Help me please!!! Useful answers only!!!
Valence Electrons:
Barium: 2
Phosphorus: 5
Oxygen: 6
b) Based on the number of valence electrons, write the monatomic ion (symbol with charge) each element may form.
c) Phosphorus and oxygen can bond to form a polyatomic ion. Write the formula of for this ion and be sure to include the ion charge.
d) Using the monatomic ions you listed above in (b), the polyatomic ion from (c), write the formulas for the three ionic compounds these ions could form
Answers
Answer:
the answer would be c
Explanation:
Related Questions
what type of chemical reaction is
10.Pb + FeSO, → PbSO, + far
Answers
Answer: To balance a chemical equation, enter an equation of a chemical reaction and press the Balance button. The balanced equation will appear above. Use uppercase for the first character in the element and lowercase for the second character.
Explanation:
What happened to the sediment?
Answers
so.. the answer is “ Sediment moves from one place to another through the process of erosion. “
hope this helps!!
Which of the following statements is true about chemical nutrients in an ecosystem?
A. They cannot be obtained from decomposition.
B. They flow through the system, losing some nutrients in the process.
C. They exit the ecosystem in the form of heat.
D. They recycle within the ecosystem, being constantly reused.
E. They depend on sunlight as their source.
Answers
The statement that is true about chemical nutrients in an ecosystem is : D.) They recycle within the ecosystem, being constantly reused. Therefore, option D) is the correct answer.
The nutrient cycle is vital to the ecosystem, and this is how nutrients are recycled in it. Nutrients that are considered chemical nutrients include carbon, hydrogen, oxygen, nitrogen, and phosphorus.What are chemical nutrients in an ecosystem
Chemical nutrients refer to essential elements that are found in an ecosystem's physical and chemical environment. These elements are necessary for life because they are responsible for different functions such as cell structure, the production of enzymes, and the production of hormones.
In conclusion, chemical nutrients recycle within the ecosystem, being constantly reused. Nutrient recycling helps to maintain the ecosystem's sustainability. It helps to maintain the balance of life forms within the ecosystem.
To know more about ecosystem, refer
https://brainly.com/question/842527
#SPJ11
a bottle of na in solution and a bottle of cl in solution are mixed together and the water evaporated. what type of bond will be created between the atoms, and what will be the product?
Answers
The bond formed by mixing the Na and Cl solutions will become IONIC and the product formed will be table salt (NaCl) after exhausting the water.
After the water is evaporated due to the temperature rise, the sodium ion binds one electron to the chlorine and becomes slightly positive and the chlorine becomes negatively charged by gaining electrons from sodium as we all know that opposites attract, and an ionic bond is formed between the two atoms resulting in the formation of NaCl.
And NaCl is usually referred to as table salt.
Na(2,8,1) → Na⁺(2,8) + e⁻
Cl(2,7) + e⁻ → Cl⁻ (2,8)
Na⁺ + Cl⁻ → NaCl
Learn more about ionic bonds at https://brainly.com/question/13526463
#SPJ4
what is the name of the organic molecule
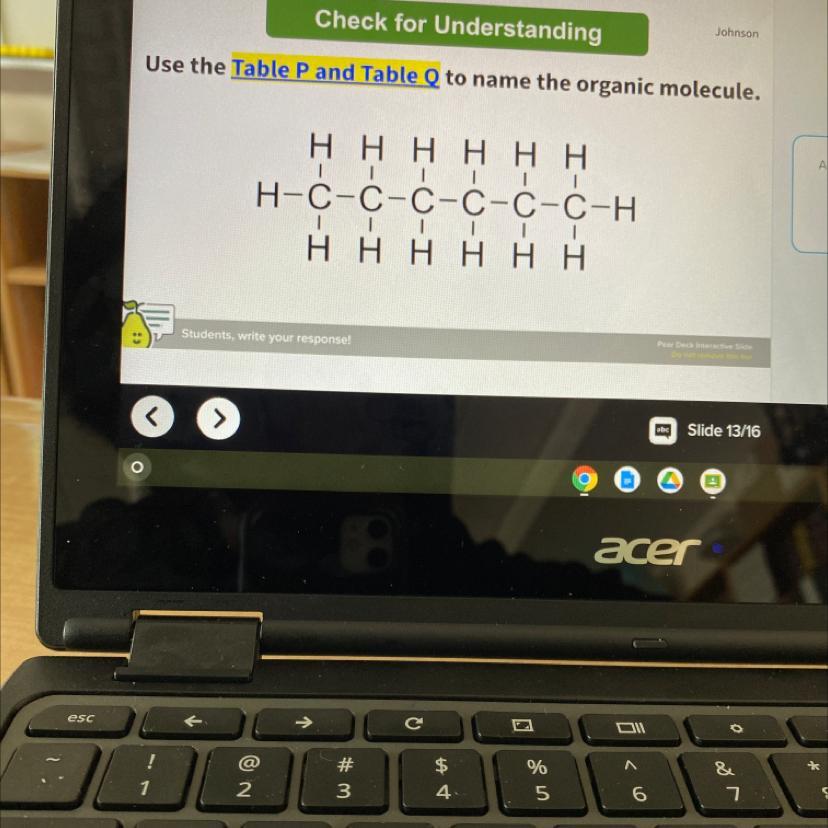
Answers
Explanation:
Although carbon is present in all organic compounds, other elements such as hydrogen ( H), oxygen ( O), nitrogen ( N), sulfur ( S) and phosphorus ( P) are also common in these molecules. An organic molecule is a molecule that contains carbon atoms (generally bonded to other carbon atoms as well as hydrogen atoms)
A woman is swimming across a cold lake. Her body temperature is 98 degrees and the lake water is 60 degrees. Which has more thermal energy, the woman or the lake? Explain your answer!
Answers
I think the lake because of how heat is transferred or something. Just check out the first law of thermodynamics.
Answer:
the woman
Explanation:
What accounts for the fact that liquid mercury forms into tight beads while ethyl alcohol do not?
Answers
Answer:
Pretty sure this is wrong but worth a shot
Explanation:
Ethyl Alcohol is a clear, colorless liquid with a wine-like odor. It is used in alcoholic . Do not rely on odor alone to determine ... This chemical is on the Special Health Hazard Substance. Ethyl Alcohol can affect you when inhaled and by passing through the Ethyl Alcohol may form an ignitable vapor/air mixture in.
A reaction vessel contains 20.0 grams of sodium metal and 10.0 grams of chlorine gas. The reaction to produce sodium chloride has gone to 100% completion. Which is the excess reagent and how much of it remains
Answers
Sodium metal is the excess reagent and after the completion of the reaction 16.8g of it is left.
What is excess reagent?In a chemical reaction, the reactant which is present in excess and don't get fully used up in the reaction is called excess reagent.
How to calculate excess reagent?To calculate excess reagent we will use the formula:no. of moles (n)/stoichiometry coefficient.
The reactant with greater value will be the excess reagent.
The balanced chemical equation for the reaction is:\(2Na+Cl_2\) ⇒ \(2NaCl\)
where given mass of Na is 20g molecular weight=23g
given mass of \(Cl_{2}\) is 10g molecular weight=71g
{n/S.C} for Na: {n/S.C} for Cl:n=given mass/ molar mass n=given mass/ molar mass
=20/23 =10/71
S.C=2 S.C.=1
so n/S.C value is 0.43 so n/S.C value is 0.14
Since value is high, Na is Since value is lower, \(Cl_{2}\) excessive reagent. is limiting reagent.
The completion of reaction will depend on the limiting reagent.according to the equation, 71g of \(Cl_{2}\) react with 23g of Na
1g ≡ 23/71
10g ≡ 23/71 *10
=3.2g of Na
the amount of Na left is 20-3.2 =16.8g
Hence Na is the excess reagent with 16.8g of it remains.
For more information refer to:
https://brainly.com/question/14222359
#SPJ4
A solution is prepared by dissolving 17. 1 g of sucrose (C12H220,1) in 275 g of H2O.
a. What is the molar mass of sucrose?
b. What is the molality of that solution?
Answers
Answer:
If you know the formula to use you should have no trouble getting the answers.
molality = mols/kg solvent.
mols sucrose = g/molar mass
kg solvent = 0.275 kg.
5.Which of the following elements was present in Mendeleev’s periodic table?
(a)Sc
(b) Tc
(c) Ge
(d) None of these
Answers
The element Sc (Scandium) was present in Mendeleev's periodic table. Therefore, the correct answer is (a) Sc.
Mendeleev's periodic table:
Mendeleev's periodic table is a chart that organizes all known elements based on their atomic number, chemical properties, and recurring patterns in their physical and chemical properties.
The periodic table consists of rows (called periods) and columns (called groups). Elements in the same group have similar chemical properties, while elements in the same period have the same number of electron shells.
Mendeleev published the first version of his periodic table in 1869, which included 63 elements known at that time. Scandium (Sc) was discovered in 1879 by Lars Fredrik Nilson and was later added to the periodic table in its proper position based on its atomic number and chemical properties.
On the other hand, Technetium (Tc) was not present in Mendeleev's periodic table because it was not discovered until 1937, long after Mendeleev's death. Similarly, Germanium (Ge) was not discovered until 1886, after the publication of Mendeleev's periodic table, but it was added to the periodic table in its proper position based on its properties.
To know more about electron shells, visit:
https://brainly.com/question/30464976
#SPJ9
how many moles of hydrogen are needed to produce 34.5 grams of ammonia
Answers
Answer:
According to the formula of mass in grams
Mass in grams= Moles/ Molar mass
Rearranging the formula
Moles= Mass/ Molar mass
Given:
Mass: 34.5 g
Molar mass: NH3
: 14 + 1×3
: 17g
Moles: ?
Solution:
Moles= Mass/ Molar mass
= 34.5 / 17
Moles= 2.025 mol
Answer:
So 2.025 moles of hydrogen are needed produce 34.5 grams of ammonia
Hope it helps
7. R, the gas constant is equal to these three values, include units;
Answers
R, the gas constant is equal to these three values, volume, temperature, pressure and number of moles.
Depending on the other units used in the equation, different units are used for the gas constant. The Gas Constant's Value The units used for pressure, volume, and temperature have an impact on the value of the gas constant "R". These were typical gas constant values prior to 2019. R = 8.3145 J/mol K R = 8.2057 m 3 atm/mol K R = 0.0821 litre atm/mol K. Work per degree every mole is what R means physically. Any system of units for measuring labour or energy, such as joules, or for measuring temperature at an absolute scale, like as kelvin or rankine, may be used to express it.
To know more about gas constant, here:
https://brainly.com/question/14279790
#SPJ1
Question 11
Which formula represents a hydrocarbon?
C₂H6
C₂H5OH
C₂H5Cl
C₂H6O
Answers
Answer:
C₂H6
Explanation:
Among the given options, the formula A) C₂H6 represents a hydrocarbon (specifically, ethane). Option A
A hydrocarbon is a compound that consists of only carbon and hydrogen atoms. It is important to identify the formula that represents a hydrocarbon among the given options:
A) C₂H6: This formula represents ethane, which is a hydrocarbon. Ethane consists of two carbon atoms bonded together with single bonds and six hydrogen atoms.
B) C₂H5OH: This formula represents ethanol, which is not a hydrocarbon. Ethanol contains a hydroxyl group (-OH), indicating the presence of oxygen in addition to carbon and hydrogen atoms. It is an alcohol, not a hydrocarbon.
C) C₂H5Cl: This formula represents ethyl chloride, which is not a hydrocarbon. Ethyl chloride contains a chlorine atom (Cl) in addition to carbon and hydrogen atoms. It is a haloalkane, not a hydrocarbon.
D) C₂H6O: This formula represents ethanol, which, as mentioned before, is not a hydrocarbon. Ethanol contains an oxygen atom (O) in addition to carbon and hydrogen atoms. It is an alcohol, not a hydrocarbon.
Among the given options, the formula A) C₂H6 represents a hydrocarbon (specifically, ethane). It consists only of carbon and hydrogen atoms, making it a suitable representation of a hydrocarbon.
In summary, the formula C₂H6 (option A) represents a hydrocarbon, while the other options contain additional elements (oxygen or chlorine) that make them non-hydrocarbon compounds. Option A
For more such questions on hydrocarbon visit:
https://brainly.com/question/21281906
#SPJ8
ILL GIVE U BRAINLYSIT

Answers
Answer:what?
Explanation:what? Huh
A year has 365 days, and a day has 24 hours. If an hour has 60 minutes and a minute has 60 seconds, how many seconds are there in 1 year?
54,750 s
525,600 s
3.15 Times. 107 s
1.89 Times. 109 s
Answers
Answer:
31,536,000 seconds
Explanation:
off google
Answer:
3.15 x 10^7s
Explanation:
Prepare a list of objects around you that are electroplated
Answers
Answer:
Hello! Some household items that are electroplated include kitchenware, such as metal pots and pans, door handles, mobile phones, and coins. An electroplated object is coated by electrolytic deposition with chromium, silver, or another metal.
PART A QUESTION 1 (a) (b) (c) (d) Use an appropriate diagram to elucidate the generation of characteristic X-ray in an atom. Explain how the X-rays are produced in an X-ray tube. C2 SP1 C2 SP3 Are X rays reflected by bone tissues? Provide your comments on the image difference between soft and hard tissue obtained in an X-ray film. C5 SP4 State ONE (1) type of physical injury where an X-ray device is used for diagnostic purpose. C2 SP3
Answers
(a) Diagram of characteristic X-ray generation in an atom:
[Note: Due to the limitations of text-based communication, I'm unable to provide a visual diagram. However, I'll explain the process in the following text.]
(b) Explanation of characteristic X-ray generation:
When high-energy electrons collide with an atom, they can knock out inner shell electrons, creating vacancies. Outer shell electrons then transition to fill these vacancies, releasing energy in the form of X-rays. These X-rays are called characteristic X-rays and have specific energies corresponding to the energy differences between different electron shells.
(c) X-ray production in an X-ray tube:
An X-ray tube consists of a cathode and an anode enclosed in a vacuum. The cathode emits a stream of high-speed electrons through a process called thermionic emission. These electrons are accelerated by a high voltage and directed towards the anode. As the fast-moving electrons collide with the anode, X-rays are produced through two main processes: bremsstrahlung radiation (braking radiation) and characteristic X-ray emission.
In bremsstrahlung radiation, the electrons are decelerated by the positively charged anode, causing them to emit X-rays with a continuous spectrum of energies. Characteristic X-ray emission occurs when the high-speed electrons displace inner shell electrons in the anode, leading to the generation of characteristic X-rays specific to the anode material.
Learn more about X-ray generation here:
https://brainly.com/question/14978779
#SPJ11
Calculate ΔH for the reaction CO(g) + H2(g) + O2(g) → CO2(g) + H2O(g)
Given:
2 C(s) + O2(g) → 2 CO(g)... ∆H = -222 kJ
C(s) + O2(g) → CO2(g)... ∆H = -394 kJ
2 H2(g) + O2(g) → 2H2O(g)... ∆H = -484 kJ
Answers
The value of ΔH for the reaction CO(g) + H₂(g) + O₂(g) → CO₂(g) + H₂O(g) is -1272 kJ.
To calculate the enthalpy change (ΔH) for the given reaction, we can use Hess's law, which states that the overall enthalpy change of a reaction is equal to the sum of the enthalpy changes of the individual reactions involved.
Given the enthalpy changes:
1. 2 C(s) + O₂(g) → 2 CO(g)... ∆H = -222 kJ
2. C(s) + O₂(g) → CO₂(g)... ∆H = -394 kJ
3. 2 H₂(g) + O₂(g) → 2 H₂O(g)... ∆H = -484 kJ
We need to manipulate these reactions to obtain the desired reaction:
1. Reverse reaction 2: CO₂(g) → C(s) + O₂(g)... ∆H = +394 kJ
2. Multiply reaction 2 by 2 to balance carbon atoms: 2 CO₂(g) → 2 C(s) + 2 O₂(g)... ∆H = -788 kJ
3. Leave reaction 3 unchanged: 2 H₂(g) + O₂(g) → 2 H₂O(g)... ∆H = -484 kJ
By adding reactions 2 and 3, we obtain the desired reaction:
CO(g) + H₂(g) + O₂(g) → CO₂(g) + H₂O(g)... ∆H = -788 kJ + (-484 kJ) = -1272 kJ
Therefore, the value of ΔH for the given reaction is -1272 kJ.
Learn more about Reaction
brainly.com/question/14025220
#SPJ11
plz help ... With explanation plz

Answers

Estimate the ethalpy change of the reaction H-H+Cl-Cl
Answers
Answer:
To estimate the enthalpy change of the reaction H-H+Cl-Cl, we need to know the enthalpies of bond dissociation of H-H and Cl-Cl, as well as the bond dissociation energy of H-Cl.
The bond dissociation energy is defined as the energy required to break a bond homolytically (i.e., each atom retains one electron from the bond). Using experimental or theoretical data, we can estimate the bond dissociation energy for each bond.
The bond dissociation energy for H-H is around 436 kJ/mol, while that for Cl-Cl is around 240 kJ/mol. The bond dissociation energy for H-Cl is around 431 kJ/mol.
Therefore, the estimated enthalpy change of the reaction H-H+Cl-Cl can be approximated as follows:
ΔH ≈ (2 × 436 kJ/mol) + (2 × 240 kJ/mol) - (4 × 431 kJ/mol)
≈ -240 kJ/mol
This estimation suggests that the reaction is exothermic, with a release of about 240 kJ/mol of energy. However, it's important to note that this is only an estimation and the actual enthalpy change of the reaction may differ depending on the experimental conditions and the specific method used to determine the bond dissociation energies.
Which of the following ions could exist in either the high-spin or low-spin state in an octahedral complex?
A. Sc3+
B. Ni2+
C. Mn2+
D. Ti4+
E. Zn2+
Answers
Ni²⁺ is the only ion on the list that can exist as both a high-spin and a low-spin octahedral complex. The correct option is B.
An electrostatic model called the crystal field theory (CFT) assumes that the metal-ligand connection is ionic and results only from electrostatic interactions between the metal ion and the ligand. When dealing with anions, ligands are viewed as point charges, and when dealing with neutral molecules, as dipoles.
The crystal field splitting theory predicts that some transition metal ions can exist as either high-spin or low-spin octahedral complexes, depending on the magnitude of the crystal field splitting parameter (Δ) relative to the pairing energy (P).
Of the ions listed, the only one that could exist as either a high-spin or a low-spin octahedral complex is Ni²⁺ (B).
Mn²⁺ (A) is a d⁵ ion and will always form a high-spin octahedral complex due to its large number of unpaired electrons.
Sc³⁺ (C) is a d⁰ ion and does not form octahedral complexes with ligands.
Cu²⁺ (D) is a d⁹ ion and typically forms a low-spin octahedral complex due to the stability of the half-filled d⁹ configuration.
Zn²⁺ (E) is a d¹⁰ ion and does not have any unpaired electrons to undergo spin pairing, so it will always form a low-spin octahedral complex.
Therefore, the correct answer is B) Ni²⁺.
To know more about crystal field theory here
brainly.com/question/23840749
#SPJ4
what is the correct method to dispose of the benzoic acid and phenanthrene produced in this experiment?
Answers
Answer:
The correct method to dispose of the benzoic acid and phenanthrene produced in this experiment is to collect them into a container marked "Hazardous Waste" and dispose of it according to the guidelines of the local waste disposal authority.
Explanation:
Chemical waste must be disposed of carefully to avoid harming the environment or people. Benzoic acid and phenanthrene are two common chemicals that are frequently used in chemistry experiments.
What is benzoic acid?
Benzoic acid is a colorless crystalline solid that is soluble in water and alcohol but insoluble in benzene, toluene, and ether. It has a sweet odor that is similar to that of benzene. Benzoic acid is used as a food preservative and to treat various medical conditions, including urinary tract infections and fungal infections. In the lab, it's often used as a reagent in organic chemistry.
What is phenanthrene?
Phenanthrene is an aromatic hydrocarbon with a formula of C14H10. It's a white crystalline solid that's insoluble in water but soluble in most organic solvents. Phenanthrene is used in the production of dyes, agrochemicals, and pharmaceuticals. In the lab, it is commonly used as a starting material in organic synthesis reactions.
To know more about benzoic acid refer here: https://brainly.com/question/28326761#
#SPJ11
a balloon has a volume of 10.2 l at 2.3 atm of pressure and 500c. what is the temperature of the same balloon at 3.3 atm and 20.4 l?
Answers
a balloon has a volume of 10.2 l at 2.3 atm of pressure and 500c. the temperature of the same balloon at 3.3 atm and 20.4 l is 77 °c;
calculation as follows: V1/T1 =V2/T2, 2.3/298 = 2.7/T2, T2 = 77 °c. The mean vertical component of a gas's moving molecules is then evaluated by it's own pressure. The pressure acts right angles to the facade (normal); the tangential (shear) element of the force is proportional to the viscosity of the gas. The standard pressure measurement unit is the pascal (Pa). Because a pascal is a very tiny quantity of pressure, the kilopascal represents the most useful unit for each and every day gas pressures (kPa). 1000 pascals corresponds one kilopascal. The atmosphere is another frequently utilised unit of pressure (atm). Temperature and pressure are basic sets of circumstances for experimental measurements that allow comparisons between different sets of data to be made.
Learn more about pressure here:
https://brainly.com/question/15265703
#SPJ4
The diagram below represents reaction of reactant A and reactant B combining to form product C.
Reactant A illustrates an example of which of the following?
Molecules of an element
0
Atoms of an element
Molecules of a compound
O A mixture
Answers
Answer:B
Explanation:
The reactant A in the diagram represents atoms of an element. These atoms combines together to form molecules and different atoms combines to form compounds.
What are compounds?Compounds are chemical substances formed from the combination of different atoms. Atoms are the basic units of every substances. Atoms are composed by subatomic particles electrons, protons and neutrons.
Atoms which are deficient in electrons or possess extra electrons undergo chemical bonding with other atoms and the bonds are formed through electrons lose or gain or electron sharing. Same kind of atoms combines to form molecules. H₂, O₂, N₂ are molecules, whereas CO₂ H₂O etc. are compounds.
The diagram A shows the atoms of an element. B is molecules of the atoms in A whereas C is compounds of atoms in A and atoms of a different element. Hence option b is correct.
To find more on compounds, refer here:
https://brainly.com/question/13516179
#SPJ2
your question was incomplete. But your complete question probably was as in the uploaded image.

. In the reaction NAD+ + H+ + 2e−→ NADH, NAD+ becomes: a.
dehydrated b. hydrolyzed c. oxidized d. reduced
Answers
In the given reaction, NAD+ + H+ + 2e− → NADH, NAD+ undergoes a transformation known as reduction. Reduction is a chemical process that involves the gain of electrons or a decrease in the oxidation state of an atom or molecule. In this reaction, NAD+ accepts two electrons (2e-) and a hydrogen ion (H+), resulting in the formation of NADH.
NAD+ (nicotinamide adenine dinucleotide) is a coenzyme involved in numerous metabolic reactions, particularly in redox reactions. It functions as an electron carrier, shuttling electrons from one molecule to another during cellular respiration and other metabolic processes.
During the reaction, the NAD+ molecule accepts the two electrons and a hydrogen ion. The two electrons reduce the positive charge on the NAD+ molecule, resulting in the formation of NADH. The hydrogen ion (H+) is also involved in the reaction, but its main role is to balance the charges.
Therefore, in the given reaction, NAD+ is reduced to NADH. This means that the NAD+ molecule gains two electrons and is thus considered the electron acceptor, making the correct answer d. reduced.
to know more about metabolic processes. click this link-
brainly.com/question/30669617
#SPJ11
Las células epiteliales del estómago producen ácido clorhídrico HCI y su producción en exceso puede producir perforaciones en la mucosa. Una de las maneras de controlar dicho exceso es tomando una solución de bicarbonato de sodio NaHCO3, porque *
Answers
Answer and Explanation:
El bicarbonato de sodio (o carbonato ácico de sodio, NaHCO₃) es utilizado como antiácido debido a que neutraliza al ácido clorhídrico (HCl) en el estómago. Al ser disuelto en agua, el NaHCO₃ se disocia en iones como sigue:
NaHCO₃ → Na⁺ + HCO₃⁻
El anión bicarbonato (HCO₃₋) sufre hidrólisis, aceptando H⁺ del agua y liberando iones hidroxilo (OH⁻):
HCO₃⁻ + H₂O → H₂CO₃ + OH⁻
Por lo tanto, la solución de NaHCO₃ es básica y sus aniones OH⁻ pueden neutralizar los H⁺ del HCl en el estómago, reduciendo la acidez.
Which of the following are not mixtures: milk, tin, sulfur, cough linctus, brass, gold?
Answers
Answer:
\(tin \: sulphar gold \: are \: not \: mixture \: \\ they \: are \: elements \: \: others \: are \: mixture \\ thank \: you\)
Tin, sulfur, and gold are not a mixture because they are pure elements.
What are the mixture and pure substance?Pure substances can be described as elements that cannot be broken down into simple substances because they have only one type of atom in the whole composition.
A pure substance can be described as composed of two or more elements that are chemically combined and have a set composition such kind of pure substance is known as a compound.
A mixture can be described as made up of two or more different substances which are just physically combined but not chemically. A mixture can be separated into its initial components.
The composition of a heterogeneous mixture is non-uniform throughout the mixture while the composition of a homogeneous mixture is always the same in the entire mixture.
Therefore, tin, sulfur, and gold are pure elements, not a mixture.
Learn more about mixture and a pure substance, here:
brainly.com/question/6243623
#SPJ2
How would you describe the size of our sun compared to a red dwarf and a supergiant?
The sun is the same size as a red dwarf.
The sun is the same size as a supergiant.
The sun is smaller than the red dwarf and the supergiant.
O The sun is larger than a red dwarf but smaller than a supergiant.
Answers
Answer:
D is the answer
Explanation:
N2O4 ⇌ 2NO2
for the following reaction at 373 K, Kc = 0.36. If initial concentration of N2O4 is 0.1 mol dm^-3, what is the equilibrium concentration of NO2? (Is there a way to solve this without using quadratics?)
Answers
Okay, let's solve this step-by-step without using quadratics:
1) The equilibrium constant Kc = 0.36 means the equilibrium lies to the left. So there will be more N2O4 than NO2 at equilibrium.
2) The initial concentration of N2O4 is 0.1 mol dm^-3. Let's call this [N2O4]initial.
3) At equilibrium, the concentrations of N2O4 and NO2 will be [N2O4]equil and [NO2]equil respectively.
4) We know the equilibrium constant expression for this reaction is:
Kc = ([NO2]equil)^2 / [N2O4]equil
5) Setting this equal to 0.36 and plugging in 0.1 for [N2O4]initial, we get:
0.36 = ([NO2]equil)^2 / (0.1 - [NO2]equil)
6) Simplifying, we get:
0.036 = [NO2]equil^2
7) Taking the square root of both sides, we get:
[NO2]equil = 0.06 mol dm^-3
So the equilibrium concentration of NO2 is 0.06 mol dm^-3.
Let me know if you have any other questions! I can also provide a more step-by-step explanation if needed.
The gaseous decomposition of N2O5 was studied at 35 ∘C.
2N2O5(g)→4NO2(g)+O2(g)
A plot of ln[N2O5] versus time has a slope of −9. 8×10−4s−1.
PART A
If 0. 100 mol of N2O5 is added to a 1. 0 L flask at 35 ∘C, calculate the concentration of N2O5 after 12. 0 minutes.
PART B
f 0. 100 mol of N2O5 is added to a 1. 0 L flask at 35 ∘C, calculate the concentration of NO2 after 12. 0 minutes.
PART C
If 0. 100 mol of N2O5 is added to a 1. 0 L flask at 35 ∘C, calculate the concentration of O2after 12. 0 minutes
Answers
The concentration of the compounds if the gaseous decomposition of \(N_2O_5\) was studied at 35°C is,
a) \(N_2O_5\) after 12 minutes is 0.0104 mol/L.
b) \(NO_2\) after 12.0 minutes is 0.0208 mol/L.
c) \(O_2\) after 12.0 minutes is 0.0052 mol/L.
To solve this problem, we need to use the integrated rate law for a first-order reaction, which is:
ln[\(N_2O_5\)] = -kt + ln[\(N_2O_5\)]₀
PART A:
We are asked to find the concentration of \(N_2O_5\) after 12.0 minutes, given that the initial concentration is 0.100 mol/L.
First, we need to find the value of the rate constant, k, using the given slope:
slope = -k = -9.8 x \(10^{-4} s^{-1}\)
k = 9.8 x \(10^{-4} s^{-1}\)
The integrated rate law may then be used to solve for ln[\(N_2O_5\)] by including the values of k, t, and [\(N_2O_5\)]:
ln[\(N_2O_5\)] = -kt + ln[\(N_2O_5\)]₀
ln[\(N_2O_5\)] = -(9.8 x \(10^{-4} s^{-1}\)) x (12.0 min x 1/60 s/min) + ln[0.100 mol/L]
ln[\(N_2O_5\)] = -0.0066 + ln[0.100]
ln[\(N_2O_5\)] = -4.714
Finally, we can exponentiate both sides of the equation to solve for [\(N_2O_5\)]:
[\(N_2O_5\)] = \(e^{(-4.714)}\)
[\(N_2O_5\)] = 0.0104 mol/L
Therefore, the concentration of \(N_2O_5\) after 12.0 minutes is 0.0104 mol/L.
PART B:
We are asked to find the concentration of \(NO_2\) after 12.0 minutes, given that the initial concentration of \(N_2O_5\) is 0.100 mol/L.
We might derive from the synthetic condition that the molar proportion of \(NO_2\) to \(N_2O_5\) is either 4:1 or 2:1. In this way, 4 moles of \(NO_2\) are made for every 2 moles of \(N_2O_5\) that separate.
Since we know the concentration of \(N_2O_5\) at 12.0 minutes from part (A), we can use the molar ratio to find the concentration of \(NO_2\):
[\(N_2O_5\)] = 0.0104 mol/L
The molar ratio of \(NO_2\) to \(N_2O_5\) = 2:1
[\(NO_2\)] = (2/1) x [\(N_2O_5\)]
[\(NO_2\)] = 2 x 0.0104 mol/L
[\(NO_2\)] = 0.0208 mol/L
Therefore, the concentration of \(NO_2\) after 12.0 minutes is 0.0208 mol/L.
PART C:
We are asked to find the concentration of \(O_2\) after 12.0 minutes, given that the initial concentration of \(N_2O_5\) 0.100 mol/L.
We may deduce from the chemical equation that \(O_2\) and \(N_2O_5\) have a molar ratio of 1:2. Therefore, 1 mole of \(O_2\) is created for every 2 moles of \(N_2O_5\) that breakdown.
Since we know the concentration of \(N_2O_5\) at 12.0 minutes from part (A), we can use the molar ratio to find the concentration of \(O_2\):
[\(N_2O_5\)] = 0.0104 mol/L
Molar ratio of \(O_2\) to \(N_2O_5\) = 1:2
[\(O_2\)] = (1/2) x [\(N_2O_5\)]
[\(O_2\)] = 0.5 x 0.0104 mol/L
[\(O_2\)] = 0.0052 mol/L
Therefore, the concentration of \(O_2\) molecule after 12.0 minutes is 0.0052 mol/L.
Learn more about the concentration at
https://brainly.com/question/10725862
#SPJ4