Answers
Answer:
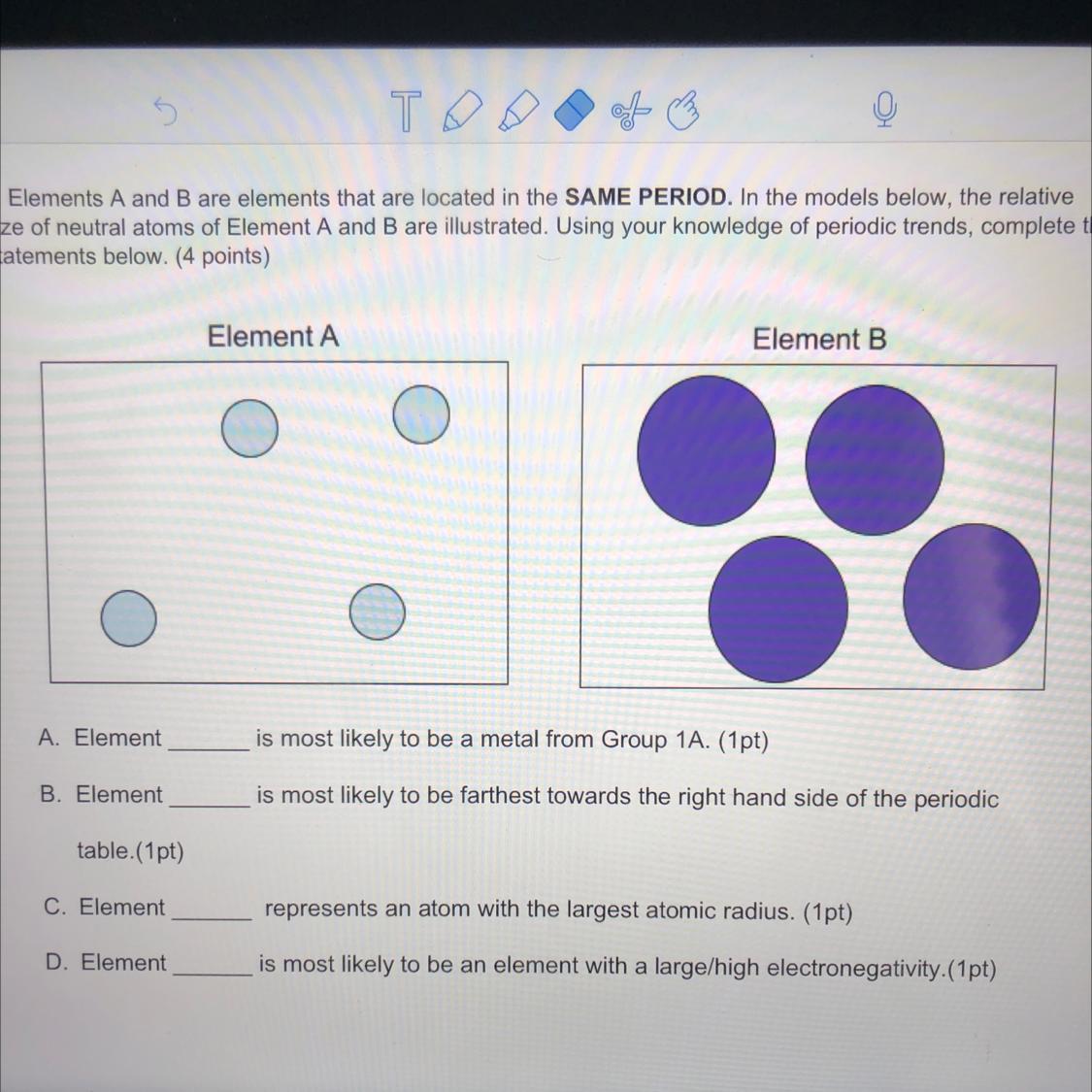
A: element B
B: element A
C: element B
D: element A
Explanation:
decrease in size leads increase in electronegativity because the smaller the size, the closer the shell is to the nucleus. Also, atomic radius decreases to the right and up on the periodic table. Atomic radius increases to the left and down a period. I hope this helps!
Related Questions
32. 2 g of ethyl alcohol is dissolved in water to make a 0. 50 L
solution. What is the molarity of the solution?
Answers
Answer:
Well, by definition,
molarity = moles of solute
volume of solution
Explanation:
Now the moles of solute are a constant. The volume of solution MAY change substantially with increasing or decreasing temperature. In some calculations molality is used in preference, which is defined by the quotien
Hope this helps!
Complete combustion of a 0.600-g sample of a compound in a bomb calorimeter releases 24.0 kj of heat. the bomb calorimeter has a mass of 1.30 kg and a specific heat of 3.41 j/(gi°c). if the initial temperature of the calorimeter is 25.5°c, what is its final temperature? use q equals m c subscript p delta t.. 30.9°c 34.5°c 44.0°c 51.5°c
Answers
The final temperature of the bomb calorimeter is 30.9°C.
What is combustion reaction?Combustion reactions are those reactions in which a compound completly decompose in to carbon dioxide and water molecule.
The final temperature will be calculated by using the equation:
Q = mc(T₂ – T₁), where
Q = relesed heat from calorimeter = 24kJ = 24000J
m = mass of calorimeter = 1.30kg = 1300g
c = specific heat of calorimeter = 3.41 J/(g°C)
T₁ = initial temperature of calorimeter = 25.5°C
T₂ = final temperature of calorimeter = to find?
On putting all these values on the above equation and calculate for T₂, we get
T₂ = 24000/(1300)(3.41) + 25.5
T₂ = 30.9°C
Hence, correct option is (1) i.e. 30.9°C.
To know more about released energy, visit the below link:
https://brainly.com/question/2279475
Answer:
30.9
Explanation:
right on edge 2022
The volume and the amount of gas are constant in a tire. The initial pressure and temperature are 1.82 atm and 293 K. At what temperature will the gas in the tire have a pressure of 2.35 atm?
What gas law will you use to solve this problem?The value for P1 is
, the value for P2 is
, the value of T1 is
, and the value for T2 is
. What Kelvin temperature will the gas in the tire have when the pressure is increased?
.
HELP PLS
Answers
PV = nRT
Where P is the pressure, V is the volume, n is the number of moles of gas, R is the gas constant, and T is the temperature in Kelvin.
Since the volume and the amount of gas are constant in this problem, we can use the following equation to find the relation between the pressure and temperature:
P1/T1 = P2/T2
where P1 and T1 are the initial pressure and temperature, and P2 is the pressure at the unknown temperature T2.
Substituting the given values, we have:
1.82 atm / 293 K = 2.35 atm / T2
Solving for T2, we get:
T2 = 1.82 atm * 293 K / 2.35 atm = 227.37 K
Therefore, the gas in the tire will have a temperature of 227.37 K, or approximately -45.78 degrees Celsius, when the pressure is increased to 2.35 atm.
3(x - 2) = 5(x + 4)
Answers
Answer:
Uh first of all this is algebra but I'll answer this
First distribute the three and 5 (Multiply them by both terms inside parenthesis.
3x-6=5x+20
Then add like terms
8x=14
Divide 8 by 8 and 8 by 14
x = 14/8
Explanation:
Answer:
The answer is \(x = -13\) .
Explanation:
Solve the equation:
\(3(x -2) = 5(x + 4)\)
Use Distributive Property:
\(3(x -2) = 5(x + 4)\)
\(3x - 6 = 5x + 20\)
-Subtract \(5x\) from \(3x\) :
\(3x - 6 -5x = 5x -5x + 20\)
\(-2x - 6 = 20\)
-Add both sides by \(6\) :
\(-2x - 6 + 6 = 20+6\)
\(-2x = 26\)
-Divide both sides by \(2\) :
\(\frac{-2x}{-2} = \frac{26}{-2}\)
\(x = -13\)
So, now you have found the answer.
How dose mass affect when objects collide??
Answers
What causes some animals to migrate from a taiga biome to the lower latitude biomes such as tropical rain forest
Answers
Answer:
The biome concept organizes large-scale ecological variation. Terrestrial biomes are distinguished primarily by their predominant vegetation, and are mainly determined by temperature and rainfall.
Terrestrial Biomes
Differences in temperature or precipitation determine the types of plants that grow in a given area (Figure 1). Generally speaking, height, density, and species diversity decreases from warm, wet climates to cool, dry climates. Raunkiaer (1934) classified plant life forms based on traits that varied with climate. One such system was based on the location of the perennating organ (Table 1). These are tissues that give rise to new growth the following season
fully reacting an aldehyde with an alcohol will produce? a. an acetal b. a primary alcohol c. no reaction d. a carboxylic acid'
Answers
correct option is a. acetal. Fully reacting an aldehyde with alcohol will produce an acetal.
Acetals are organic compounds that have the general formula R2C (OR ') 2, where R and R' are alkyl groups. An acetal is formed by the reaction of an aldehyde with two equivalents of alcohol under acidic or basic conditions.
What is the reaction between aldehydes and alcohols?
The reaction of an aldehyde with alcohol produces an acetal. This reaction requires the presence of an acid catalyst to convert the alcohol to its corresponding alkoxide, which then reacts with the carbonyl group of the aldehyde to form the acetal.
In the reaction, the carbonyl group is transformed into an ether, and the alcohol is transformed into an ether. The reaction equation is:
Aldehyde + Alcohol + Acid → Acetal + Water
Therefore, the correct option is a) An acetal.
To learn more about aldehyde and ketone refer - https://brainly.com/question/29756072
#SPJ11
Where did the 2 come from?
Answers
Answer:
somewhere
Explanation:
10. Cesium-137 has a half-life of about 30 years. Is it normal that after more than 20 years, levels of
radioactivity in the ground near Chernobyl are still very high? Explain your answer.
/2 marks
Answers
Answer:
see below
Explanation:
There is still more than 1/2 of the original amount of cesium in the environment because it has been less than one half life ...so levels if high to begin with, are still high.
Balance the following equations.
a. _CH4 + _O2 → _CO2 + _H2O
b. _Zn + _HCl → _ZnCl2 + _H2
c. _H2SO4 + _HI → _H2S + _I2 + _H2O
d. _NaCl + _SO2 + _H2O + _O2 → _Na2SO4 + _HCl
Answers
Answer:
a. CH4+202→CO2+2H2O
b.Zn+2HCl→ZnCl2+H2
c.H2SO4+8HI→H2S+4I2+4H2O
d.4NaCl+2SO2+2H2O+O2→2Na2SO4+4HCl
What phrase Best defines energy 
Answers
Answer:
the ability to do work.
Explanation:
The atmospheric gas that forms a mild acid when dissolved in water is _____.
Answers
Answer:
Carbon dioxide
HAVE A GOOD DAY!
Answer:
Carbon Dioxide
Explanation:
Carbon dioxide is in the atmosphere and is an end product in organisms that obtain energy from breaking down sugars, fats and amino acids with oxygen as part of their metabolism.
what’s the number of moles 105.9 g NaCl
Answers
the number of moles 105.9 g NaCl is 1.8121.
How does NaCl function?The substance our body needs to absorb and transfer tape is chloride (NaCl), also referred to as salt. keep the heart rate steady. keep the appropriate fluid balance.
How do NaCl and regular salt differ from one another?The chemical formula for both is NaCl. However, sodium chloride is just a salt that is composed of one metal and one non-metal i.e., sodium metal, and chlorine non-metal, whereas table salt is indeed a refined salt that contains 97 to 98% sodium chloride.
To know more about NaCl visit:
https://brainly.com/question/1550455
#SPJ1
At the UNAM's Science Faculty, the probability that a student who is admitted for studies takes Chemistry AND Geology is 0.08. The probability that a student takes Geology is 0.45. Let C denote the event: student chooses Chemistry; and let G denote the event: student chooses Geology. What is the probability that a student will choose Chemistry given that the student is taking Geology? 2. An X-ray test is used to detect a certain disease that is common in 3% of the population. The test has the following error rates: 7% of people who are disease-free do get a positive reaction and 2% of the people who have the disease do get a negative reaction from the test. A large number of people are screened at random using the test, and those with a positive reaction are further examined. What is the probability that a person tested at random indeed has the disease given that the test result shows positive?
Answers
P(C|G) = P(C and G) / P(G)
Given that the probability of a student taking Chemistry AND Geology is 0.08 (P(C and G) = 0.08) and the probability of a student taking Geology is 0.45 (P(G) = 0.45), we can substitute these values into the formula:
P(C|G) = 0.08 / 0.45 ≈ 0.1778
Therefore, the probability that a student will choose Chemistry given that the student is taking Geology is approximately 0.1778, or 17.78%.
To find the probability that a person tested at random indeed has the disease given that the test result shows positive, we can use Bayes' theorem:P(Disease|Positive) = (P(Positive|Disease) * P(Disease)) / P(Positive)
Given that the disease is common in 3% of the population (P(Disease) = 0.03), the error rate for people who are disease-free getting a positive reaction is 7% (P(Positive|Disease-free) = 0.07), and the error rate for people who have the disease getting a negative reaction is 2% (P(Negative|Disease) = 0.02), we can calculate the probability of a positive result (P(Positive)) as follows:
P(Positive) = (P(Positive|Disease) * P(Disease)) + (P(Positive|Disease-free) * P(Disease-free))
= (0.98 * 0.03) + (0.07 * 0.97)
≈ 0.0294 + 0.0679
≈ 0.0973
Now, we can substitute these values into Bayes' theorem:
P(Disease|Positive) = (0.98 * 0.03) / 0.0973
≈ 0.0294 / 0.0973
≈ 0.3026
Therefore, the probability that a person tested at random indeed has the disease given that the test result shows positive is approximately 0.3026, or 30.26%.
Learn more about Probability
brainly.com/question/31828911
#SPJ11
A solution is prepared by dissolving 108.3 gHCl(g) in enough water to make 135.0 L ofsolution. The pH of this solution isa. 1.66b. 12.34c. 0.096d. 2.97e. none of these
Answers
The pH of the solution prepared by dissolving 108.3 g HCl(g) in enough water to make 135.0 L of solution is (a) 1.66
To determine the pH of the given solution, we need to first calculate the concentration of H⁺ ions in the solution. HCl is a strong acid, which means it completely dissociates in water to form H⁺ and Cl- ions.
The balanced chemical equation for the dissociation of HCl is: HCl (g) → H⁺ (aq) + Cl⁻ (aq)
The molar mass of HCl is 36.46 g/mol. Therefore, the number of moles of HCl in 108.3 g is:
n = mass/molar mass = 108.3 g / 36.46 g/mol = 2.97 mol
The volume of the solution is 135.0 L, so the concentration of H⁺ ions is:
[H⁺] = n/V = 2.97 mol/135.0 L = 0.022 M
To calculate the pH, we use the equation:
pH = -log[H⁺]
Substituting the value of [H⁺], we get:
pH = -log(0.022) = 1.66
Therefore, the pH of the given solution is 1.66, which corresponds to option (a).
In summary, the given solution is prepared by dissolving 108.3 g of HCl in enough water to make 135.0 L of solution. The pH of the solution is 1.66, which is calculated based on the concentration of H⁺ ions in the solution, which is determined from the number of moles of HCl and the volume of the solution.
Learn more about pH here: https://brainly.com/question/26424076
#SPJ11
2. which atoms (or groups of atoms) can have expanded octets?
a. F
b. Cl
c. N
d. B
e. O
Answers
The atoms (or groups of atoms) that can have expanded octets are option c. N and option e. O.
An octet is a set of 8 electrons in an atom's outer shell. The octet rule is a chemical rule that explains how atoms combine and behave chemically by requiring them to have complete outer electron shells consisting of eight electrons. When considering covalent bonding in a compound, the octet rule states that atoms will donate, accept, or share electrons to achieve a full valence shell of eight electrons.
Expanded octets occur when an atom in a compound has more than eight electrons in its outer shell. Expanded octets are only observed for elements in period three and above because of the d orbitals in the valence shell, which allows for more electrons to be held.
Expanded octets can exist due to the existence of d-orbitals in elements. The most popular expanded octets are found in phosphorus, sulfur, and chlorine.
To learn more about atoms visit;
https://brainly.com/question/1566330
#SPJ11
the _________ in the equation means a reaction is happening
Answers
Which one of the equations below is an exothermic reaction?
A) CO2 (g) - C(s) + O2 (g) AH = 394 kJ/mol
B) CaO (s) + H2O (D = Ca(OH)2 (aq) AH = -64
kJ/mol
C) C (s) + 2 F2 (g) - CF. (9) AH = 141.3
kJ/mol
2 NO (9) AH° = 180.6
D) N2 (g) + O2 (9)
kJ/mol
Answers
Answer:
B) CaO(s) + H2O(l) --> Ca(OH)2(aq)
Explanation:
This is the only reaction with a negative enthalpy value. Exothermic reactions have a negative enthalpy.
in lactic acid fermentation, pyruvate functions as an: electron acceptor for the reduction of nad electron acceptor for the oxidation of nadh electron donor for the reduction of nad electron donor for the oxidation of nadh
Answers
In lactic acid fermentation, pyruvate functions as an electron acceptor for the reduction of NAD+.
Lactic acid fermentation is a process that occurs in the absence of oxygen and involves the conversion of pyruvate to lactate. During this process, NADH is oxidized to NAD+, providing the cell with a continuous supply of NAD+ for glycolysis. The conversion of pyruvate to lactate is catalyzed by the enzyme lactate dehydrogenase and involves the transfer of electrons from NADH to pyruvate. As a result, pyruvate acts as an electron acceptor for the reduction of NAD+.
Overall, the reaction can be summarized as follows:
Pyruvate + NADH → Lactate + NAD+
Know more about pyruvate
https://brainly.com/question/25886015
#SPJ11
Help for brainliest

Answers
Answer:
those things that looks like cristals
How is percent yield calculated? (2 points) Measured mass of limiting reactant divided by the measured mass of the excess reactant given at the beginning of the reaction Measured mass of excess reactant divided by the measured mass of the limiting reactant used in the reaction Measured mass of product actually produced divided by calculated mass of product that should be produced by the given amount of reactants Calculated mass of product that should be produced by the given amount of reactants divided by the measured mass of product actually produced
Answers
Measured mass of product actually produced divided by calculated mass of product that should be produced by the given amount of reactants gives the percent yield. The correct option is option C.
The % ratio of the theoretical yield to the actual yield is known as the percent yield. It is calculated as the theoretical yield times by 100% divided by the experimental yield. The percent yield equals 100% if the theoretical and actual yields are equal.
Because the real yield is frequently lower than the theoretical value, percent yield is typically lower than 100%. This may be due to incomplete or conflicting reactions or sample loss during recovery. Measured mass of product actually produced divided by calculated mass of product that should be produced by the given amount of reactants gives the percent yield.
Therefore, the correct option is option C.
To know more about percent yield, here:
https://brainly.com/question/2506978
#SPJ1
If 50 mL of a 3.6 M solution is diluted to a total volume of 785 mL, what is the new
molarity of the dilute solution?
Answers
The new molarity of the dilute solution is 0.23 M.
To solve this problem, we can use the formula for dilution: M1V1 = M2V2, where M1 is the initial molarity, V1 is the initial volume, M2 is the final molarity, and V2 is the final volume.
In this case, we have M1 = 3.6 M, V1 = 50 mL, and V2 = 785 mL. We want to find M2, the new molarity of the dilute solution.
First, we need to convert the volumes to liters, since molarity is defined as moles of solute per liter of solution. So V1 = 0.050 L and V2 = 0.785 L.
Next, we can plug in the values we have into the dilution formula and solve for M2:
M1V1 = M2V2
(3.6 M)(0.050 L) = M2(0.785 L)
0.18 mol = M2(0.785 L)
M2 = 0.23 M
Therefore, the new molarity of the dilute solution is 0.23 M.
The new molarity of the dilute solution is 0.23 M. This solution requires a dilution process in order to achieve the desired molarity. Dilution is the process of reducing the concentration of a solution by adding more solvent to it. The formula for dilution is M1V1 = M2V2, where M1 is the initial molarity, V1 is the initial volume, M2 is the final molarity, and V2 is the final volume. In this case, the initial molarity is 3.6 M, and the initial volume is 50 mL. The final volume is 785 mL, which is 0.785 L. After solving for M2, the new molarity of the dilute solution is 0.23 M.
To know more about dilution visit:
https://brainly.com/question/28997625
#SPJ11
a compound with the formula secln has a formula mass of 220.77 amu. what is the value for n in the formula secln?
Answers
Molecular mass/ empirical formula mass = n
Where n is a whole number.
For the above question, n = 2
Explain the above formula.The above formula is used to calculate the formula mass of a compound.
Where n, that is the answer, should be a whole number or very close to a whole number.
Molecular mass = 220.771 g/mol
Se molecular weight =78.96
Cl molecular weight = 35
The formula mass = 78.96+35.45 = 114.41
Thus, n = 220/114 = 2
Hence value of n=2
To know more about empirical formula, click on https://brainly.com/question/1439914
#SPJ4
If the density of a gas is 1.2 g/L at 745 torr and 20 degree celsius, what is its molecular mass?R = 0.0821 L.atm/K.mol
Answers
The question requires us to calculate the molecular mass of a gas, given its density (1.2 g/L) and conditions of pressure (745 torr) and temperature (20°C).
Density is defined as the mass of a compound over its volume. From this definition, we can calculate the molecular mass of the gas knowing the density, as given by the question, and the volume of 1 mol of the gas.
\(\text{density = }\frac{mass}{\text{volume}}\to\text{mass = volume }\times\text{ density}\)We can calculate the volume of a gas considering the equation of Ideal Gases:
\(P\times V=n\times R\times T\)where P is the pressure of the gas, V is its volume, n is the number of moles, R is the constant of gases and T is the temperature.
Note that the constant of gases R was given in units of L.atm/K.mol, while the pressure and temperature were given in Torr and °C, respectively. Thus we need to convert these values to the appropriate units.
Knowing that 1 Torr corresponds to 0.00131579 atm:
1 Torr --------------------- 0.00131579 atm
745 Torr ---------------- x
Solving for x, we have that 745 Torr corresponds to 0.980 atm.
To convert the temperature from Celsius degrees to Kelvin, we must add 273.15:
T = 20 + 273.15 K = 293.15 K
Therefore, the pressure and temperature we'll use in our calculation are 0.980 atm and 293.15 K. Also, since we are calculating the molecular mass, we'll consider 1 mol of gas.
Rearranging the equation of ideal gases to calculate the volume and applying the values to the equation, we'll have:
\(\begin{gathered} P\times V=n\times R\times T\to V=\frac{n\times R\times T}{P} \\ V=\frac{(1mol)\times(0.0821L.atm/K.mol)\times(293.15K)}{(0.980\text{atm)}}=24.6L \end{gathered}\)Therefore, the volume of 1 mol of the gas under the conditions given is 24.6L.
Next, we'll use this value to calculate the molecular mass using the density given by the question:
\(\begin{gathered} \text{mass = volume }\times\text{ density} \\ \text{mass = 24.6L}\times1.2g/L \\ \text{mass = }29.5g/\text{mol} \end{gathered}\)Therefore, the gas given by the question presents 29.5g per mol.
What is the "percent
abundance" of the size 14d
nails in this sample?
Nail Size
4d
14d
Number
of Nails
123
77
Abundance Mass
(%)
(g)
3.65
11.95
[?]
Percent Abundance
Weighted
Average (g)
6.8

Answers
The percent abundance of each nail sample would 61.5 and 38.5% respectively.
What is percent abundance?
The percent abundance of a component of a sample is the ratio of the amount of the component and the total amount of the sample itself.
The percent abundance can be mathematically expressed as:
Percent component in a sample = amount of component/amount of total sample x 100
In this case, the total number of nails can be calculated as:
123 + 77 = 200 nails.
Percent of 4d nails = 123/200 x 100 = 61.5%
Percent of 14d nails = 77/200 x 100 = 38.5%
This means that the percent component of each of the nail sizes in the sample is 61.5 and 38.5% respectively.
More on percent composition can be found here: https://brainly.com/question/17505281
#SPJ1
what is the name of this organic compound?

Answers
The name of the organic compound is 2-methyl pentane. The given organic compound is a five-carbon system with a substitution at the C-2 carbon. The naming of an organic compound is done according to the rules given by IUPAC.
The given organic compound has 5 carbon in its main chain. So It has the root word Pent. Since, all the bonds are single bonds, the organic compound is saturated, hence it has the suffix -ane. Hence the unsubstituted straight chain is pentane.
Numbering is done from right to left, because when the numbering is from right to left, the substituted carbon gets C-2, when it is numbered from left to right, the substituted carbon gets C-4. So the numbering is from the right and the substituted carbon is C-2. The substituent is a single carbon system, a methyl substituent. So the organic compound is named 2-methyl pentane .
Learn more about naming of organic compounds here,
https://brainly.com/question/27843604?
what is the ph of 1.00 l of a buffer that is 0.110 m nitrous acid (hno2) and 0.160 m nano2? (pka of hno2
Answers
We need to use the Henderson-Hasselbalch equation, which relates the pH of a buffer solution to the pKa of the weak acid and the concentrations of the acid and its conjugate base. The equation is pH = pKa + log([A-]/[HA]), where [A-] is the concentration of the conjugate base and [HA] is the concentration of the weak acid.
In this case, the weak acid is nitrous acid (HNO2) and its conjugate base is nitrite ion (NO2-). The pKa of HNO2 is 3.35. We are given the concentrations of HNO2 and NaNO2, which are 0.110 M and 0.160 M, respectively.
First, we need to calculate the concentration of the conjugate base, which is given by [A-] = NaNO2. Therefore, [A-] = 0.160 M.
Next, we need to calculate the concentration of the weak acid, which is given by [HA] = HNO2. Therefore, [HA] = 0.110 M.
Now we can substitute these values into the Henderson-Hasselbalch equation:
pH = 3.35 + log(0.160/0.110)
pH = 3.35 + 0.23
pH = 3.58
Therefore, the pH of 1.00 L of the buffer solution is 3.58.
To know more about Acid visit :
https://brainly.com/question/29796621
#SPJ11
A student bends a paperclip rapidly back and forth. When he touches the point where he was bending the paperclip, he finds that its temperature has increased. why did it increase?
Answers
A student bends a paperclip rapidly back and forth when he touches the point where he was bending the paperclip, he finds that its temperature has increased because temprature goes up at the point of the bending
Temprature is the degree of hotness and coldness and here when student bends a paperclip rapidly back and forth he touches the point where he was bending the paperclip then the temprature is increases because of temprature goes up at the point of the bending and molecule of clip start moving slower
Know more about temperature
https://brainly.com/question/28446445
#SPJ1
what are the benefits of a lilac bonnet?
Answers
Answer:
Maintains natural hair moisture.
Prevents hair frizz.
Reduces hair breakage & tangles.
Longer lasting hair styles.
Explanation:
.What is the KE of a baseball that has a mass of 100 kg and is traveling at 5 m/s?
Answers
Answer:
1250joules
Explanation:
The kinetic energy is energy in motion and it's formula is
KE =1/2MV^2
M represent mass which is 100Kg.
V represent velocity which is 5m/s
Therefore, KE = 1/2×100×5× 5
= 50 × 25
KE= 1250joules