Answers
Answer:
a = repulsion
b = attraction and obliteration
c = repulsion
Explanation:
same charges repulse
opposite charges attract
Related Questions
Which of the following is the weakest reducing agent?
a. C
r
3
+
(
a
q
)
b. K
(
s
)
c. C
a
2
+
(
a
q
)
d. C
r
(
s
)
e. F
−
(
a
q
)
Answers
The weakest reducing agent among the options given is Ca\(_{2}\)+(aq). Option C is answer.
The strength of a reducing agent is determined by its ability to donate electrons and undergo oxidation. In this case, we can compare the reduction potentials of the species listed.
Cr\(_{3}\)+(aq) is a stronger reducing agent than Ca\(_{2}\)+(aq) because it has a higher tendency to donate electrons and get reduced. Similarly, Cr(s) is a stronger reducing agent than Ca\(_{2}\)+(aq) because it has a greater tendency to donate electrons.
K(s) is a very strong reducing agent as it readily donates its electron, making it the strongest reducing agent among the options.
F−(aq) is also a strong reducing agent because it readily accepts electrons and gets reduced.
Option C is answer.
You can learn more about reducing agent at
https://brainly.com/question/7484765
#SPJ11
Consider the following generic reaction for which k = 2. 54: 3 z q ⇄ 2 x what is the value of k for the following reaction: 4 x ⇄ 6 z 2 q.
Answers
The equilibrium constant of the second reaction is 0.155.
What is equilibrium constant?The equilibrium constant is a numerical value that shows the extent to which reactants are converted into products at a given temperature.
Now, we have the reaction; 3Z + Q ⇄ 2 X and 4 X ⇄ 6 Z + 2 Q.
In the first reaction;
K =[ X]^2/[Z]^3 Q
In the second reaction;
[Z]^6 [Q]^2/[X]^4
Let [ X]^2 = A
[Z]^3 Q = B
Then the second reaction can be;
K = (B)^2/(A)^2 = (B/A)^2
The equilibrium constant of the second reaction therefore is; (1/2.54)^2
K = 0.155
Learn more about equilibrium constant:https://brainly.com/question/17960050
How many grams of iron are produced from 300. moles of carbon monoxide reacting with 15,000. grams of ferric oxide? 3CO + Fe2O3 →2Fe + 3C02
Answers
11,169 grams of iron is produced from 300 moles of carbon monoxide reacting with 15,000 grams of ferric oxide.
The balanced chemical equation shows that 3 moles of CO react with 1 mole of \(Fe_2O_3\) to produce 2 moles of Fe. Therefore, we can calculate the number of moles of Fe produced from 300 moles of CO reacting with \(Fe_2O_3\) as follows:
1 mole \(Fe_2O_3\) produces 2 moles Fe
300 moles CO produces (2/3) x 300 = 200 moles Fe (by stoichiometry)
Next, we can use the molar mass of Fe to convert moles to grams:
1 mole Fe = 55.845 g Fe
200 moles Fe = 200 x 55.845 = 11,169 g Fe
To know more about carbon monoxide, here
brainly.com/question/22530423
#SPJ1
A. How many electrons will the lithium atom give up to become stable?
B. How many electrons does the oxygen atom need to become stable?
C. Can a stable compound be made from these two atoms? Explain why or why not.
Answers
A. Lithium atom gives 1 electron to become stable.
B. Oxygen atom needs 2 electrons to become stable.
C. No, a stable compound cannot be formed from only these two atoms.
Ionic compounds are held together by electrostatic forces and comprise an equal number of cations (positive ions) and anions (negative ions) that combine to form neutral compounds. An atom's outer orbit must be finished for it to be more stable. Three electrons make up a lithium atom, with one in the outer orbit. Therefore, for lithium to become stable, it must shed its additional electron from the outermost orbit, which results in lithium becoming a positive ion. The oxygen atom has 8 electrons, 6 of which are in its outer orbit. Therefore, it needs two additional electrons to stabilize. As a result, it acquires 2 electrons and becomes a negative ion. Equal charges exist in the cations and anions of a stable compound. Since the oxygen atom and the lithium atom have different charges it is not possible to form a stable compound with only 2 atoms.
Learn more about ionic compound
brainly.com/question/9167977
#SPJ4
A ball rolled down from the top of the slope. What kind of energy
conversion occurs from the top to bottom of the inclined plane?
a Kinetic to gravitational potential
b Elastic potential to kinetic
C Gravitational potential to kinetic
d Internal energy to kinetic
e Electric to kinetic
Answers
Answer:
C
Explanation:
The ball is undergoing a gravitational potential to kinetic energy transformation. When the ball is at the top of the slope, it has the potential to be moved down by gravity, hence the name. When it's rolling down the slope, it turns to kinetic energy, which is when something moves.
Consider the following B+-decay: p < n + et + ve Question 2. What is the name of the interaction which is involved in the B+-decay? Question 3. What are the conserved quantities in the reaction above? Is the quark flavour a conserved quantity?
Answers
2. The interaction involved in the B⁺-decay is known as beta decay.
3. The conserved quantities in the reaction are:
Conservation of electric chargeConservation of lepton numberConservation of baryon numberThe quark flavor is not a conserved quantity in the given reaction of B⁺-decay.
The B⁺-decay is a type of beta decay, specifically beta plus decay. In beta plus decay, a proton (p) decays into a neutron (n), emitting a positron (e+) and an electron neutrino (νe):
p → n + e⁺ + νe
2. The interaction involved in the B⁺-decay is the weak nuclear force. The weak force is responsible for processes involving the transformation of particles, such as the conversion of a proton into a neutron in this case.
The interaction involved in the B⁺-decay is known as beta decay. Specifically, the B⁺-decay refers to the decay of a positively charged (B⁺) meson, which is a type of subatomic particle.
3. The conserved quantities in the reaction are:
Conservation of electric charge: The total charge on both sides of the reaction is conserved. The proton (p) has a charge of +1, while the neutron (n) has no charge. The positron (e⁺) has a charge of +1, which balances out the charge.
Conservation of lepton number: The total lepton number is conserved in the reaction. The lepton number of the proton and neutron is 0, while the lepton number of the positron and electron neutrino is also 0. Hence, the lepton number is conserved.
Conservation of baryon number: The baryon number is conserved in the reaction. The baryon number of the proton is 1, and the baryon number of the neutron is also 1. Therefore, the total baryon number is conserved.
Regarding quark flavor, it is not conserved in the B⁺-decay. The decay process involves the transformation of a up-type quark (u) in the proton to a down-type quark (d) in the neutron. This change in quark flavor is allowed by the weak force.
Learn more about Weak Nuclear Force at
brainly.com/question/31753095
#SPJ4
If a car travels 150 km in 3.0 hours, the speed is ?
Answers
Answer:
50km per hour.
Part of the speed, distance, time triangle.
To get speed, just divide distance by time. 150/3=50
A student prepared four solutions of known [Cu2+] and measured the absorbance of each solution using the same cuvette. The graph shows the data for two absorbance measurements done for each solution. Which of the following identifies the most likely error that affected the absorbance recorded for the solution with [Cu2+]≈7×10−3M in the second trial?
a. The cuvette was rinsed with water between measurements.
b. A fingerprint was left on the side of the cuvette facing the detector.
c. The absorbance was measured at a wavelength where Cu2+ has a lower molar absorptivity.
d. The cuvette was not filled with the same volume of solution.
![A student prepared four solutions of known [Cu2+] and measured the absorbance of each solution using](https://i5t5.c14.e2-1.dev/h-images-qa/contents/attachments/92LSYUncGKZzNJ8k2ffHRCx69TimdpWG.png)
Answers
A fingerprint was left on the side of the cuvette facing the detector identifies the most likely error that affected the absorbance recorded for the solution with [Cu²⁺]≈7×10⁻³M in the second trial and the correct option is option B.
The Beer-Lambert law relates the attenuation of light to the properties of the material through which the light is traveling.
The Beer-Lambert law states that for a given material sample path length and concentration of the sample are directly proportional to the absorbance of the light. The factors which influence absorbance are:
The concentration of the sample.The thickness of the medium.The temperature at which we will measure the absorbance.The wavelength.Learn more about Beer Lambert Law, here:
https://brainly.com/question/30404288
#SPJ1
what effect does acid have on the gelatinization of a starch paste
Answers
Acid can inhibit the gelatinization of a starch paste.
Gelatinization is the process of breaking down the intermolecular bonds of starch molecules in the presence of water and heat, allowing the hydrogen bonding sites to engage more water.
However, when an acid is introduced to the starch paste, it can partially hydrolyze the starch molecules, breaking the glycosidic bonds and reducing the size of the starch granules.
This inhibits the gelatinization process by preventing the granules from swelling and absorbing water as effectively as they would in a neutral or alkaline environment.
Summary: Acid negatively affects the gelatinization of a starch paste by partially hydrolyzing the starch molecules, which in turn hinders the starch granules' ability to swell and absorb water.
Learn more about acid click here:
https://brainly.com/question/25148363
#SPJ11
What role does vitamin K play in the body?
carbohydrate production
functions of the brain
helps with blood clots
fat disposal
Answers
Vitamin K plays a crucial role in the body as it is involved in blood clotting and bone metabolism.
- Blood clotting: Vitamin K helps in the production of proteins that are responsible for blood clotting. Without enough vitamin K, blood may not clot properly and can lead to excessive bleeding.
- Bone metabolism: Vitamin K helps in the activation of proteins that are important for bone health. It promotes the absorption of calcium, which is necessary for bone formation and strength.
- Carbohydrate production: Although not as well known, vitamin K also plays a role in carbohydrate production in the body.
- Fat disposal: Vitamin K has been shown to play a role in fat metabolism and disposal in the body.
Long Answer:
Vitamin K is a fat-soluble vitamin that is involved in various processes in the body. One of its main functions is in blood clotting. Vitamin K helps in the production of proteins that are important for blood clotting, such as prothrombin. Without enough vitamin K, blood may not clot properly and can lead to excessive bleeding.
In addition to its role in blood clotting, vitamin K is also important for bone metabolism. It helps in the activation of proteins that are important for bone health, such as osteocalcin. Osteocalcin is responsible for the absorption of calcium, which is necessary for bone formation and strength.
Although not as well known, vitamin K also plays a role in carbohydrate production in the body. It helps in the conversion of glucose into glycogen, which can be stored in the liver and muscles for energy.
Finally, vitamin K has been shown to play a role in fat metabolism and disposal in the body. It helps in the activation of proteins that are involved in fat metabolism, such as lipoprotein lipase. This protein is responsible for breaking down fats and using them for energy.
In conclusion, vitamin K plays a crucial role in the body, particularly in blood clotting and bone metabolism. It also plays a lesser-known role in carbohydrate production and fat metabolism.
To know more about osteocalcin visit:
brainly.com/question/15016932?
#SPJ11
Fields allow some objects to exert forces on each other even when they are not ________.
Answers
Answer:
in contact
Explanation:
Fields allow some objects to exert force on each other even when they are not contact with one another.
The region of space where the effect of a force is felt is know as a field.
Field forces for provides a non-contact means of exerting forces. They objects do not have to be in contact before the force is felt. An example is the gravitational force field.Fields allow some objects to exert forces on each other even when they are not in contact .
Which statement does NOT describe a relationship shown in the food web?
A Elk are prey for mountain lions.
B Mice are herbivores that consume grasses and are preyed on by snakes.
COwls prey on rabbits and frogs.
Rabbits consume shrubs and are parasites of grasses.
D
PLEASE HELP
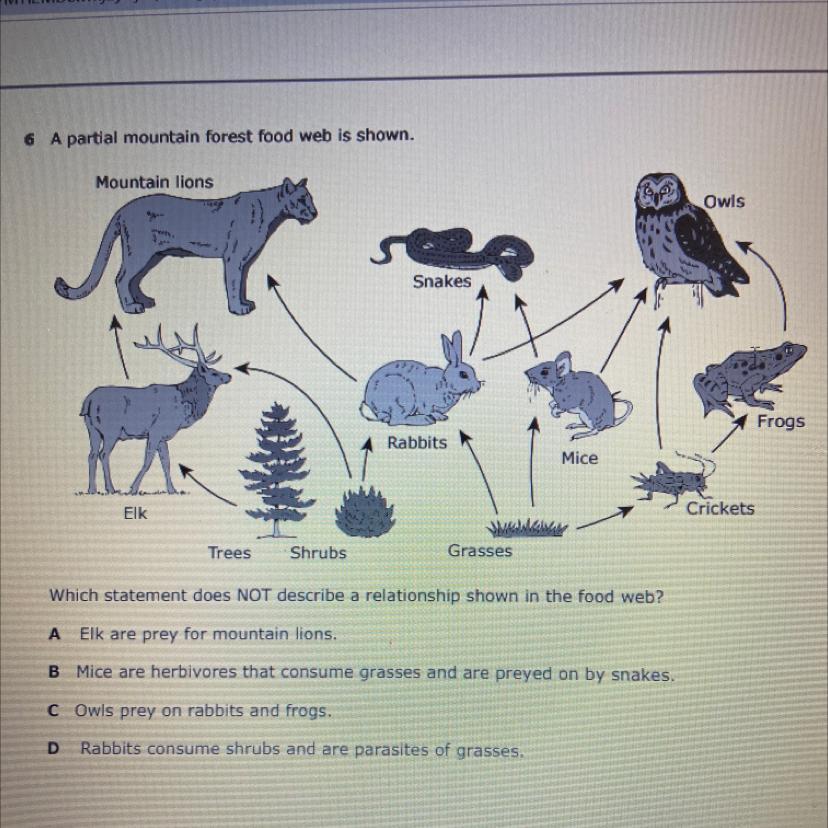
Answers
I know this because rabbits aren’t parasites
Why do reactions need to be balanced?
A. Because the reactants must be the exact same as the products
B. Because of the conservation of energy
C. Because the volume of reactants must equal the volume of
products
D. Because atoms are not lost or gained in a chemical reaction
Answers
Answer:
A part.
Explanation:
Because the reactants must be the exact same as the the products.
The depictions of ionic compounds do not show distinct compound units but rather a collection of ions in the ratio given by the formula. Explain how the drawing of NaCl both illustrates that the compound exists as a large collection of ions and that the ratio of sodium ions to chloride ions is 1-to-1 as indicated by the formula NaCl.
Answers
Answer:
The ionic compound NaCl has a Face Centered Cubic unit cell
The site for atoms are Face, center and Edges corner.
Effective number of atoms
Na+
1centers + 12edges x\(\frac{1}{4}\) = 4 Na+
Cl-
6face x\(\frac{1}{2}\) + \(\frac{1}{8}\) x8 corners = 4Cl-
This is just one unit cell effective atom, there are thousand's of such unit cells are combined to form one such structure of NaCl. The real formula of NaCl is Na4Cl4 but for simplicity we use empirical formula that is NaCl.
Compare the wavelength and energy of ultraviolet rays to visible light
Answers
Answer:
Visible wavelengths range from 0.0007 milimeters for red light, through orange, yellow, green, and blue, to 0.0004 milimeters for violet light.
Ultraviolet is shorter wavelengths than violet.
Hope This Helps.
C AL= -4.184 × 39.85 × 4.7 ÷ 11.98 × (-72.9)
Answers
Answer:
4,768.57
Explanation:
If you are asking us to calculate, your answer is above. Easiest and fastest way is to plug it into the calc.
How many molecules are in 47. 93 g sample of magnesium nitrate ? Please show The whole work
Answers
The number of molecules in 37.93g of magnesium nitrate will be : 0.2568 mol * 6.022x10^23 molecules/mol = 1.55x10^24 molecules.
To determine the number of molecules in a 37.93 gram sample of magnesium nitrate, you would need to know the molar mass of the compound. Magnesium nitrate has a molar mass of 148.31 g/mol.
we can use the formula:
Number of moles = mass (in grams) / molar mass (in g/mol)
Number of moles = 37.93 g / 148.31 g/mol = 0.2568 mol Avogadro's number (6.022x10^23) is the number of atoms, ions, or molecules in one mole of a substance. Therefore, the number of molecules in 37.93g of magnesium nitrate will be : 0.2568 mol * 6.022x10^23 molecules/mol = 1.55x10^24 molecules.
Learn more about molar mass here:
https://brainly.com/question/22997914
#SPJ4
A solution contains a mixture of cl- and br- ions. can both be positivevly identified?
Answers
Yes, \(Br^{-}\) and \(Cl^{-}\) ions both can be positively identified through precipitation reaction or precipitimetry.
Through titration employing precipitation reaction or precipitimetry, these two ions can both be positively identified. When exposed to Cl- and Br- ions, AgNO3 transforms into silver halides. AgNO3 with Cl- ions precipitates white because AgCl is not particularly soluble in water, whereas AgNO3 with Br- ions precipitates cream.
A very light cream precipitate results from mixing cream and white ppt.
Both halides react as described below:
\(AgNO_{3}+ XCl\)\(= AgCl_{whiteppt.}\)
\(AgNO_{3}+ XBr\) \(= AgBr_{creamppt.}\)
Now, While AgBr does not dissolve in diluted ammonia, this AgCl precipitate does to create an Ag-diammonium ion combination. Two facts, including the fact that the ppt shade is now darker than the prior pale cream, demonstrate this. As a result of the addition of an ammonia solution, it becomes less concentrated, although some cream precipitates persist.
Second, concentrated ammonia dissolves the AgBr precipitate. AgBr precipitates dissolve when cream precipitate is filtered and concentrated ammonia is added. In solution Br- ions are confirmed by this.
\(Ag^{+}+NH_{3}\) ⇄ \((AgNH_{3} )_{2} ^{+}\)
The foregoing reaction switches in the right direction after the addition of diluted ammonia solution, and more and more Ag+ ions are complexed, producing the soluble form of Ag-diammonium complex.
Brown globules are produced when CHCl3 is added to the mixture and agitated.
Learn more about ions here:
https://brainly.com/question/269828
#SPJ4
what is change in science??
Answers
Answer:
A transition from one accepted theory to another or from one employed method to another.
Explanation:
Fe(s)+2HBr(aq)→FeBr2(aq)+H2(g)Fe(s)+2HBr(aq)→FeBr2(aq)+H2(g) Part A What mass of HBrHBr (in gg) would you need to dissolve a 3.2-gg pure iron bar on a padlock?
Answers
The mass of HBr (in g) you would need to dissolve a 3.2-gg pure iron bar on a padlock is 9.27 g.
The given chemical equation is:
Fe(s) + 2HBr(aq) → FeBr₂(aq) + H₂(g)
The balanced chemical equation indicates that 1 mole of Fe reacts with 2 moles of HBr and produces 1 mole of H₂ and 1 mole of FeBr₂. We are to determine the mass of HBr required to dissolve a 3.2 g pure iron bar on a padlock.
First, find the molar mass of Fe. To find the molar mass of Fe, we need to find its atomic mass from the periodic table.
Atomic mass of Fe = 55.85 g/mol
Molar mass of Fe = 55.85 g/mol
Find the number of moles of Fe present in a 3.2 g sample using the following formula:
Number of moles = Mass/Molar mass
Number of moles of Fe = 3.2 g/55.85 g/mol = 0.057 mol
Find the number of moles of HBr required for the reaction. To dissolve 0.057 mol of Fe, we need 0.057 mol x 2 mol HBr/1 mol Fe = 0.114 mol of HBr
Find the mass of HBr required. To find the mass of HBr required, we can use the following formula:
Mass = Number of moles x Molar mass
Mass of HBr required = 0.114 mol x 80.91 g/mol = 9.27 g
Therefore, the mass of HBr required to dissolve a 3.2-g pure iron bar on a padlock is 9.27 g.
Learn more about balanced chemical equation here: https://brainly.com/question/26694427
#SPJ11
________________________ is the amount of space a substance takes up and is measured with a graduated cylinder.
Answers
Volume is measured inside a graduated cylinder
Use the following chemical equation to answer the following problems:
2 C4H6+11 02-> 8 CO2 + 6 H20
If 20 moles of fuel are combusted in the above equation, how many moles
of 02 are consumed?
What is the limiting reactant for this equation based on the previous
question?
Answers
110 moles of O2 are consumed when 20 moles of C4H6 are combusted ; Based on the amount of O2 available, it is the limiting reactant in this equation.
What is meant by combustion reaction?A reaction in which substance reacts with the oxygen gas and producing energy in the form of light and heat is called as combustion reaction.
2 C4H6 + 11 O2 -> 8 CO2 + 6 H2O
(11 moles O2 / 2 moles C4H6) x 20 moles C4H6 = 110 moles O2
Therefore, 110 moles of O2 are consumed when 20 moles of C4H6 are combusted.
From the previous calculation, we know that 110 moles of O2 are consumed when 20 moles of C4H6 are combusted. This means that we need 20/2 = 10 moles of O2 to react with the available 20 moles of C4H6.
However, if we only have 8 moles of O2 available, then O2 will be the limiting reactant, as there is not enough O2 to react with all of the C4H6.
Therefore, based on the amount of O2 available, it is the limiting reactant in this equation.
To know more about combustion reaction, refer
https://brainly.com/question/13251946
#SPJ1
There were 6 students in the class wearing white sneakers. This would be an example of an observation that is
1. Qualitative only
2. Quantitative only
3. Both qualitative and qualitative
4. Neither qualitative and quantitative
Answers
Answer:
4.
Explanation:
It's neither qualitative and quantitative. Qualitative is speculation on quality, and quantitative is speculation on quantity, or the amount of something.
In which of the following compounds is the octet expanded to include 12 electrons?PC13H2SSF6PCIE
Answers
The octet rule tells us that for an atom and a bond to be stable there must be 8 electrons in its last energy level, in other words it must have 8 valence electrons.
Now, if we look at the structure of PCl3 we can see that it complies with the octet rule as shown in the following figure:
Each line corresponds to two electrons and the dots correspond to one electron each. We have 3 lines (6 electrons) and two dots (2 electrons) for a total of 8 electrons.
Now for H2S we have, see the figure above. We have 2 lines (4 electrons) and four dots (4 electrons) for a total of 8 electrons.
For SF6 we have, see the figure above. For SF6 we have six lines (12 electrons), that is to say, that this is the compound that does not fulfill the octet rule.
The answer will be SF6

CuCl2 + NaNO3 -------> Cu(NO3)2 + NaCl
Answers
Answer:
what
Explanation:
Draw the structural formulas for the organic products of hydrolysis of this acetal in aqueous HCl.
Answers
The structural formula for acetal hydrolysis in water HCl. (View image)
This problem is based on the concept of hydrolysis. Addition of aqueous acids to acetals results in the formation of aldehydes and ketones. Acetals are derivatives of aldehydes and ketones. When a hemiacetal is subjected to nucleophilic attack by an alcohol molecule, acetal formation occurs.
When the cyclic acetal undergoes hydrolysis in the presence of an aqueous acid i.e. HCl, the formation of a ketone occurs. In this case, methanol (CH₃OH) is a by-product. The stereochemistry of the products will be the same as the reactants. If the binding is drawn on the plane of the paper, the binding that is off the page is a wedge bond. On the other hand, ties that are at the back of the page are dotted bonds.
Learn more about hydrolysis acetal in aqueous HCl at https://brainly.com/question/9652379.
#SPJ4

The empirical formula for trichloroisocyanuric acid, the active ingredient in many household bleaches, is ocncl. The molar mass of this compound is 232. 41g/mol. What is the molecular formula of trichloroisocyanuric acid.
Answers
Answer:
\(C{3}Cl{3}N{3}O{3}\\\)
Explanation:
Isocyanuric acid is C3H3N3O3. The "trichloro" means that there are also 3 Cl atoms attached. They likely replace the hydrogens, especially since the H's also form a single bond. So replace the 3 H's with 3 Cl's:
Isocyanuric Acid: C3H3N3O3
Trichlioroisocyanuric acid: C3Cl3N3O3
3C = 3*12 = 36
3Cl = 3*35.45 = 106.4
3N = 3*14 = 42
3O = 3*16 = 48
Total is 232.40 g/mole. This matches the provided molar mass figure of 232.41 g/mole.
.One ballon is filled with Hydrogen (H2) gas, the other balloon is filled with methane (CH4). Both balloons have the same temperature, pressure, and volume. Which statement is true?
A. The balloon with CH4 has more moles of gas molecules than the balloon with H2
B. The balloon with CH4 has less moles of gas molecules than the balloon with H2
C. The balloon with CH4 has the same moles of gas molecules as the balloon with H2
D. We do not have enough information to support any of the answer choices listed above
Answers
Answer:
C. The balloon with CH4 has the same moles of gas molecules as the balloon with H2
Explanation:
Based on combined gas law, gases under the same pressure, temperature and volume have the same number of moles. With this information we can say the rigth statement is:
C. The balloon with CH4 has the same moles of gas molecules as the balloon with H2Addition of Diamine and Diacid results to O Nylon O Styrofoam O Polyester O Polyurethane
Answers
The combination of diamine and diacid results in the formation of a polymer known as polyester. Polyesters are polymers that contain an ester functional group in their primary chain.
The most common type of polyester is a thermoplastic polymer that is created by the addition of a dicarboxylic acid and a diol or diamine monomer.
This reaction results in the production of a polymer that is commonly used in a wide range of applications, including textiles, packaging, and coatings.
Polyester, as a thermoplastic polymer, has a high tensile strength, chemical resistance, and dimensional stability. It is also known for its excellent resistance to UV radiation, moisture, and fire. Its physical properties, combined with its affordability, make it a popular choice for use in a variety of applications.
The addition of diamine and diacid does not result in the formation of Nylon, Styrofoam, or Polyurethane. Nylon is created by the reaction of diamine and dicarboxylic acid in a process known as polycondensation.
Styrofoam is made from polystyrene, a thermoplastic polymer that is created from the addition of styrene monomers. Polyurethane is made by the reaction of isocyanates with polyols or diamines in a process known as polyaddition.
To know more about polyester here
https://brainly.com/question/30711926
#SPJ11
4. When the mole fraction of solute is 1, there is
(a) a 1:1 ratio of solute to solvent.
(b) no solute present.
(c) only solute present.
(d) only solvent present.
(e) 1 mole of solute and 99 moles of solvent.
Answers
Answer:
(c) only solute present
Explanation:
In chemistry, the mole fraction, denoted by X, refers to the number of moles of a substance in a compound/mixture divided by the total number of substances in the same compound or mixture.
In this case, we can say that mole fraction represents the number of solutes to the number of solutes and solvent in the solution i.e. X = nA/nA + nB
Where; nA = number of solutes
nB = number of solvent
X = mole fraction.
Based on this analogy, When the mole fraction of solute is 1, there is only solute present. That is; X = 1 / 1 + 0
X = 1/1 = 1.
