Answers
Answer:
I think it's 8????????
Explanation:
I don't know
Answer:
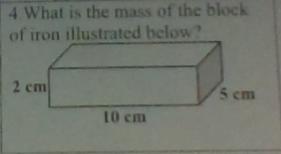
787 g
Explanation:
m=VxD
V=100cm3
D=7.87 g/cm3
m=100cm3 x787g/cm3
m=787 g
Related Questions
Can somebody please go over how to solve this correctly? I keep getting stuck.

Answers
I need help on this. It’s kinda confusing...

Answers
Answer: here u go
Explanation:
A. 3.7 x 10^4
B. 4.56 × 10^-8
C. 8.01 × 10^6
Work for A:
Step 1
To find a, take the number and move a decimal place to the right one position.
Original Number: 37,000
New Number: 3.7000
Step 2
Now, to find b, count how many places to the right of the decimal.
New Number: 3 . 7 0 0 0
Decimal Count: 1 2 3 4
There are 4 places to the right of the decimal place.
Step 3
Building upon what we know above, we can now reconstruct the number into scientific notation.
Remember, the notation is: a x 10b
a = 3.7 (Please notice any zeroes on the end have been removed)
b = 4
Now the whole thing:
3.7 x 104
Step 4
Check your work:
104 = 10,000 x 3.7 = 37,000
Work for B:
Step 1
To find a, take the number and move a decimal place to the right one position.
Original Number: 456
New Number: 0.0000000456
Step 2
Now, to find b, count how many places to the right of the decimal.
New Number: 0 . 0 0 0 0 0 0 0 4 5 6
Decimal Count: 1 2 3 4 5 6 7 8 9 10
There are 2 places to the right of the decimal place.
Step 3
Building upon what we know above, we can now reconstruct the number into scientific notation.
Remember, the notation is: a x 10b
a = 4.56
b = 2
Now the whole thing:
4.56 x 102
Step 4
Check your work:
102 = 100 x 4.56 = 456
Work for C:
Step 1
To find a, take the number and move a decimal place to the right one position.
Original Number: 8,010,000
New Number: 8.010000
Step 2
Now, to find b, count how many places to the right of the decimal.
New Number: 8 . 0 1 0 0 0 0
Decimal Count: 1 2 3 4 5 6
There are 6 places to the right of the decimal place.
Step 3
Building upon what we know above, we can now reconstruct the number into scientific notation.
Remember, the notation is: a x 10b
a = 8.01 (Please notice any zeroes on the end have been removed)
b = 6
Now the whole thing:
8.01 x 106
Step 4
Check your work:
106 = 1,000,000 x 8.01 = 8,010,000
Hope this helps!
Part C Before you begin, keep in mind these two points: The timer runs fast, so the minutes go faster than actual minutes. The temperature will rise during the experiment. If the temperature gets very high, lower it to around 300 K. Follow these steps, and then record your observations: Locate the orange reset button on the bottom right side of the screen. Press reset to start the reaction over. Drag the top of the ruler upward until it reaches the 40 mark. Drag the left platform upward until the top of the platform coincides with the 30 mark on the ruler. Toggle the blue play/pause button to the play position at the bottom of the screen to ensure that the reaction doesn’t start before you’re ready. Add 50 A particles, and press the play button on the bottom. Immediately start the timer using the play button on the blue box. At every minute on the timer, pause the simulation and record the number of A and B particles.

Answers
The given instructions outline the steps to follow in a simulation or experiment involving particles A and B. The purpose is to observe and record the number of A and B particles at each minute on the timer. The exact observations and data collection will depend on the specific simulation or experiment being conducted.
Locate the orange reset button: Find the reset button on the bottom right side of the screen and press it to start the reaction over. This ensures that the previous data is cleared and the experiment begins from the initial state.
Drag the ruler: Use the mouse or touch screen to drag the top of the ruler upward until it reaches the 40 mark. This step sets the reference point for measuring the positions of the particles.
Adjust the left platform: Drag the left platform upward until the top of the platform aligns with the 30 mark on the ruler. This step positions the platform for the particles to interact within the desired range.
Toggle the blue play/pause button: Locate the blue play/pause button at the bottom of the screen. Make sure it is in the play position to prevent the reaction from starting before you are ready. This allows you to control the timing of the experiment.
Add 50 A particles: Use the appropriate tool or feature to add 50 A particles to the simulation or experimental setup. This step ensures that the initial condition includes a specific number of A particles.
Start the timer and record observations: Press the play button on the blue box to start the simulation or experiment. Immediately start the timer using the play button on the timer itself. At every minute on the timer, pause the simulation and record the number of A and B particles. This step allows you to track the changes in the particle population over time.
Note: The specific details and actions may vary depending on the simulation or experiment being conducted. It is important to follow the given instructions accurately and record the observations as instructed.
For more such questions on experiment , click on:
https://brainly.com/question/26117248
#SPJ8
• What are the types of luster and which two are the most common
Answers
Answer:
Luster refers to how light is reflected from the surface of a mineral. The two main types of lustre are metallic and nonmetallic.
Vitreous lustre is one of the most commonly seen, and occurs in transparent or translucent minerals with relatively low refractive indices.
Hope that answers your question
The same mass of 5 different potential fuels was used to heat the same mass of water in a simple calorimeter. The results are shown below. Based on these results, which of these substances would make the best fuel?
Answers
We can see here that the best fuel is the one that produces the most heat per unit mass. In this case, the fuel that produces the most heat per unit mass is methanol.
What is fuel?Fuel is a substance that is used to produce energy through combustion or other chemical reactions. It is commonly utilized to power various forms of transportation, generate heat or electricity, and operate machinery and appliances.
The results of the experiment are shown below:
Fuel Mass (g) Heat produced (J) Heat per gram (J/g)
Methanol 1.0 350 350
Ethanol 1.0 250 250
Propane 1.0 200 200
Butane 1.0 150 150
Pentane 1.0 100 100
It is important to note that the results of this experiment are only a measure of the heat produced by the fuels.
Learn more about fuel on https://brainly.com/question/10172005
#SPJ1
When 106 g of water at a temperature of 21.4 °C is mixed with 64.3 g of water at an unknown temperature, the final temperature of the resulting mixture is 46.8 °C. What was the initial temperature of the second sample of water? (The specific heat capacity of liquid water is 4.184 J/g ⋅ K.)
Answers
Answer:
THE INITIAL TEMPERATURE OF THE SECOND SAMPLE IS 4.93 C OR 277.93 K
Explanation:
Mass of first sample of water = 106 g
Initial temp of first sample = 21.4 °C = 21.4 + 273 K = 294.4 K
Mass of second sample = 64.3 g
Final temp of theresulting mixture = 46.8 °C = 46.8 + 273 K = 319.8 K
Specific heat capacity of water = 4.184 J/g K
It is worthy to note that;
Heat gained by the first sample = Heat lost by the second sample
Since heat = mass * specific heat capacity * change in temperature, we have
Mass * specific heat * change in temp of the first sample = Mass * specific heat * change in temp. of the second sample
MC (T2 - T1) = MC (T2-T1)
106 * 4.184 * ( 319.8 - 294.4) = 64.3 * 4.184 * ( 319.8 - T1)
106 * 4.184 * 25.4 = 269.0312 ( 319.8 - T1)
11 265.0016 = 269.0312 (319.8 - T1)
Since the change in temperature = 319.8 -T1
Change in temperature =11265.0016 / 269.0312
Change in temperature = 41.87
Change in temperature = 319.8 -T1
41.87 = 319.8 - T1
T1 = 319.8 - 41.87
T1 = 277.93 K
T1 = 4.93 °C
So therefore, the initial temperature of the sacond sample is 4.73 °C or 277.93 K
In the balanced equation
2C₂H6+702--> 4CO2+6H₂O
if 21 g of C₂H6 react with 32 g O2, what is the limiting reactant?
02
C₂H6
CO₂
H₂O
Answers
In the balanced equation \(2C_{2} H_{6}\) + \(7 O_{2}\) --> \(4 CO_{2}\) + \(6H_{2}O\) if 21 g of \(C_{2} H_{6}\) reacts with 32 g O₂, C₂H6 is the limiting reactant.
To determine the limiting reactant, we need to compare the amount of each reactant to the stoichiometric ratio in the balanced equation.
Let's calculate the number of moles for each reactant using their molar masses:
For \(C_{2} H_{6}\) (ethane):
Molar mass of \(C_{2} H_{6}\) = 2(12.01 g/mol) + 6(1.01 g/mol) = 30.07 g/mol
Number of moles of C₂H6 = 21 g / 30.07 g/mol ≈ 0.698 mol
For O₂ (oxygen):
Molar mass of O₂ = 2(16.00 g/mol) = 32.00 g/mol
Number of moles of O₂ = 32 g / 32.00 g/mol = 1.00 mol
Next, we compare the moles of each reactant to the stoichiometric ratio in the balanced equation:
2 moles of \(C_{2} H_{6}\) react with 7 moles of O₂ to produce 4 moles of CO₂ and 6 moles of H₂O.
From the given amounts, we have:
0.698 mol \(C_{2} H_{6}\) and 1.00 mol O₂.
Using the stoichiometric ratio, we can calculate the expected amount of CO₂ and H₂O produced for each reactant:
For C₂H6:
Expected moles of CO₂ = 0.698 mol C₂H6 * (4 mol CO₂ / 2 mol C₂H6) = 1.396 mol CO₂
For O₂:
Expected moles of CO₂ = 1.00 mol O₂ * (4 mol CO₂ / 7 mol O₂) ≈ 0.571 mol CO₂
Comparing the expected moles, we see that the calculated amount of CO₂ is greater when used \(C_{2} H_{6}\) as the limiting reactant. Therefore, the limiting reactant in this reaction is \(C_{2} H_{6}\).
Know more about the Balanced equation here:
https://brainly.com/question/13451900
#SPJ8
PLEASE HELP.
B. What is the equation that relates these factors? What is it called?
(background to the question shown in photo)

Answers
The enthalpy could be positive or negative and this would affect the spontaneity of the reaction. The equation that relates the enthalpy and the entropy to the spontaneity of the reaction is;
ΔG = ΔH - TΔS
How does enthalpy and entropy affect the spontaneity of a reaction?Enthalpy and entropy are two important thermodynamic parameters that can affect the spontaneity of a reaction.
The spontaneity of a reaction is determined by the change in Gibbs free energy (ΔG) of the reaction, which takes into account both enthalpy and entropy. The equation for calculating ΔG is:
ΔG = ΔH - TΔS
where T is the temperature in Kelvin.
In general, a reaction will be spontaneous if ΔH is negative (i.e., the reaction releases heat) and ΔS is positive (i.e., the reaction increases disorder or randomness in the system). However, there are cases where entropy may dominate over enthalpy. For example, a reaction that is endothermic (ΔH>0) but highly disordered (ΔS>>0) may still be spontaneous at high temperatures (i.e., when TΔS > ΔH).
Learn more about enthalpy:https://brainly.com/question/13996238
#SPJ1
equivalent weight of cacl2
Answers
Answer:
55 grams
Explanation:
55 grams of calcium chloride is the equivalent weigh because 55 grams of calcium chloride would supply one mole of positive or negative charges when dissolved in water.
hope it helps you and give me a brainliest
Which of these mixtures are heterogeneous? a. oil and water b. salt and water c. brass (an alloy of copper and zinc) d. granite
Answers
The mixture that has been heterogeneous has been oil and water. Thus, option A is correct.
The mixture has been defined as the combination of two or more substances. The mixture has been categorized as pure and heterogeneous mixture based on the solubility of the constituents.
Heterogeneous mixtureThe pure mixture has been defined as the one in which the constituent substances are completely soluble, and are not visible with the bare eyes.
The heterogeneous mixture has been defined as the combination in which the constituents elements are not completely soluble and are easily differentiated from each other by physical means.
The mixture of oil and water has been a heterogeneous mixture, as the two liquids are immiscible and can be separated based on density.The salt and water is a pure mixture, as the two are completely soluble, and they can not be distinguished easily in the solution.The brass is the pure mixture as they have properties different from the constituent elements and has been completely miscible form.The granite has been a compound of carbon. It has been the mixture of carbon atoms in the specified arrangement, thereby is a pure form.Thus, the mixture that has been heterogeneous has been oil and water. Thus, option A is correct.
Learn more about heterogeneous mixture, here:
https://brainly.com/question/24898889
Which of the following involves a change in chemical properties
Answers
Answer: A chemical change occurs when the substance's composition is changed. When bonds are broken and new ones are formed a chemical change occurs.
Explain the difference between mass and weight and
how they are measured.
Answers
\(\bold{Hiya...}\)
Explain the difference between mass and weight and how they are measured ?The difference between of mass, cause- mass can be best understood as the amount of matter present in any object or body. Whereas weight is the force exerted by the gravity on that object mg. Note that mass is independent of everything but weight is different on the earth.Hope It Helps~
A n s w e r : -⋆ \(\sf{NicoChu}\) ⋆
Partner A: Writer.
Partner B: Calculato
1. How many moles of bromine are in 2.8 L at 1.38 atm and 327 K?
Answers
The number of moles of the gas can be determined using ideal gas equation. The number of moles of Br gas in 2.8 L at 1.38 atm and 327 K is 0.144 moles.
What is ideal gas equation ?Ideal gas law states the relation between temperature, pressure and volume with the number of moles of a gas as written below:
PV = nRT
where, R is the universal gas constant equal to 0.082 L atm/K mol
Given that, T = 327 K
P = 1.38 atm
V = 2.8 L.
Then, n = PV/RT
Number of moles of Br gas, n = (1.38 atm ×2.8 L)/(327 K × 0.082 L atm/K mol ) = 0.144 moles.
Therefore, the number of moles of Br gas in in 2.8 L at 1.38 atm and 327 K is 0.144 moles.
Find more on ideal gas law:
https://brainly.com/question/13821925
#SPJ1
Net ionic equation for potassium sulfide and magnesium iodide
Answers
The net ionic equation for the reaction between potassium sulfide and magnesium iodide is S2- + Mg2+ -> MgS, as the potassium and iodide ions are spectator ions and do not participate in the reaction.
To determine the net ionic equation for the reaction between potassium sulfide (K2S) and magnesium iodide (MgI2), we first need to identify the ions present in each compound and then determine the products formed when they react.
Potassium sulfide (K2S) dissociates into two potassium ions (K+) and one sulfide ion (S2-):
K2S -> 2K+ + S2-
Magnesium iodide (MgI2) dissociates into one magnesium ion (Mg2+) and two iodide ions (I-):
MgI2 -> Mg2+ + 2I-
Now, we need to determine the possible products when these ions combine. Since potassium (K+) has a +1 charge and iodide (I-) has a -1 charge, they can combine to form potassium iodide (KI):
K+ + I- -> KI
Similarly, magnesium (Mg2+) and sulfide (S2-) can combine to form magnesium sulfide (MgS):
Mg2+ + S2- -> MgS
Now, we can write the complete ionic equation by representing all the ions present before and after the reaction:
2K+ + S2- + Mg2+ + 2I- -> 2KI + MgS
To obtain the net ionic equation, we remove the spectator ions, which are the ions that appear on both sides of the equation and do not participate in the actual reaction. In this case, the spectator ions are the potassium ions (K+) and the iodide ions (I-).
Thus, the net ionic equation for the reaction between potassium sulfide and magnesium iodide is:
S2- + Mg2+ -> MgS
For more such questions on ionic equation visit:
https://brainly.com/question/25604204
#SPJ8
Which of the following electron models is the one currently accepted by modern science?
A.) Dalton's Billiard Ball.
B.) Thomson's Plum Pudding Model.
C.) Schrodinger's Quantum Mechanical Model.
D.) Rutherford-Bohr Planetary Model.
Answers
C.) Schrodinger's Quantum Mechanical Model is the electron model currently accepted by modern science. This model is based on the principles of quantum mechanics, which describe the behavior of particles at the atomic and subatomic level.
What is Electron?
An electron is a subatomic particle that carries a negative electric charge. It is one of the three fundamental particles that make up atoms, along with protons and neutrons. Electrons are much smaller than protons and neutrons, and they orbit around the nucleus of an atom. Electrons play a crucial role in chemical reactions, since they determine the chemical properties of atoms and molecules.
It represents electrons as probability clouds or orbitals, rather than as discrete particles following classical trajectories. This model has been successful in explaining the behavior of electrons in atoms and molecules, and has led to many important discoveries in modern physics and chemistry.
Learn more about Electron from the given link
https://brainly.com/question/860094
#SPJ1
Which of these pairs of solutions result in a precipitate when they are mixed?A.magnesium chloride and silver nitrate B.sodium sulfate and magnesium nitrate C.sodium chloride and potassium nitrate D.sodium hydroxide and nitric acid

Answers
Answer: the pair magnesium chloride and silver nitrate (letter A) would result in a precipitate when mixed
Explanation:
The question requires us to determine which mixture among the options given , would result in a precipitate.
To olve this problem, we'll need to analyze the chemcical reacton that happens when the mixtures o f solutions are made.
Note that most of the reactions between the soluions wgiven ill ahappen through a double displacement mechanism, which can be written as:
\(AB+CD\rightarrow AD+CB\)A) mixing magnesium chloride (MgCl2) and silver nitrate (AgNO3) would produce silver choride (AgCl) and magnesium nitrate (Mg(NO3)2):
\(MgCl_2+2AgNO_3\rightarrow2AgCl+Mg(NO_3)_2\)B) Mixing sodium sulfate (Na2SO4) and magnesium nitrate (Mg(NO3)2) produces sodium nitrate (NaNO3) and magnesium sulfate (MgSO4):
\(Na_2SO_4+Mg(NO_3)_2\rightarrow2NaNO_3+MgSO_4\)C) Mixing sodium chloride (NaCl and opotassium nitrate (KNO3) produces sodium nitrate (NaNO3) and potassium chloride (KCl):
\(NaCl+KNO_3\rightarrow NaNO_3+KCl\)D) Mixing sodium hydroxide (NaOH) and nitric acid (HNO3) results in the formation of the salt odium nitrate (NaNO3) and water (H2O):
\(NaOH+HNO_3\rightarrow NaNO_3+H_2O\)Among the list of possible products obtained (AgCl, Mg(NO3)2, NaNO3, MgSO4, KCl and H2O), only silver chloride presents low solubility in water, thus it would form a precipitate in aqueous medium.
Therefore, the pair magnesium chloride and silver nitrate (letter A) would result in a precipitate when mixed.
12. 5.6g of solid copper was heated with 476.2 J at room temperature (25°C). Given that
copper has a C of 0.38 J/g°C, what would its final temperature be?
Answers
Answer:
The final temperature of the copper would be 311.3°C.
I hope this helps you
EXPERIMENT
You have learned that carbon dioxide is a compound consisting of two atoms of oxygen united with one atom of carbon. This substance is a gas which you exhale (breathe out) as waste material. In this experiment, you will combine carbon dioxide with limewater (calcium hydroxide) to make a new compound called calcium carbonate. You may do this experiment as required with the supplies listed below, or watch the video demonstration.
Answers
Answer:
6
Explanation:
I somehow know just cause im smart and stuff.
Which of the followings are true or false.
a. The total internal energy ( E ) of a chemical system equals the sum of the kinetic and potential energies of all the particles in the system.
1. True
2. False
b. The sum of all the energies due to the motions of the particles in a system is a form of specifically called thermal energy.
1. True
2. False
c. The energy in a chemical system that results primarily from the arrangement of the nuclei and electrons within an atom or compound is a form of specifically called Changes in the internal energy of a system take place when energy is transferred as work, or both.
1. True
2. False
When a chemical reaction produces a gas, the newly formed gas pushes away the atmosphere and creates space for itself such that the system loses energy as:_______
Answers
Answer:
A. True
B. True
2. It losses energy as heat.
Explanation:
Internal energy is the sum of the potential and kinetic energy of all the particles in the system that form the system.
2. Atoms in a chemical system consist of nucleus which are positively charge and the electrons surrond it which are negatively charged. The protons are positively charged and the neutrons are neutral and this require energy to move, a change in the internal energy convert it to work.
Statement that there is equality between internal energy ( E ) of a chemical system as well as sum of the kinetic and potential energies is True.
Statement that the summation of all the energies in the system is regarded as Thermal energy as a result of motion is True.
The statement that energy in a chemical system is formed due to arrangement of the nuclei and electrons within an atom is True.
When a gas is produced from chemical reaction , the newly produced gas will create a space for itself by pushing atmosphere a way and energy will be lose as Heat.
The internal energy of a thermodynamic system can be regarded as energy that is found within the system.The thermal energy serve as the overall energies as a result of motion in the systemIt can be concluded that the energy in chemical system can be occur as a result of arrangement of the nuclei and electrons within an atom
Learn more at:
https://brainly.com/question/18757696?referrer=searchResults
One of the fastest growing plants is the bamboo which can grow as fast as 1.0 yards in a day. What is the speed of bamboo growth in centimeters per hour?
Answers
Answer
The speed of bamboo growth in centimeters per hour is 3.81
Explanation
Note: 1 yard = 91.44 centimeters
1 day = 24 hours
Therefore, 1.0 yard in a day will be equivalent 91.44 centimeters in 24 hours.
The required speed of the bamboo growth in centimeters per hour will be =
\(\frac{91.44\text{ cm}}{24\text{ h}}=3.81\text{ centimeters per hour}\)Is the number of total molecules on the left side of a balanced equation always equal to the number of total molecules on the right side of the equation
Answers
Answer:
No
Explanation:
No, but the total mass of reactants must equal the total mass of products to be a balanced equation.
Example: Consider the following reaction ...
3H₂ + N₂ => 2NH₃ and 'amu' is atomic mass units (formula weights from periodic table)
In terms of molecules, there are 4 molecules on the left (3 molecular hydrogens (H₂) and 1 molecular nitrogen (N₂) and 2 molecules of ammonia on the right side of equation arrow. ∑reactant molecules ≠ ∑product molecules.
In terms of mass of reactants & mass of products, the 3H₂ + N₂ => 6amu + 28amu = 34amu & mass of products (2NH₃) => 2(14amu) + 6(1amu) = 34amu for sum of product masses.
∑mass reactants = ∑mass products <=> 34amu = 34amu.
The expression '∑mass reactants = ∑mass products' as applied to chemical equations is generally known as 'The Law of Mass Balance'.
What is the IUPAC name of the following substance?

Answers
The International Union of Pure and Applied Chemistry (IUPAC) provides a standard system for naming organic compounds.
It is essential to learn this nomenclature system to communicate correctly about the chemical structures of compounds and how they relate to each other. Here is the IUPAC name of the following substance.Below is the structure of the given compound: In the given compound, there are four carbon atoms that are connected with single bonds. Carbon atoms are also attached to hydrogen atoms. Since it has four carbons in the main chain, the root name will be "but-". The functional group present in the molecule is the carboxylic acid group (-COOH), which gives the suffix "-oic acid." Therefore, the IUPAC name of the given substance is Butanoic acid.Thus, the IUPAC name of the given compound is Butanoic acid. It is essential to know the IUPAC naming of organic compounds to communicate correctly about the chemical structures of the compounds.
for such more questions on Chemistry
https://brainly.com/question/29886197
#SPJ8
help me with questions 6,11,15,16 and 17 please
ayúdame con las preguntas 6,11,15,16 y 17 por favor

Answers
11-positive and negative
15- photons for the sunlight
16-complete
17-false
What total volume of ozone measured at a pressure of 24.5 mmHg and a temperature of 232 K can be destroyed when all of the chlorine from 15.5 g of CF3Cl goes through 10 cycles of these reactions
Answers
Answer:
\(1.75272\ \text{m}^3\)
Explanation:
The breakdown reaction of ozone is as follows
\(CF_3Cl + UV \rightarrow CF_3 + Cl\)
\(Cl + O_3 \rightarrow ClO + O_2\)
\(O_3 + UV \rightarrow O_2 + O\)
\(ClO + O \rightarrow Cl + O_2\)
It can be seen that 2 moles of ozone is required in the complete cycle
So for 10 cycles, 20 moles of ozone is required
m = Mass of \(CF_3Cl\) = 15.5 g
M = Molar mass of \(CF_3Cl\) = 104.46 g/mol
P = Pressure = 24.5 mmHg
T = Temperature = 232 K
R = Gas constant = \(62.363\ \text{L mmHg/K mol}\)
Number of moles is given by
\(n=\dfrac{m}{M}\\\Rightarrow n=\dfrac{15.5}{104.46}\\\Rightarrow n=0.1484\ \text{moles}\)
\(20\ \text{moles} = 20\times 0.1484 = 2.968\ \text{moles}\)
From ideal gas law we have
\(PV=nRT\\\Rightarrow V=\dfrac{nRT}{P}\\\Rightarrow V=\dfrac{2.968\times 62.363\times 232}{24.5}\\\Rightarrow V=1752.72\ \text{L}=1.75272\ \text{m}^3\)
For 20 cycles of the reaction the volume of the ozone is \(1.75272\ \text{m}^3\).
The Total volume of Ozone measured is = 1.7527 m³
Given data :
Mass of CF₃Cl = 15.5 g
Molar mass of CF₃Cl = 104.46 g/mol
Pressure ( P ) = 24.5 mmHg
T = 232 K
Gas constant ( R ) = 62.363 L mmHg/K mol
Note : For a complete ozone cycle 2 moles of ozone is required therefore for 10 cycles 20 moles of ozone will be required
First step : Determine number of moles in each cycle reaction
n = m / M ----- ( 1 )
m = mass = 15.5 g
M = molar mass = 104.46 g/mol
∴ n = 15.5 / 104.46 = 0.1484
Hence the number of moles in 10 cycle = 20 * 0.1484 = 2.968 moles
Next step : Apply Ideal gas law to determine the volume of Ozone
PV = nRT ----- ( 2 )
n = 2.968
V = ?
R = 62.363 L mmHg/K mol
T = 232 K
P = 24.5 mmHg
∴ V = nRT / P --- ( 3 )
= ( 2.968 * 62.363 * 232 ) / 24.5
= 1752.72 L ≈ 1.7527 m³
Hence we can conclude that the Total volume of Ozone measured is = 1.7527 m³
Learn more : https://brainly.com/question/20335707
Patrick is a 16 year old boy whose body has stopped producing osteoclasts. What does this mean for his bones? What other parts of his body will be affected by this?
Answers
Hope this helps!
Have an AWESOME day! :)
Milk of magnesia, Mg(OH)2, is prepared from magnesium sulfate and
sodium hydroxide. If a solution containing 100.0 g of MgSO4 is added to a
solution with 70.0g of NaOH, what is the limiting reactant? Calculate the
theoretical mass of milk of magnesia produced.
MgSO4 (aq) + 2 NaOH(aq) →Mg(OH)2 (s) + Na2SO4(aq)
Answers
From the equation, MgSO₄ is the limiting reagent and the mass of Magnesium Hydroxide produced is 48.3g.
It is given that the mass of MgSO₄ is 100g and the mass of Sodium Hydroxide is 70g. Then the number of moles of Magnesium Sulfate and Sodium Hydroxide is given by,
Moles of MgSO₄ = 100/120
Moles of MgSO₄ = 0.833 mol
Moles of NaOH = 70/40
Moles of NaOH = 1.75 mol
It is given that 1 mol MgSO₄ needs 2 mol of NaOH, then 0.833mol of MgSO₄ needs 2x0.833 mol of NaOH which is given by 1.67mol. This is less than 1.75 mol of NaOH.
Thus, MgSO₄ is the limiting reagent. To find the mass of Magnesium Hydroxide the following steps are to be taken,
1mol of MgSO₄ forms 1mol of Magnesium Hydroxide
Then 0.833mol of MgSO₄ forms 0.833mol of Magnesium Hydroxide
Therefore, the mass of Magnesium Hydroxide is the product of moles and molar mass which is given by,
Mass of Magnesium Hydroxide = 0.833x58
Mass of Magnesium Hydroxide = 48.3g
Therefore the mass of Magnesium Hydroxide is 48.3g
To know more about limiting reagents, click below:
https://brainly.com/question/23661051
#SPJ1
How can you describe ideal gas particles? Check all that apply.
They have no mass.
They have a small mass.
They have no volume.
They have a small volume.
They have no intermolecular forces.
They experience intermolecular forces.
Answers
The description of an ideal gas is as follows:
They have a small massThey have a small volumeThey have no intermolecular forcesWHAT IS AN IDEAL GAS?An ideal gas is a theoretical gas composed of many randomly moving particles that do not engage in any inter-particle interactions.
An ideal gas obeys all the gas laws and possess the following characteristics:
They have a small massThey have a small volumeThey have no intermolecular forcesLearn more about gas laws at: https://brainly.com/question/1437490
Answer:
-They have no mass.
-They have no volume.
-They have no intermolecular forces.
Explanation:
Loren is examining a hair found at a crime scene. He notes radical changes in the coloring of the hair. What is the term for this type of coloring?
Answers
Answer:
Granules of the hair cortex.
Explanation:
hope it helpfully...Answer:
It is banding for plato / edmentum
Explanation:
I took notes in the class and in my notes it says -
Human hairs have consistent coloring, whereas animal hair coloring may change radically. This radical change is called banding.
What is the difference between the R.M.M. and the molar mass of a compound?
Answers
Answer:
rmm doesn't need to include unit and molar mass need to include unit
Choose the equation below that is balanced correctly.
S8 +24 028 SO3
S8+ 12 0₂8 SO3
6 S8+8 026 SO3
2 S8 +3 022 SO3
Answers
The balanced equation for the reaction between sulfur (S₈) and oxygen (O₂) to form sulfur trioxide (SO₃) is 2S₈ + 16O₂ → 16SO₃.
What is the balanced chemical equation?Balancing chemical equations involves the addition of stoichiometric coefficients to the reactants and products.
The balanced equation for the reaction between sulfur (S₈) and oxygen (O₂) to form sulfur trioxide (SO₃) is determined as;
2S₈ + 16O₂ → 16SO₃
From the reactants side we can see that sulfur is 16 and also 16 in the product side. The number of oxygen in the reactant side is 32 and also 32 in the product side.
Thus, the balanced equation for the reaction between sulfur (S₈) and oxygen (O₂) to form sulfur trioxide (SO₃) is 2S₈ + 16O₂ → 16SO₃.
Learn more about balanced chemical equation here: https://brainly.com/question/26694427
#SPJ1