Answers
Answer:
a
Explanation:
Matter can be volume or density. So, this concludes that it is when it takes up space.
Answer: A.
Explanation:
it takes up space
Related Questions
What is the mass percentage of C in morphine, C₁7H19NOs? Provide an
answer to two decimal places.

Answers
The mass percentage of C in morphine would be 4.21%.
What is mass percentage?The mass percentage of a composition in a compound is the mass of the composition relative to the mass of the entire compound. This can be mathematically expressed as:
Mass percentage = mass of component/mass of substance x 100%
In this case, we are looking for the mass percentage of C in \(C_{17}H_{19}NO_3\).
Molar weight of C = 12 g/mol
Molar mass of \(C_{17}H_{19}NO_3\) = (12x17) + (1x19) + (14x1) + (16x3)
= 285 g/mol
Mass percentage of C = 12/285
= 4.21% to 2 decimal places.
In other words, the mass percentage of C in morphine is 4.21%.
More on mass percentage can be found here: https://brainly.com/question/16885872
#SPJ1
Find the SDS for regular bleach
Answers
The Safety Data Sheet (SDS) for regular bleach can be obtained from the manufacturer or supplier of the specific brand or product. It contains important information regarding the hazardous properties, handling, storage, and emergency procedures related to the bleach.
An SDS typically includes details such as the product's chemical composition, physical and chemical properties, potential hazards to health and the environment, precautionary measures for safe handling and storage, first-aid procedures, and information about proper disposal. It also provides guidance on personal protective equipment (PPE) to be used when handling the product and steps to take in case of accidental release or exposure.
The SDS serves as a crucial resource for understanding the potential risks associated with the use of regular bleach and helps ensure that appropriate safety measures are implemented. It is important to review and follow the information provided in the SDS to minimize the risk of accidents, injuries, or adverse health effects associated with the use of the product.
for such more questions on product
https://brainly.com/question/30667391
#SPJ8
Jonathon is conducting an experiment to determine how much precipitate (solid product) will form when combining measured volumes of Aich, and NaOH. According to his calculations the reaction should produce 26.0 grams of solid AKOH), when combined. However, when Jonathon measures the mass of the solid precipitate formed in his experiment, he finds that the experiment actually produced 24.5 grams of Al(OH).
Answers
Jonathon's experiment produced 24.5 grams of Al(OH), which is less than the predicted amount of 26.0 grams of AKOH. The discrepancy could be due to measurement errors, incomplete reaction.
What is discrepancy?
To determine the cause of the discrepancy, Jonathon should first review his experimental procedure and make sure that all measurements and calculations were performed accurately. He should also check that the reactants were mixed thoroughly and that the reaction was allowed to proceed to completion. If any errors or inconsistencies are identified, Jonathon should correct them and repeat the experiment to obtain more accurate results.
If the experimental procedure was carried out correctly and the discrepancy cannot be attributed to measurement errors, Jonathon should consider the possibility of impurities in the reactants. Even small amounts of impurities can affect the outcome of a chemical reaction, so it is important to use high-quality, pure chemicals in experiments whenever possible.
Overall, the most important thing for Jonathon to do in this situation is to carefully review his experimental data and methodology, and to identify any potential sources of error or uncertainty. By doing so, he can improve the accuracy and reliability of his results and draw more meaningful conclusions from his experiment.
To know more about reactants, visit:
https://brainly.com/question/13005466
#SPJ9
In a nuclear fission reaction, a freely moving neutron is introduced to a
nuclear fuel, such as uranium-235. What happens next?
O A. A neutron is absorbed by an atom's nucleus.
B. The neutron breaks apart.
O C. Heat is released by the neutron.
D. The nucleus becomes unstable.
Answers
The nucleus becomes unstable when a freely moving neutron is introduced to a nuclear fuel.
What is Nuclear fission?This is a reaction in which the nucleus of an atom splits into two or more smaller nuclei.
This reaction produces more neutrons and the nucleus becomes unstable when a freely moving neutron is introduced to a nuclear fuel to form fission fragments.
Read more about Nuclear fission here https://brainly.com/question/3992688
#SP2
The circles, or orbits, for electrons are called energy levels. Each level can hold only a certain number of electrons. Add electrons to each level until you can add any more. How many electrons can each level hold?
IF YOU GIVE ME THE RIGHT ANSWER I WILL GIVE YOU BRAINIEST OR WHATEVER ITS CALLED!!!!!!!
Btw this subject is Science but I couldn’t find it so I just put chemistry.

Answers
Answer: n = 1 can hold a maximum of 2 electrons
n = 2 , maximum 8 electrons
n = 3, maximum 18 electrons
As per the principles of quantum mechanics, the number of electrons that can be added to a given energy level is deduced based on the three quantum numbers: n, l , m(l) and m(s)
'n' is the principal quantum number which defines the energy level. It can take on integer values: 0,1,2,3...
'l' is the angular momentum quantum number which defines the shape of the orbital that an electron occupies
l = 0,1,2...(n-1)
where: l = 0 corresponds to s-orbital
l = 1 corresponds to p-orbital
l = 2, corresponds to d-orbital
'm(l)' is the magnetic moment quantum number which defines the orientation of an orbital in space.
m(l) = -l, 0, +l
'm(s)' is the spin quantum number which defines the orientation of an electron is an orbital m(s) = +1/2 or -1/2
An s, p or d-orbital can accommodate a maximum of 2, 6 and 10 electrons respectively For energy level with n= 1
l = 0, i.e. s-orbital or 1s.
Therefore, the maximum number of electrons for a 1s orbital would be 2 resulting in an electron configuration of 1s²
For energy level with n= 2
l = 0, 1 i.e. s and p-orbitals
The maximum number of electrons would be
Electron configuration: 2s²2p⁶
For energy level with n= 3
l = 0, 1, 2 i.e. s, p and d-orbitals
The maximum number of electrons would be
Electron configuration: 3s²3p⁶3d¹⁰
how many molecules of potassium chloride will react if 21.89 grams KCl are added to the solution
Answers
There are approximately 1.765 x 10²³ molecules of KCl in 21.89 grams of KCl.
What is meant by potassium chloride ?Potassium chloride (KCl) is a compound made up of potassium and chloride ions. It is a colorless, odorless salt that is commonly used in a variety of applications.
Molar mass of KCl is 74.55 g/mol; number of moles = Mass/ Molar mass
So, the number of moles = 21.89 g ÷ 74.55 g/mol = 0.2936 mol
and the number of molecules = Number of moles * Avogadro's number
Number of molecules = 0.2936 mol x 6.02 x 10²³ molecules/mol
Number of molecules = 1.765 x 10²³ molecules
Therefore, there are approximately 1.765 x 10²³ molecules of KCl in 21.89 grams of KCl.
To know more about potassium chloride, refer
https://brainly.com/question/25380525
#SPJ1
Consider the cell Pt |Cr²+ (aq, 1.0 M), Cr3+ (aq, 2.2 mM) || Pb2+ (aq, 1.3M)| Pb. EºCell -0.37. What is the value of K at 25 °C
Answers
Answer:
1
Explanation:
To determine the value of K (equilibrium constant) at 25 °C, we can use the Nernst equation, which relates the cell potential (E) to the equilibrium constant (K) and the standard cell potential (EºCell). The Nernst equation is given by:
E = EºCell - (RT / nF) * ln(K)
Where:
E = cell potential
EºCell = standard cell potential
R = gas constant (8.314 J/(mol·K))
T = temperature in Kelvin (25 °C = 298 K)
n = number of electrons transferred in the balanced redox equation
F = Faraday's constant (96,485 C/mol)
ln = natural logarithm
In this case, the given standard cell potential (EºCell) is -0.37 V.
The balanced redox equation for the cell reaction is:
Pt + Cr²+ -> Pt + Cr³+
Since there is no change in the oxidation state of Pt, no electrons are transferred in the reaction (n = 0).
Substituting the known values into the Nernst equation, we have:
E = -0.37 V - (8.314 J/(mol·K) * 298 K / (0 * 96,485 C/mol)) * ln(K)
E = -0.37 V
Since n = 0, the term (RT / nF) * ln(K) becomes 0, and we are left with:
-0.37 V = -0.37 V - 0
This implies that the value of K is 1, since any number raised to the power of 0 is equal to 1.
Therefore, the value of K at 25 °C for the given cell is 1.
A 105.5mg sample of a white substance, suspecte of being cocaine (C17 H21 NO4), from 279.3mg of H2O on combustion chemical analysis shows that the compound contain 4.68%N by mass. would you conclude that the white solid is cocain?
Answers
Yes, we can conclude that a white solid that weighs 105.5 mg and is presumed to be cocaine (C₁₇H₂₁NO₄) produces 279.3 mg of CO2 and 66.46 mg of water upon combustion.
Briefing:Cocaine is a tropane alkaloid that has both local and central nervous system (CNS) stimulating effects. Cocaine inhibits the re-uptake of dopamine, serotonin, and norepinephrine into pre-synaptic neurons by binding to the transport proteins for these neurotransmitters. The salt form of cocaine is called cocaine hydrochloride. The taste is bitter and numbing, and it is a fine white powder.
Types Of Cocaine:Pure CocaineCrack CocaineSynthetic CocainePink CocaineBlack CocaineFish Scale CocaineCocaine HydrochlorideYellow CocaineBrown CocaineTo know more about Cocaine visit:
https://brainly.com/question/15865826
#SPJ9
The complete question is -
A 105.5mg sample of a white substance, suspected of being cocaine (C17H21NO4), forms 279.3mg of CO2 and 66.46mg of water on combustion. Chemical analysis shows that the compound contains 4.68% N by mass. Would you conclude that the white solid is cocaine?
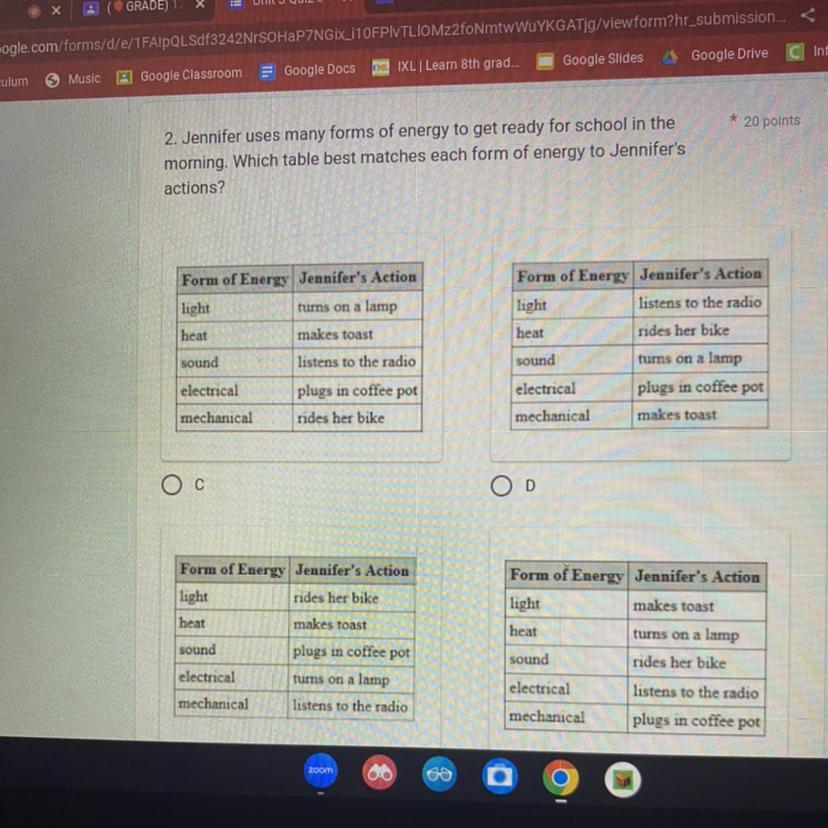
2. Jennifer uses many forms of energy to get ready for school in the
morning. Which table best matches each form of energy to Jennifer's
actions?
Form of Energy Jennifer's Action
light
turns on a lamp
makes toast
listens to the radio
plugs in coffee pot
rides her bike
JOSTAGENS
sound
O C
E
electrical
mechanical
Form of Energy Jennifer's Action
Light
rides her bike
makes toast
plugs in coffee pot
turns on a lamp
listens to the radio
sound
electrical
mechanical
S
Form of Energy Jennifer's Action
Light
listens to the radio
heat
rides her bike
sound
turns on a lamp
electrical
plugs in coffee pot
mechanical
makes toast
O D
* 20 points
Form of Energy Jennifer's Action
light
heat
sound
electrical
mechanical
makes toast
turns on a lamp
rides her bike
listens to the radio
plugs in coffee pot
Answers
The table best matches each form of energy to Jennifer's actions is A.
What is energy?Energy is defined as the capacity to labor long hours or be extremely busy without becoming exhausted. Red blood cells, which carry oxygen in the blood throughout the body, are created by vitamin B12 in the body. Your body's cells use the oxygen once it gets there to produce energy.
Electrical energy, chemical energy obtained from fuels, food, and energy derived from the sun are the major types of energy used in our homes. Everyday appliances convert electrical energy into a variety of forms, including mechanical/kinetic, sound, heat, light, and other types of electromagnetic radiation.
Thus, the table best matches each form of energy to Jennifer's actions is A.
To learn more about energy, refer to the link below:
https://brainly.com/question/1932868
#SPJ1
Which is one factor that scientists use to classify orders of soil?
Answers
Answer:
The scientist classify the types of soil based on the texture of the soil, the type of Rock from which they are formed and the type of organisms which live in the soil. For example red soil is formed by the weathering of reddish type of Rock which is found in that territory.
Explanation: google and me.....hope this helps
One factor which scientists used to classify orders of is Soil texture
Soil textureSoil texture is a classification strategy that scientists used to determine soil classes orders based on their physical texture.
Soil texture and soil structure are unique properties of the soil that will have a great effect on the behavior of soils, such as water holding capacity, nutrient retention and supply, drainage, and nutrient leaching.
Learn more:
https://brainly.com/question/25761065
Matter includes all of the following EXCEPT-
Osmoke
о
water vapor
light
air
Answers
Answer: Light is not a matter.
Explanation:
Answer:light
Explanation:
For the reaction below, the amounts at equilibrium in a
1.0 L
container at a certain temperature are:
2.626
moles
BaCl 2
,3.732
moles
K 2
SO 4
,7.592
moles
BaSO 4
, and
7.632
moles
KCl
. What is the equilibrium constant, K? Enter your answer with 3 sig figs.
∗
Hint: check the phases for each substance!
BaCl 2
(aq)+K 2
SO 4
(aq)−−−>BaSO 4
( s)+2KCl(aq)
Question 16 1 pts On Earth, naturally occurring potassium contains 3 isotopes:
39
K, 20
K
, and
41
K
Select ALL of the following that are true for a sample of naturally occurring potassium. A mole of naturaly occurring potassium has a mass of
39.098
grams K-41 has 41 electrons All of the potassium isotopes have more neutrons than protons All potassium isotopes have an atomic number of 19
K−39
is the most abundant potassium isotope A mole of K-39 atoms has a mass of
39.098
grams Potassium 41 has 19 protons and 22 neutrons
Answers
The equilibrium constant, K, is \(8.24\) with 3 sig figs.
A mole of naturally occurring potassium has a mass of\(39.098 grams\)
The equilibrium constant, K, for the reaction
BaCl2 (aq) + K2SO4 (aq) → BaSO4 (s) + 2KCl (aq)
can be calculated using the following equation:
K = \(\frac{[BaSO4]*[2KCl]}{[BaCl2]*[K2SO4]}\)
K = \(\frac{3.71 mol/L * (2*4.65 mol/L)}{(2.78 mol/L * 3.25 mol/L)}\)
K =\(8.24\)
Using the values provided, we can determine that K = 8.24.
Thus, the equilibrium constant, K, is 8.24 with 3 sig figs.
This is because a mole of potassium atoms is made up of 39.098 grams of K-39 atoms, 20.042 grams of K-20 atoms and 0.950 grams of K-41 atoms. The atomic number of all potassium isotopes is 19, which means they all have 19 protons. The number of neutrons in each isotope differs. K-39 has 20 neutrons, K-20 has 18 neutrons and K-41 has 22 neutrons. K-39 is the most abundant potassium isotope, making up 93.2581% of naturally occurring potassium.
learn more about equilibrium constant refer:brainly.com/question/10038290
#SPJ4
complete question:
For the reaction below, the amounts at equilibrium in a 1.0 L container at a certain temperature are: 2.78 moles BaCl2, 3.25 moles K2SO4, 3.71 moles BaSO4, and 4.65 moles KCl. What is the equilibrium constant, K? Enter your answer with 3 sig figs.
BaCl2 (aq) + K2SO4 (aq) ----> BaSO4 (s) + 2KCl (aq)
On Earth, naturally occurring potassium contains 3 isotopes:
39K, 20K, and 41K.Select ALL of the following that are true for a sample of naturally occurring potassium.
a.A mole of naturaly occurring potassium has a mass of 39.098 grams K-41 has 41 electrons
b.All of the potassium isotopes have more neutrons than protons
c.All potassium isotopes have an atomic number of 19 K−39 is the most abundant potassium isotope
d.A mole of K-39 atoms has a mass of 39.098 grams
Potassium 41 has 19 protons and 22 neutrons
What does hydrogen fuel mix with in a chemical reaction? What products are produced in the chemical reaction? How do the products affect the atmosphere?
Answers
Answer:
Hydrogen fuel typically reacts with oxygen in a chemical reaction to produce water (H2O) as the main product. The chemical equation for this reaction is:
2H2 + O2 → 2H2O
The reaction releases energy, which can be harnessed to power engines or generate electricity. When hydrogen fuel is burned in an engine or fuel cell, the only byproduct is water vapor, which is harmless to the atmosphere.
However, the production of hydrogen fuel can have environmental impacts if it is not produced using renewable energy sources. The most common method of producing hydrogen involves using natural gas, which releases carbon dioxide and other greenhouse gases into the atmosphere. Therefore, it is important to consider the source of the hydrogen fuel and the environmental impact of its production when evaluating its benefits for reducing emissions and improving air quality.
The chemical equation for the combustion of hydrogen can be represented as follows: 2H₂ + O₂ → 2H₂O
In this reaction, two molecules of hydrogen gas (H₂) combine with one molecule of oxygen gas (O₂) to form two molecules of water vapor (H₂O). The reaction releases a significant amount of energy in the form of heat.
The products of the combustion of hydrogen, which are water molecules, do not have significant adverse effects on the atmosphere. Water vapor is a natural component of the Earth's atmosphere, and its presence is essential for various natural processes, such as the water cycle.
However, it is important to note that the combustion of hydrogen as a fuel can indirectly affect the atmosphere in certain scenarios. One consideration is the generation of the primary energy used to produce the hydrogen fuel.
Additionally, the transportation and storage of hydrogen as a fuel may present challenges. If there are any leaks or improper handling, hydrogen gas can contribute to the formation of local air pollution. However, these issues can be managed through appropriate safety measures and technology advancements.
Overall, the combustion of hydrogen fuel with oxygen produces water as a product, and the water vapor does not have direct adverse effects on the atmosphere.
However, the environmental impact associated with hydrogen as a fuel depends on the methods used for its production and transportation. Sustainable production methods, such as electrolysis using renewable energy sources, can minimize the environmental impact of hydrogen fuel.
For more such questions on combustion visit:
https://brainly.com/question/13251946
#SPJ8
A compound consists of 75% magnesium and 25% oxygen. Find the empirical formula.
Answers
% Composition of elements:
Magnesium = 75%Oxygen = 25%Atomic mass of given elements:
Magnesium = 24 gOxygen = 16 gNow, divide % composition by Atomic mass:
\(\footnotesize\implies Mg = \dfrac{\% \: Composition}{Atomic \: mass} = \dfrac{75}{24} = \bf 3.125\)
\(\footnotesize\implies O = \dfrac{\% \: Composition}{Atomic \: mass} = \dfrac{25}{16} = \bf 1.5625\)
Simplest Ratio:
\(\footnotesize\implies Mg = \dfrac{3.125}{1.5625} = 2\)
\(\footnotesize\implies O = \dfrac{1.5625}{1.5625} = 1\)
Empirical Formula:
\(\footnotesize\implies \underline{ \boxed{ \red{ \bf Empirical \: Formula = Mg_2O}}}\)
The empirical formula will be \(Mg_{2}\) .
What is empirical formula?The simplest entire number fraction of atoms contained in a chemical molecule is its empirical formula.
Calculation of empirical formula:
It is given that, composition of Mg = 75 %, composition of oxygen = 25%.
It is known that, atomic mass of Mg = 24 g, atomic mass of oxygen = 16g.
By dividing % composition by atomic mass of given elements:
Mg = % composition / %atomic mass
= 75 / 24
= 3.125
Oxygen (O) = % Composition / atomic mass
= 25 / 1.56
Now, calculate simplest ratio of Mg and O.
For Mg = 3.125 / 1.56
= 2.
For O = 1.56 / 1.56
= 1
So, the empirical formula will be \(Mg_{2}O\).
To know more about empirical formula.
https://brainly.com/question/14044066.
#SPJ2
The ionic compound MX(s) is formed from the metal M(s) and the diatomic gas X2(g) at standard conditions. Calculate the lattice energy given the following data( data in picture)

Answers
The lattice energy of MX is 459.2 kJ/mol.
The lattice energy (ΔH° lattice) of an ionic compound is the energy released when one mole of the solid is formed from its constituent gaseous ions under standard conditions. The lattice energy is calculated using the Born-Haber cycle, which involves several steps including atomization, ionization, dissociation, and sublimation energies.
The lattice energy is related to the Coulombic attraction between the oppositely charged ions in the solid. To calculate the lattice energy for MX, we can use the following equation:
ΔH° lattice = ΔH° sub + ΔH° ion + ΔH° diss + ΔH° formation
where ΔH° sub is the sublimation energy of M(s), ΔH° ion is the first ionization energy of M(g), ΔH° diss is the dissociation energy of X2(g), and ΔH° formation is the enthalpy of formation of MX(s).
Using the given data, we can calculate each of these values and substitute them into the equation to obtain the lattice energy. The final answer should be in units of kJ/mol.
ΔH° sub (M) = 107.3 kJ/mol
ΔH° ion (M) = 577.5 kJ/mol
ΔH° diss (X2) = 242 kJ/mol
ΔH° formation (MX) = -467.6 kJ/mol
ΔH° lattice = 107.3 + 577.5 + 242 + (-467.6) = 459.2 kJ/mol
As a result, MX has a lattice energy of 459.2 kJ/mol.
To know more about the Ionic compound, here
https://brainly.com/question/1603676
#SPJ1
The half-life of a radioactive isotope is 27 years. How long will its
mass take to fall from 2.00 g to 0.25 g?
years.
Answers
Life will come out to be 10 days. This is the required value of half life.
what is radioactive isotope?
An unstable form of a chemical element that releases radiation as it breaks down and becomes more stable. Radioisotopes may occur in nature or be made in a laboratory. In medicine, they are used in imaging tests and in treatment. Also called radionuclide. Radioactive isotopes have many useful applications. In medicine, for example, cobalt-60 is extensively employed as a radiation source to arrest the development of cancer. Other radioactive isotopes are used as tracers for diagnostic purposes as well as in research on metabolic processes.Atoms of the same element with different numbers of neutrons are called isotopes. Many elements have one or more isotopes that are radioactive. Their nuclei are unstable, so they break down, or decay, and emit radiation.To learn more about isotope refers to:
https://brainly.com/question/364529
#SPJ1
How many grams of mercury would be contained in 15 compact fluorescent light bulbs?
Answers
Answer:
Grams of mercury= 0.06 g of Hg
Note: The question is incomplete. The complete question is as follows:
A compact fluorescent light bulb contains 4 mg of mercury. How many grams of mercury would be contained in 15 compact fluorescent light bulbs?
Explanation:
Since one fluorescent light bulb contains 4 mg of mercury,
15 such bulbs will contain 15 * 4 mg of mercury = 60 mg
1 mg = 0.001 g
Therefore, 60 mg = 0.001 g * 60 = 0.06 g of mercury.
Compact fluorescent lightbulbs (CFLs) are tubes containing mercury and noble gases. When electricity is passed through the bulb, electron-streams flow from a tungsten-coated coil. They collide with mercury atoms, exciting their electrons and creating flashes of ultraviolet light. A phosphor coating on the inside of the tube absorbs this UV light flashes and re-emits it as visible light. The amount of mercury in a fluorescent lamp varies from 3 to 46 mg, depending on lamp size and age.
Total amount of mercury in 15 compact fluorescent light bulbs is 0.06 gram of mercury.
Compact fluorescent light bulbs and mercury:What information do we have?
Number of compact fluorescent light bulbs = 15 bulb
Amount of mercury in each bulb = 4 mg
Total amount of mercury = Number of compact fluorescent light bulbs × Amount of mercury in each bulb
Total amount of mercury = 15 × 4
Total amount of mercury = 60 mg
Total amount of mercury = 60 / 1000
Total amount of mercury = 0.06 gram of mercury
Find out more information about 'Mercury'.
https://brainly.com/question/2279846?referrer=searchResults
What type of chemical reaction occurs between AgNO3 (sliver nitrate) and Cu (Copper)?

Answers
Answer:
The answer is option c.
I hope this helps you.
What is the median reaction of second end point in HCL and NaOH titration
Answers
The median reaction at the second end point in the HCl and NaOH titration is: HCl + NaOH → NaCl + H2O
In a titration between hydrochloric acid (HCl) and sodium hydroxide (NaOH), the reaction involved is the neutralization reaction between an acid and a base. The balanced equation for this reaction is:
HCl + NaOH → NaCl + H2O
In this reaction, one mole of HCl reacts with one mole of NaOH to form one mole of NaCl (sodium chloride) and one mole of water.
During the titration process, the reaction occurs gradually as the base is added to the acid solution.
The first end point of the titration is reached when the moles of HCl and NaOH are stoichiometrically equivalent, meaning they react in a 1:1 ratio. At this point, all the HCl has been neutralized by the NaOH, and no excess of either reagent remains.
However, if the titration is continued beyond the first end point, the reaction between HCl and NaOH can still occur, albeit in a different ratio.
The second end point refers to the point where the moles of NaOH added exceed the stoichiometrically required amount to neutralize the HCl completely. As a result, any excess NaOH added after the second end point reacts with the excess HCl in a 1:1 ratio.
Therefore, the median reaction at the second end point in the HCl and NaOH titration is:
HCl + NaOH → NaCl + H2O
For more such question on median reaction visit:
https://brainly.com/question/14189499
#SPJ8
Using the formula for the ideal gas law and the value for the gas law constant of 0.08206 L.atm/K/mol, what is the volume (in L) of 9.84 grams of dry hydrogen at 23.4 degrees C and 757 torr?
Answers
PV=NRT
P=755torr
V=?
n=Mass/Molarmass…… mass=9.84g molarmass=1g/mol
R=0.8206L.atm/K/mol
T=23.4 degrees C so to kelvin that would be 23.4+273K=296.4K
Changing 757torr to atm:
760torr=1atm
757totr=X
Sooo X=757torrx1atm/760torr
X=757x1atm/760
X=757atm/760
X=0.996atm
Then we use our ideal gas equation which is:
PV=nRT
0.996atmxV=9.84molX0.8206X296.4K
V=9.84molX0.8206L.atm/K/molX296.4K/0.996atm
V=9.84X0.8206LX296.4/0.996
V=2,393.3L/0.996=2,402.9L approximately 4significant figure=2,403L
name the acid and base needed to form the salt ammonium nitrate give the chemical reaction
what is neutrallization
Answers
Answer:
here
Explanation:
(NH4NO3), a salt of ammonia and nitric acid, ....4 and 3 are sub script
A neutralization reaction is a chemical reaction where an acid and a base are combined with the intent of producing a neutral pH level.
HNO3+NH4OH ----------------NH4NO3 + H2O
If you had 6H2 molecules and 4O2 molecules, how many H2O molecules could you produce?
Answers
Answer:
6
Explanation:
As , 2H2 + O2 = 2H2O
with 6 H2, 4O2 is excess.
H2O molecules formed = 6
Which of the following statements is true about the strength of the intermolecular forces in CH4 and NH3?
a.CH4 ≥ NH3 because CH4 is tetrahedral but NH3 is pyramidal.
b.CH4 < NH3 because δ− on C in the CH bond is greater than δ− on N in the NH bond.
c.CH4 < NH3 because the NH bond is more polar than the CH bond.
d.CH4 ≥ NH3 because CH4 has H bonding but NH3 has dispersion forces.
Answers
Answer:
c.CH4 < NH3 because the NH bond is more polar than the CH bond.
Explanation:
Which hormones are secreted from the posterior pituitary gland?
Answers
Answer:
growth hormone is ur answer
Answer:
Explanation:
two hormones are secreted from posterior lobe i.e. oxytocin and vasopressin
Can someone please convert this for brainiest answer
2.4 x 10^24 atoms c to grams
Answers
Answer:
48 g C
Explanation:
To find the grams, you need to (1) convert atoms to moles (using Avogadro's Number) and then (2) convert moles to grams (using atomic mass). It is important to arrange the conversions in a way that allows for the cancellation of units. The final answer should have 2 sig figs to match the sig figs of the given value.
Avogadro's Number:
6.022 x 10²³ atoms = 1 mole
Atomic Mass (C): 12.011 g/mol
2.4 x 10²⁴ atoms C 1 mole 12.011 g
----------------------------- x -------------------------------- x --------------- = 48 g C
6.022 x 10²³ atoms 1 mole
Calculate the [H+] in a solution that has a pH of 10.36
Answers
Answer:
[H+] = 4.365x10⁻¹¹
Explanation:
The pH is a measurement widely used in chemistry. Is used in quality control to determine if a product is good for human or pet consumption. The equation to obtain the pH is:
pH = -log [H+]
To solve [H+]:
10^pH = -[H+]
10^-pH = -[H+]
In the problem:
10^-10.36 = -[H+]
[H+] = 4.365x10⁻¹¹Which of the following is an example of an environmental impact of
agriculture?
O high use of gold, copper, and silver
O high use of rock supplies
O high use of mineral resources
O high use of water
Ne
Answers
Answer:
B
self explanatory
Explanation:
____ Are subatomic particles found in the nucleus and carry positive electrical charge.
A. Atoms
B. Electrons
C. Protons
D. Neutrons
E. Electron Shell
Answers
Protons are subatomic particles found in the nucleus and carry positive electrical charge.
Answer:
C
Explanation:
there's no need to rush just calm down baby!!!
what are some potential fire startersfor a wildfire
Answers
how much 0.100 m h2so4 are needed to make 25.0 ml of 0.00500 m solution

Answers
Answer:
3) 1.25
Explanation:
I had the same question and somehow got it right :)
The new volume will be determined by molarity and which will be 1.25 ml.
What is volume?The volume of an object is just a measurement of how much space it takes up.
What is Molarity?
The molality refers to the moles of a solute in relation to the mass of the solvent, whereas the molarity refers to the moles of a solute in based on the volume of the solution. It can be denoted by M.
The formula of Molarity is
\(M_{1} V_{1} =M_{2}V_{2}\)
where, M is molarity and V is volume.
Calculation of volume.
It is given that,\(M_{1} = 0.1 M, M_{2} =0.0025 M.\)
Now, puts the value of given data in molarity equation.
\(M_{1} V_{1} = M_{2} V_{2}\)
\(V_{1} = M_{2} V_{2} /M_{1}\)
\(V_{1}\) = 0.005×25/0.1 = 1.25 ml
Therefore, the correct answer will be option 3.
To know more about molarity and volume click here.
https://brainly.com/question/20366625.
#SPJ2