the following chemical equation: H2SO4 + 2KOH → K2SO4 + 2H2OThe reaction between sulfuric acid and potassium hydroxide (KOH) can be used to illustrate the basicity of tetraoxosulphate VI acid. When sulfuric acid is mixed with potassium hydroxide, the reaction produces potassium sulfate (K2SO4) and water (H2O). Since sulfuric acid has a basicity of 2, two molecules of potassium hydroxide are needed to react with one molecule of sulfuric acid.For more such questions on chemical
https://brainly.com/question/28053119
#SPJ8
Ask BRIA
Water vapor
Liquid water
Which of the following labels should be added to all of the black arrows in the above
illustration?
O temperature increases
O temperature decreases
heat removed
O heat added
Answers
Answer:
o temperature increases
The following mechanism has been proposed for the gas-phase reaction of chloroform (CHCl3) and chlorine:
Step 1: Cl2(g)⇌2Cl(g)(fast)
Step 2: Cl(g)+CHCl3(g)→HCl(g)+CCl3(g)(slow)
Step 3: Cl(g)+CCl3(g)→CCl4(fast)
Part A
What is the overall reaction?
Express your answer as a chemical equation. Identify all of the phases in your answer.
Part B
What are the intermediates in the mechanism?
Express your answers as a chemical expression. If there is more then one answer, separate them by commas. Identify all of the phases in your answer.
Part G
What is the rate law predicted by this mechanism? (Hint: The overall reaction order is not an integer.)
Use a for [Cl2], b for [Cl], c for [CHCl3], d for [HCl], e for [CCl3] and k for the rate constant.
Answers
Answer:
Overall reaction equation is:
Cl2(g) + CHCl3(g) --> HCl(g) + CCl4(g)
The intermediates are Cl(g), CCl3(g).
Rate law= ka^1/2 c
Explanation:
This reaction is a non elementary reaction. A non elementary reaction is one that does not take place in a single reactive encounter. In other words, the reaction is comprised of many steps.
The overall reaction equation is the sum of all the reaction steps after all intermediates have been cancelled out. The rate law is obtained from the slowest step in the reaction sequence.
the difference between Major purchase Consumer good
Answers
A major purchase refers to a high-cost item or service that is considered a significant investment for an individual or household, such as a car, home, or college education. Major purchases usually involve a large sum of money and require careful planning and consideration before a final decision is made.
Any item that a person or a household buys for their own use or consumption is called a consumer good. Durable and non-durable goods are two types of consumer goods that can be classified. Consumer goods are tangible items that people or households buy for their own use or consumption.
Learn more about major purchase, here:
https://brainly.com/question/29998590
#SPJ1
Your question is incomplete, most probably the complete question is:
The difference between Major purchase and Consumer good?