HCl + NaOH 2. Write the balanced chemical reaction for reaction 3. Be sure to include the states of matter and correct formulas. If applicable (ie: if you classified it as a precipitation reaction) include the net ionic equation as well. CH₃Cool & Noot 1. Write the balanced chemical reaction for reaction 1. Be sure to include the states of matter and correct formulas. If applicable (ie: if you classified it as a precipitation reaction) include the net ionic equation as well. Write the balanced chemical reaction for reaction 5. Be sure to include the states of matter and correct formulas. If applicable (ie: if you classified it as a precipitation reaction) include the net ionic equation as well. + Naz po + Fecla 3. Write the balanced chemical reaction for reaction 5. Be sure to include the states of matter and correct formulas. If applicable (ie: if you classified it as a precipitation reaction) include the net ionic equation as well. Naz pa + Fecha 4. Write the balanced chemical reaction for reaction 7. Be sure to include the states of matter and correct formulas. If applicable (ie: if you classified it as a precipitation reaction) include the net ionic equation as well. (NH4)2CO₃ + Cucla
Answers
The balanced chemical equation is HCl + NaOH2 ----> NaCl2 + H2O. This is double displacement reaction.
Chemical formulas and symbols used in chemical equations are used to represent chemical reactions symbolically. The reactant entities are supplied on the left, and the product entities are given on the right, with a plus sign separating the entities in both the reactants and the products, and an arrow pointing in the direction of the products to show the direction of the reaction. Chemical equations can be either mixed, structural, or both. Coefficients are displayed next to the symbols and formulas of the various entities, together with the absolute values of the stoichiometric numbers. A chemical equation is made up of a list of reactants (the chemicals used to start the reaction) on the left, an arrow symbol, and a list of products (the substances created during the reaction) on the right. Each substance is described by its chemical formula, which may be followed by a stoichiometric coefficient, a numerical value. The coefficient describes the number of molecular entities (such as molecules) of that chemical that are engaged in the process. If not stated specifically, the coefficient is + sign separates two or more substances on any side of the equation from one another.
To know more about chemical equation please refer: https://brainly.com/question/28294176
#SPJ4
Related Questions
during a laboratory experiment, 44.18 grams of al2o3 was formed when o2 reacted with aluminum metal at 290.0 k and 1.3 atm. what was the volume of o2 used during the experiment? 3o2 4al → 2al2o3 (5 points)
Answers
During a laboratory experiment, 44.18 grams of al2o3 was formed when o2 reacted with aluminum metal at 290.0 k and 1.3 atm , the volume of O2 used during the experiment 3o2 4al → 2al2o3 is approximately 12.58 liters.
To find the volume of O2 used during the experiment, we can use the ideal gas law equation PV = nRT,
where P is the pressure, V is the volume, n is the number of moles, R is the ideal gas constant, and T is the temperature.
First, we need to calculate the number of moles of Al2O3 formed. The molar mass of Al2O3 is 101.96 g/mol. Therefore, 44.18 grams of Al2O3 is equal to 44.18 g / 101.96 g/mol = 0.433 moles of Al2O3.
According to the balanced chemical equation, 3 moles of O2 react with 4 moles of Al to form 2 moles of Al2O3. Therefore, the number of moles of O2 used can be calculated as follows:
(0.433 moles of Al2O3) * (3 moles of O2 / 2 moles of Al2O3) = 0.6495 moles of O2.
Next, we can use the ideal gas law equation to find the volume of O2. The temperature is given as 290.0 K and the pressure is given as 1.3 atm.
(1.3 atm) * (V) = (0.6495 moles of O2) * (0.0821 L*atm/mol*K) * (290.0 K)
Solving for V, we get:
V = (0.6495 moles of O2) * (0.0821 L*atm/mol*K) * (290.0 K) / (1.3 atm)
V ≈ 12.58 liters
To know more about volume refer to :
https://brainly.com/question/14197390
#SPJ11
Which one of the following systems has the highest entropy?
10 mL of water at 100°C
10 mL of water at 50°C
10 mL of water at 10°C
Answers
Answer: a
Explanation:
The highest entropy will be 0 mL of water at 100°C.
What is entropy?Entropy is just a measured physical quality that will be most usually linked with disorder, unpredictability, or uncertainty.
The quantity of random movement in a system increases as more energy is put into it. Entropy rises like a volume increases in a system.
Therefore, the correct answer will be option 1.
To know more about entropy.
https://brainly.com/question/13146879
#SPJ2
Identify the error in the following statement:
A scientific theory is well-substantiated, fact-based, and unchangeable.
O A. Scientific theories can change as scientists continue to test ideas and gather evidence.
Please help me on this! I’ll mark you the brainliest
B. The statement is describing a hypothesis rather than a scientific theory.
O
C. A scientific theory is not well-substantiated.
D. A scientific theory is not fact-based.
Answers
Which of the following statements about the energy of a pendulum is true.
A. The kinetic energy is always greater than both the potential energy and total energy
B. The potential energy is always greater than both the kinetic energy and the total energy
C. The total energy is always greater than both the potential energy and the kinetic energy
D. None of the above is true
Answers
What is the one way that the thermal energy of a system can be explained?
Answers
Answer: here is 1 way:
Explanation:
Meh explaination :1. Thermal energy refers to the energy contained within a system that is responsible for its temperature. Heat is the flow of thermal energy. A whole branch of physics, thermodynamics, deals with how heat is transferred between different systems and how work is done in the process (see the 1ˢᵗ law of thermodynamics).
Easy explaination:2. The energy of motion in the molecules of a substance.
Hard explanation: 3. Thermal energy is defined as the total of all kinetic energies within a given system.
Hope this helps!;)
if a student reacts 4.40 ml of acetic acid with 3.35 ml of isopentyl alcohol and obtains 3.25 ml of isopentyl acetate as the product, what was the percent yield
Answers
The percent yield of isopentyl acetate is 71.23% when reacting 4.4 ml f acetic acid with 3.35 ml of isopentyl alcohol
Percentage yield calculationPercentage yield can be defined as the ratio of the actual yield of a reaction to the theoretical yield of a reaction, multiplied by 100.
The percentage yield is calculated as follows:
Percentage yield = (actual yield/theoretical yield) × 100Given data:
Volume of acetic acid used = 4.40 ml
Volume of isopentyl alcohol used = 3.35 ml
Volume of isopentyl acetate obtained = 3.25 ml
Density of acetic acid = 1.049 g/mL and its molar mass = 60.05 g/mol
Density of isopentyl alcohol = 0.809 g/mL and its molar mass = 88.15 g/mol
Density of isopentyl acetate = 0.876 g/mL and its molar mass = 130.19 g/mol
The balanced chemical equation for the reaction is:
C5H11OH + CH3COOH → CH3COOC5H11 + H2O
First, we need to determine which reactant is the limiting reactant. We can do this by calculating the number of moles of each reactant:
Number of moles of acetic acid = (volume in mL) x (density) / (molar mass)Number of moles of acetic acid = (4.40 mL) x (1.049 g/mL) / (60.05 g/mol) = 0.0767 molNumber of moles of isopentyl alcohol = (volume in mL) x (density) / (molar mass)Number of moles of isopentyl alcohol = (3.35 mL) x (0.809 g/mL) / (88.15 g/mol) = 0.0307 molThe mole ratio of acetic acid to isopentyl alcohol in the reaction is 1:1, so the limiting reactant is isopentyl alcohol because there are fewer moles of it.
The theoretical yield of isopentyl acetate can be calculated from the number of moles of limiting reactant:
Number of moles of isopentyl acetate = (number of moles of limiting reactant) = 0.0307 molNow we can calculate the percent yield:
Percent yield = (actual yield / theoretical yield) x 100%The actual yield is given as 3.25 mL of isopentyl acetate, but we need to convert this to moles:
Number of moles of isopentyl acetate = (volume in mL) x (density) / (molar mass)Number of moles of isopentyl acetate = (3.25 mL) x (0.876 g/mL) / (130.19 g/mol) = 0.022 molPercent yield = (0.022 mol / 0.0307 mol) x 100% = 71.23%Therefore, the percent yield of isopentyl acetate is 71.23%.
learn about percentage yield
https://brainly.com/question/11963853
#SPJ11
Michelle drove 300 miles in 6 hours. What was Michelle's speed?
30 miles per hour
80 miles per hour
50 miles per hour
60 miles per hour
Answers
Michelle drove 50 miles per hour
Explanation:
300 miles divided by 6 hours equals 50 miles per hour.
Miles per hour basically just means
\(\frac{Total number of miles}{total number of hours}\)
You can infer from the use of the word 'per' that you should divide the number of miles by the number of hours to get an average speed.
Similarly, to work out the number of miles driven you could say you travelled 50 miles per hour for 6 hours, so you would multiply 50mph x 6 hours to work the equation in reverse.
Hi!, please help :)
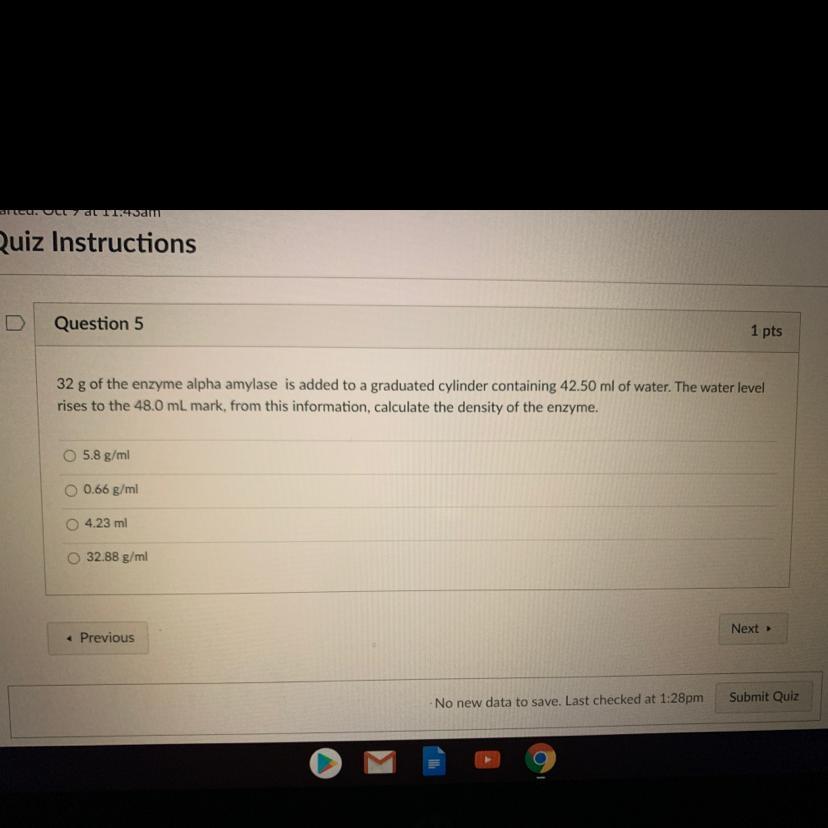
Answers
Answer:
5.8
Explanation:
humans are heating up the Earth's atmosphere is this an example of the law or theory support your answer
Answers
Answer:qui
qzasui
Explanation:
qzasui
A solid rubber ball with a mass of 5.9 grams sank in water. A hollow rubber ball with the same mass floats in water. Explain why this might be. Please answer ASAP!
Answers
Answer:
The hollow ball is more buoyant than the solid ball.
Explanation:
The hollow ball will float while the solid ball sinks because the volume of water displaced by the hollow ball is more than that displaced by the solid ball.
An object floats when it displaces a volume of water equal to its weight. A solid ball is more compact, and hence, does not displace a lot of water when dropped into a bowl full of water. On the other hand, the hollow ball will have a larger curved surface area, since it has no fill. This will make it displace a larger volume of water and hence make it float.
Microscopic interface asymmetry and spin-splitting of electron subbands in semiconductor quantum structures. Solid State Commun
Answers
The microscopic interface asymmetry of grown semiconductor heterostructures.
The dispersion of restricted electrons. beginning from a multiband envelope formulation we practice matrix perturbation theory to derive specific expressions. Interface asymmetry, which in the conduction band Hamiltonian appear as a warping and a spin-splitting term. The warping term consequences in an inequivalence of the dispersion.
The microscopic interface asymmetry of grown semiconductor heterostructures that gives upward thrust to heavy-light hole coupling even at 0 in-plane wave vector, modifies also the dispersion of restricted electrons. beginning from a multiband envelope method we practice matrix perturbation principle to derive explicit expressions as a result of this interface asymmetry, which inside the conduction band.
Learn more about Microscopic interface here:-https://brainly.com/question/26348652
#SPJ4
1. When old cells, which normally die, instead grow uncontrollably and
form new, abnormal cells, it is called:
A. lymphoma
B. a tumor
C. carcinoma
D. cancer
Answers
1. if a jar test demonstrates that the optimum dosage for coagulation is 25 ppm al3 , how many lbs alum are required for a 45 mgd water treatment plant?
Answers
To calculate the amount of alum required for a 45 mg/d (million gallons per day) water treatment plant, we need to convert the units from ppm (parts per million) to lbs (pounds).
Given that the optimum dosage for coagulation is 25 ppm of Al₃+:Convert ppm to lbs per million gallons:
25 ppm Al₃+ x 1 lb Al₂(SO₄)₃ / 1000 ppm Al₃+ = 0.025 lbs Al₂(SO₄)₃ per million gallons
Calculate the total amount of alum required for the entire treatment plant:0.025 lbs Al₂(SO₄)₃ per million gallons x 45,000,000 gallons per day = 1,125 lbs Al₂(SO₄)₃ per day
Therefore, a 45 mg/d water treatment plant would require approximately 1,125 lbs of alum per day at an optimum dosage of 25 ppm Al₃+.
To know more about water treatment plants, visit:
https://brainly.com/question/30696756
#SPJ1
The graph shows the speed of a car
traveling east over a 12 second
period. Based on the information in
the graph, it can be concluded that
in the first 6 seconds, the car is -
A changing its direction.
B experiencing less and less
friction.
moving at a constant speed
D climbing a steep incline.

Answers
Answer:
Letter B.
Explanation:
Changing its direction and climbing a steep incline can't be proven only by the graph info. You can see that it's speed isn't constant in the graph.
We have letter B left.
In order to make 1 batch of cookies it requires 2 cups flour, 3 eggs, and 1 cup sugar. What would be the coefficients for the flour, eggs, and sugar, assuming you wanted to make 10 batches of cookies? Write the coefficients below!
Answers
Answer:
Explanation:
Let e=eggs
Let f=flour
2/3*f=e
Although a certain molecule is involved in a specific reaction, its structure and chemical composition are exactly the same after the reaction as before the reaction. This molecule is most likely classified as.
Answers
This molecule is most likely classified as an enzyme.
In chemical reactions, the total mass of all substances involved in the reaction remains the same. The number of atoms in the reaction also remains the same. Mass cannot be created or destroyed by chemical reactions. synthetic. A chemist is said to be synthesizing or synthesizing a new substance when he makes a new substance out of other substances.
Reactants are converted to products and the process is represented by a chemical formula. The substances that enter a chemical reaction are called reactants, and the substances that are formed at the end of the reaction are called products. A reversible reaction occurs when the forward and reverse directions of the reaction proceed at the same rate and the amounts of reactants and products do not change overall.
Learn more about A specific reaction here:-https://brainly.com/question/26724488
#SPJ4
A student argued that there is less gravity on the Moon than on Earth. The student explained, "You need an atmosphere to have gravity. The Moon doesn't have an atmosphere and therefore doesn't have a lot of gravity. Earth has a thick atmosphere and therefore has much more gravity." What do you think about the student's claim?
A. The student is correct because the atmosphere is what creates the gravitational pull on Earth
B. The student is correct because the mass of the object determines how thick the atmosphere will be
c. The student is incorrect because gravity is determined by the mass of the object and not the atmosphere
D. The student is incorrect because the thickness of the atmosphere will determine the amount of gravity on the object
Answers
Answer:
C
Explanation:
The student is incorrect.
The atmosphere weighs very little compared to the material the earth is made of. Gravity is effected by mass. The more mass, the more gravity. The only correct (and it is totally correct) answer is C
Which of the following statements is true?
A. The change in freezing point of a solution is directly proportional to the number of
solute particles in the solution and dependent on the type of particle.
B. The change in freezing point of a solution is directly proportional to the number of
solute particles in the solution and independent of the type of particle.
C. The change in freezing point of a solution is inversely proportional to the number
of solute particles in the solution and dependent on the type of particle.
D. The change in freezing point of a solution is inversely proportional to the number
of solute particles in the solution and independent of the type of particle.
Answers
Answer:
The effect of adding a solute to a solvent has the opposite effect on the freezing point of a solution as it does on the boiling point. A solution will have a lower freezing point than a pure solvent. The freezing point is the temperature at which the liquid changes to a solid.
Explanation:
Hope this helps :3
The change in freezing point of a solution is inversely proportional to the number of solute particles in the solution and dependent on the type of particle,is the the TRUE statement.
What is meant by freezing point?Freezing point, temperature at which a liquid becomes a solid. As with the melting point, increased pressure usually raises the freezing point. The freezing point is lower than the melting point in the case of mixtures and for certain organic compounds such as fats.The change in freezing point of a solution is inversely proportional to the number of solute particles in the solution and dependent on the type of particle,is the the TRUE statement.
To learn more about Freezing point refer:https://brainly.com/question/11977850
#SPJ2
Synthetic materials are not found in nature and therefore they are typically developed in laboratories.
True
False
Answers
Answer: True
Explanation: Synthetic materials are not found in nature and can be created in labs using chemicals and compounds.
HELP MY CHEMISTRY NERDS PLEASE PLEASE PLEASE !!!!! I WILL LUV U 4EVER

Answers
Answer:
A. 40 kPa
Explanation:
P(total) = P(O2) + P(N2) + P(CO2)
300kPa = 160 kPa + 100kPa + P(CO2)
P(CO2) = 300-160-100= 40 kPa
12.7 l of argon at 33°c and 735 torr are dissolved in enough water to give a final volume of 0.750 l. what is the molarity of the resulting solution
a. 6.02 M
b. 0.652 M
c. 0.0644 M
d. 0.489 M
e. 4.95 M
Answers
Option (b) is correct. The molarity of the resulting solution is 0.652 M. This is calculated using the expression of ideal gas equation and molarity.
12.7 l of argon at 33°c and 735 torr are dissolved in enough water to give a final volume of 0.750l. so,
P = 735.0 torr
= (735.0/760) atm.
= 0.9671 atm.
V = 12.7 L
T = 33.0 o C
= (33.0+273) K
= 306 K
We can find the number of moles using ideal gas equation. The ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas. It is a good approximation of the behavior of many gases under many conditions. The ideal gas equation is,
P * V = n*R*T
0.9671 atm. * 12.7 L = n * 0.08206 atm. L/mole . K * 306 K
n = 0.4891 mole
Now, we will calculate Molarity. Molarity or molar concentration is the number of moles of solute per liter of solution, which can be calculated using the following equation,
Molarity = number of mole / final volume
= 0.4891 mole / 0.750 L
= 0.652 M
To learn more about Ideal gas equation please visit:
https://brainly.com/question/13248885
#SPJ4
Using the thermodynamic data provided below, calculate the standard change in entropy when one mole of sodium nitrate is dissolved in water?
S° (J/K•mol)
NaNO3(s) 116.3
Na+(aq) 60.25
NO3–(aq) 146.4
Will the solubility of sodium nitrate increase or decrease if the temperature of the system is increased?
Answers
Thermodynamic data is data that relates to the physical properties of a system related to temperature, pressure, and energy.
What is Thermodynamic data?
Thermodynamic data is data that describes the physical properties of a material system in terms of temperature, pressure, and other thermodynamic variables. It is used to predict the behavior of materials in different conditions and to analyze processes such as heat transfer or chemical reactions. It is also used to design and optimize energy systems, such as engines and power plants.
The standard change in entropy when one mole of sodium nitrate is dissolved in water is:
ΔS° = S° (products) - S° (reactants)
ΔS° = (60.25 + 146.4) - 116.3 = 90.3 J/K•mol
The solubility of sodium nitrate will increase if the temperature of the system is increased.
To know more about thermodynamic data,
https://brainly.com/question/14265296
#SPJ4
use the term reclamation in a sentence
Answers
Answer:
Explanation:
The increase of the arable land has been affected partly by the reclamation of the marshes, but mostly by the transformation of large tracts of the puszta
An example of a reclamation is how landfill employees sift through garbage to find usable items. An example of a reclamation is trees growing on land that was heavily logged.
Which of the following is true about a redox reaction?
In a redox reaction, the reducing agent loses electrons..
In a redox reaction, the oxidizing agent loses electrons.
In a redox reaction, the oxidized species gains electrons.
In a redox reaction, the reduced species loses electrons
Answers
AnswerThe correct (A).
Explanation:
The true statement about the redox reaction is In a redox reaction, an electron is lost by the oxidizing agent.
Answer:A
Explanation:i took the test
List down the observations made by Rutherford during the gold foil experiment.
Answers
Answer:
1) The volume occupied by an atom is composed of mainly empty space
2) Atoms have a very small, relatively dense, central nucleus that is positively charged
3) The region around the nucleus of an atom are orbited by negatively charged electrons in a the same fashion planets orbit around the Sun.
Explanation:
The selection of gold for the gold foil experiment was due to its ability to be rolled into extremely thin sheets such that it was expected for alpha particle to perforate or pass through the foil.
What is your worst fear?????????????????????????????????????
Answers
Answer:
Everyone has a fear.
Fear is something that everyone have and fear itself is worse.... so there wont be any other wort fear when it itself is the worst
Answer:
apocaplyse
Explanation:
how much energy is required to ionize hygrogen in each of the following states? (a) ground state
Answers
The energy needed is the energy that changes. The electron in a hydrogen atom is initially assumed to be in the ground state with n=1. The energy of the electron in its ground state is therefore 13.6 eV.
As a result, 12.75eV of the energy is needed to transfer electrons from the ground state to the third excited state. The 4th and 5th ionisation the energies differ significantly from one another. The fourth electron is attracted to the nucleus atom considerably less strongly than the fifth electron because it is in an inner main shell that is closer to the nucleus.
To learn more about electron, click here.
https://brainly.com/question/1255220
#SPJ4
Which statement best describes why most individual organisms never fossilized?(1 point)
Only organisms that lived in the ocean fossilize.
Conditions for fossilization are rare.
Chemicals that fossilize organisms are rare.
Only organisms with hard parts fossilize.
Answers
The statement which best describes why most individual organisms never fossilized is that Conditions for fossilization are rare.
What is fossilization?Fossilization is a term which ia defined as the mechanisms which are chemical, physical, and biological which results in the preservation for long term of animals and plants renants. Fossilization is a type of negative representation of the creatures which is preserved in the substrates as fossil.
Reason for lack of decomposition of fossilizationThe reason for the lack of these fossilized parts can be explained as these parts quickly decompose.
The process of the recycling nutrients which have been consumed by an organism which is used to create its body begins with decomposition.Thus, we concluded that the statement which best describes why most individual organisms never fossilized is that Conditions for fossilization are rare.
learn more about fossilization:
https://brainly.com/question/6867325
#SPJ1
How are hydrogens removed from polyprotic acids? how does this relate to the ka of these same species?.
Answers
Many acid contain two or more ionizable hydrogen. There are two in carbonic acid H₂CO₃ and three in phosphoric acid H₃PO₄. For any such multiple hydrogen acid the first hydrogen is easily removed and the last hydrogen is removed with great difficulty.
Define polyprotic acids.Polyprotic Acid is a chemical that can donate more than one proton. Diprotic and Triprotic are two distinct varieties of polyprotic acid that can, respectively, donate two and three protons.
Depending on how frequently dissociation takes place, polyprotic acids have a variety of dissociation constants, including Ka1, Ka2, Ka3, and equivalence points.
Examples:
As an illustration of a diprotic polyprotic acid, consider sulfuric acid (H2SO4). Sulfuric acid (H2SO4) is successively deprotonated to produce HSO4- and SO42-, respectively.As an illustration of a diprotic polyprotic acid, consider sulfuric acid (H2SO3). Sulfuric acid (H2SO3) is successively deprotonated to produce HSO3- and SO32-, respectively.To know more about polyprotic acids, visit:
https://brainly.com/question/28605275
#SPJ4
At a high concentration do you have more or less particles per unit volume
Answers
Answer:
More particles per unit volume
Explanation:
Concentration means the amount of solute in a solution. Now, the amount of solute also means the number of particles of solute present in a solution.
Hence, when we use the term "high concentration", we imply that the amount of solute present or the number of particles present in a solution is high.
Thus, at high concentration, there are more solute particles than solvent particles in a solution.