Hat is the major organic product obtained from the following sequence of reactions? (ch3)2chch2mgbr
Answers
The major organic product obtained is
The right response is (D) (CH3)2CH2CH2CH2OH.
Alcohols react with PBr3 and become bromides.
When bromides and magnesium combine, Grignard reagent is created.
What is a major product?
The main product of a reaction would be the one that is more stable and hence most likely to form. Propene added electrophilically to HBr would be one instance.
The right response is (D) (CH3)2CH2CH2CH2OH.
Alcohols react with PBr3 and become bromides.
When bromides and magnesium combine, Grignard reagent is created.
To learn more about major product from the given link below,
https://brainly.com/question/15005839
#SPJ4

Related Questions
rbcl has a high boiling point. which of the following compounds is also likely to have a high boiling point, and why?
a. NO, because its elements are in the same period of the periodic table.
b. ClF, because its elements are in the same group of the periodic table.
c. Cl2O, because its elements have similar electronegativities and it is a covalent compound.
d. CsCl, because its elements have very different electronegativities and it is an ionic compound.
Answers
Option d is the correct answer that is CsCl has a high boiling point because its elements have very different electronegativities and it is an ionic compound.
CsCl has a high boiling point because its elements have very different electronegativities and it is an ionic compound. In CsCl, Cs is an electropositive element whereas Cl is an electronegative element. Due to the strong electrostatic attraction forces between the positive ions Cs and negative ions Cl, a lot of heat energy is required to break the bond and overcome these forces of attraction.
CsCl being an ionic compound contains a large number of strongly bound ions and a lot of energy is needed to overcome this ionic bonding.
Know more about ionic compounds
brainly.com/question/13058663
#SPJ4
2. Each family in the periodic table has its own characteristic properties based on
the number of neutrons
the atomic masses
the number of valence electrons
the atomic numbers
Answers
Answer:
the answer is
The atomic number
POINTS POINTSSSS :)))
Hopefully they don’t delete it but answer for free if you need some :)
Answers
thank youuuuu :)
thank means a great honour of joy and peace have a good day
Which example is correctly paired with its appropriate category?
1. carbon monoxide : mixture
2. cigarette smoke : element
3. none of these
4. ice : element
Answers
Carbon monoxide : mixture, cigarette smoke : element and ice : element ,none of them are correctly matched, therefore option 3 is correct'
Crystalline structures are created when water molecules have been organized in an ordered, cage-like form. Crystalline compounds, such as ice particles as well as water molecules, are composed of particles or molecules which are arranged in a particular order to produce an ordered component known as just a crystalline compound.
Even though it is made up of a chemical link between an atom of carbon and an atom of oxygen, carbon monoxide would be a pure material. There is only one kind of molecule that makes up carbon monoxide, when nothing is physically mixed with that as well.
carbon monoxide = pure substance
cigarette smoke = mixture
Ice = element
Hence, carbon monoxide : mixture, cigarette smoke : element and ice : element ,none of them are correctly matched, therefore option 3 is correct'
To know more about correctly paired
https://brainly.com/question/14563579
#SPJ4
4Al + 3O2 —> 2Al2 O3
10.7g of powdered Al is placed into a container containing 10.7g O2. What is the limiting reactant? How many grams of aluminum oxide can be produced? Calculate the mass of excess reactant that remains after the reaction is complete
Answers
Limiting reactant : Al
Al2O3 produced = 18.36 g
mass O2 remains = 11.904 g
Further explanationGiven
Reaction
4Al + 3O2 —> 2Al2O3
10.7 g O2
Required
Limiting reactant
mass Al2O3
Mass of excess reactant remains
Solution
mol Al : 10.7 : 27 = 0.396
mol O2 = 10.7 : 32 = 0.334
1. limiting reactant
mol : coefficient of Al : O2 = 0.396/4 : 0.334/3 = 0.099 : 0.111
Al as a limitng reactant(smaller ratio)
2. mass Al2O3
mol Al2O3 based on Al : 2/4 x 0.396 = 0.18
mass Al2O3(MW=102 g/mol) = 0.18 x 102 = 18.36 g
3. mass O2 remains
mol O2 reacted : 3/4 x 0.396 = 0.297
mol O2 remains = 0.669 - 0.297 =0.372
mass O2 remains = 0.372 x 32 = 11.904 g
Budeme stát na lodi s plachtou, na klidné hladině bez větru a někoho napadne foukat velkým větrákem do plachet lodi.
Answers
Answer:
english?
Explanation:
What is the molarity of a solution of h3po4 if 10.2 ml is neutralized by 53.5 ml of 0.20m koh what is the molarity of a solution of h3po4 if 10.2 ml is neutralized by 53.5 ml of 0.20m koh 0.67m h3po4 0.15m h3po4 0.35m h3po4 0.37m h3po4
Answers
The molarity of the solution of H₃PO₄ needed to neutralize the KOH solution is 0.35 M
Balanced equationH₃PO₄ + 3KOH —> K₃PO₄ + 3H₂O
From the balanced equation above,
The mole ratio of the acid, H₃PO₄ (nA) = 1The mole ratio of the base, KOH (nB) = 3How to determine the molarity of H₃PO₄ Volume of acid, H₃PO₄ (Va) = 10.2 mLMolarity of base, Ca(OH)₂ (Mb) = 0.2 MVolume of base, Ca(OH)₂ (Vb) = 53.5 mL Molarity of acid, H₃PO₄ (Ma) =?MaVa / MbVb = nA / nB
(Ma × 10.2) / (0.2 × 53.5) = 1 / 3
(Ma × 10.2) / 10.7 = 1 / 3
Cross multiply
Ma × 10.2 × 3 = 10.7
Ma × 30.6 = 10.7
Divide both side by 30.6
Ma = 10.7 / 30.6
Ma = 0.35 M
Learn more about titration:
https://brainly.com/question/14356286
#SPJ1
nguyên tử sắt có điện tích hạt nhân là 26+. trong nguyên tử, số hạt mang điện nhiều hơn số hạt không mang điện là 22. Hãy xác định số khối của nguyên tử sắt
Answers
Answer:
I don't know yr language
Mark scheme
Remember how we work out relative formula mass: Mr = Sum of (Ar of element x number
of atoms in element)
Multiply the number of atoms in each element by the element's relative atomic mass and
add these up:
Mr (1 x 23) + (1 x 23) + (1x 16)
Work out the answer: Mr = 23 + 23 + 16
V
Mr= 62
Feedback?

Answers
The given relative mass formula is correct. The weight in grams of the number of atoms of an element contained in 12.00 g of carbon-12 is known as the relative atomic mass of the element.
What is mass relative to?The ratio of an element's average atomic mass to the unified atomic mass unit is the relative atomic mass, or Ar. The average mass of an element's isotopes is used to calculate the relative atomic mass.
What exactly are absolute and relative masses?Absolute mass is the total mass of all protons and neutrons, whereas relative mass is the average atomic mass of all the isotopes present in a given percentage. As an illustration, the average atomic mass of carbon, calculated using the proportions of the isotopes C-12, C-13, and C-14, is 12.01 while the absolute mass of carbon is 12.0 amu.
What is the atomic mass equation?An element's mass number is determined by the sum of its proton and neutron counts: Protons and neutrons together make up mass.
To know more about relative mass visit:
https://brainly.com/question/29963883
#SPJ1
Which of the following describes the length of a football field using the metric system?
Answers
Answer:
91.44 meters
Explanation:
There are no choices listed but an American pro football field is 100 yards long, which is 91.44 meters. Rounded to the nearest whole number that would be 91 meters.
I’ll give brainliest, pls help with this mole concept. Due in 2hours

Answers
Answer:
20 g of copper (II) oxide, CuO
Explanation:
The balanced equation for the reaction is given below:
CuO + 2HCl —> CuCl₂ + H₂O
From the balanced equation above,
1 mole of CuO reacted to produce 1 mole of CuCl₂.
Next, we shall determine the number of mole of CuO needed to produce 0.25 mole of CuCl₂. This can be obtained as follow:
From the balanced equation above,
1 mole of CuO reacted to produce 1 mole of CuCl₂.
Therefore, 0.25 mole of CuO will also react to produce 0.25 mole of CuCl₂.
Finally, we shall determine the mass of 0.25 mole of CuO. This can be obtained as follow:
Mole of CuO = 0.25 mole
Molar mass of CuO = 64 + 16
= 80 g/mol
Mass of CuO =?
Mole = mass /Molar mass
0.25 = mass of CuO /80
Cross multiply
Mass of CuO = 0.25 × 80
Mass of CuO = 20 g
Therefore, 20 g of copper (II) oxide, CuO is needed for the reaction.
Please help! Thanks :D4. How could you tell a Cu(NO3)2 solution from a Ni(NO3)2 solution?

Answers
To tell a Cu(NO3)2 solution from a Ni(NO3)2 solution, you can add KI to the solution. If the solution contains Cu(NO3)2, the brown precipitate will be formed, but if the solution contains Ni(NO3)2, no precipitate will be formed.
This is the best way to distinguish between these two solutions, since adding KOH or Na2C2O4 will result in blueish precipitates, being harder to identify if it is Cu(NO3)2 or Ni(NO3)2 and with Na2SO4 no reaction will happen.
It means that the answer is: By adding KI and seeing if a brown precipitate is formed.
This is how fluorine appears in the periodic table.
Which is one piece of information that "9" gives about an atom of fluorine?
The atomic mass is different than the atomic number, and the number of neutrons is the difference between the atomic mass and the atomic number.
the atomic number
the atomic mass
the mass of protons
the number of neutrons
Answers
The only piece of information we can deduce from the number "9" about a Fluorine atom is its atomic number.
Fluorine is an element with the atomic symbol F and the number 9. It is found in group 17 (group VIIa), at the top of the halogen family, on the opposite side of Oxygen and Neon. The lightest, riskiest, and most reactive of all the halogens is fluorine, which is positioned above chlorine on the periodic table.
With an electronegativity of 3.98, the fluorine atom is the most electronegative element in the periodic table. Its electron configuration is [He] 2s²2p⁵, or 1s²2s²2p⁵. It is extremely challenging to isolate and will ferociously shred an electron off practically any other atom.
Seven valence electrons make up fluorine. It is particularly reactive and electronegative because it only requires one more electron to complete its second shell.
To know more about halogen
brainly.com/question/11156152
#SPJ4
How many moles of magnesium hydroxide, used to treat heartburn and indigestion, is
manufactured from the reaction of 2.55 moles of magnesium oxide and excess water?
Answers
2.55 moles of magnesium oxide will produce 2.55 moles of magnesium hydroxide.
What is magnesium hydroxide ?
Magnesium hydroxide (Mg(OH)2) is an inorganic compound that is commonly used as an antacid and laxative to treat heartburn, indigestion, and constipation. It is a white, odorless, and tasteless powder that is slightly soluble in water.
The balanced chemical equation for the reaction between magnesium oxide and water to form magnesium hydroxide is:
MgO + H2O → Mg(OH)2
From the equation, we can see that 1 mole of MgO reacts with 1 mole of H2O to produce 1 mole of Mg(OH)2.
Therefore, if 2.55 moles of MgO react with excess water, then the number of moles of Mg(OH)2 produced will also be 2.55 moles.
So, 2.55 moles of magnesium oxide will produce 2.55 moles of magnesium hydroxide.
Learn more about magnesium hydroxide here : brainly.com/question/29637511
#SPJ1
can someone answer this 4 me?

Answers
The statement among the available options that describes the result of the study is that less than 50% of the people never felt phantom pain. Option 2.
Data interpretationIn order to interpret the data and find the best description of the outcome of the experiment, the response needs to be grouped into 2 from the 3 available responses.
Since those that phantom pain sometimes and always belong to the same category, the respondents can be summed up into one:
Number of those that sometimes feel phantom pain = 534
Number of those that always feel phantom pain = 193
Total number of people that feel phantom pain = 534 + 193
= 727 people
Number of people that never feel phantom pain = 183
Total number of respondents in the experiment = 183 + 534 + 193
= 910 people
Percentage of respondents that felt phantom pain = 727/910 x 100
= 79.89%
Percentage of people that never felt phantom pain = 183/910 x 100
= 20.11%
Percentage of people that felt phantom pain all the time = 193/910 x 100
= 21.21%
Thus, the statement that correctly describes the outcome of the experiment of all the statements is that less than 50% never felt phantom pain.
More on data interpretations can be found here: https://brainly.com/question/25529708
#SPJ1
23 Which statement best describes how a model can be used to illustrate the chemical reaction at station 1
Answers
A model can be used to visually represent the chemical reaction occurring at station 1, which can help to understand the reaction mechanism and the role of each reactant in the reaction.
What is chemical reaction?A chemical reaction is a process in which one or more substances (known as reactants) are transformed into new substances (known as products) through the breaking and formation of chemical bonds.
A model can be used to represent a chemical reaction by showing the reactants and products involved and the changes that occur during the reaction. Specifically, for station 1, a model can illustrate the chemical reaction by depicting the reactants (such as hydrogen and oxygen) and the products (such as water) involved in the process.
One way to create a model of this chemical reaction is through a chemical equation, which uses chemical formulas to represent the reactants and products and shows the chemical changes that occur during the reaction. The chemical equation for the reaction at station 1 is:
2 H2 + O2 → 2 H2O
This equation shows that two molecules of hydrogen (H2) react with one molecule of oxygen (O2) to form two molecules of water (H2O). The coefficients in front of each molecule indicate the number of molecules of each substance involved in the reaction.
To know more about chemical reaction:
https://brainly.com/question/29039149
#SPJ1
Complete question:
Can you explain how a model can be used to illustrate the chemical reaction at station 1?
Steel density of 7.8 g/cm3.what is the volume of a 780 gram piece of steel?
Answers
Answer:
The answer is 100 cm³Explanation:
The volume of a substance when given the density and mass can be found by using the formula
\(volume = \frac{mass}{density} \\ \)
From the question we have
\(volume = \frac{780}{7.8} \\ \)
We have the final answer as
100 cm³Hope this helps you
Calcium carbonate decomposes in this balanced equation:
CaCO3 CaO + CO2 ΔH = +110 kJ/mol
Answers
The decomposition of calcium carbonate is an endothermic reaction.
The given equation for the decomposition of calcium carbonate is:
CaCO₃ → CaO + CO₂
ΔH = +110 kJ/mol
where ΔH is the enthalpy change for the reaction.
The positive value of ΔH in the equation indicates that the reaction is endothermic, meaning that heat is absorbed by the reactants from the surroundings during the reaction. This means that the reaction requires an input of energy to proceed, and the products (CaO and CO₂) have higher potential energy than the reactant (CaCO₃).
To know more about calcium carbonate, here
brainly.com/question/13565765
#SPJ4
--The complete question is, Calcium carbonate decomposes in this balanced equation:
CaCO3 + CaO + CO2 ΔH = +110 kJ/mol
Is this reaction endothermic or exothermic?--
Are toolboxes homogeneous or heterogeneous
Answers
Answer:
Homogenous thats it ig
Explanation:
taatjsn
Pls helppppppp if u cannnnn
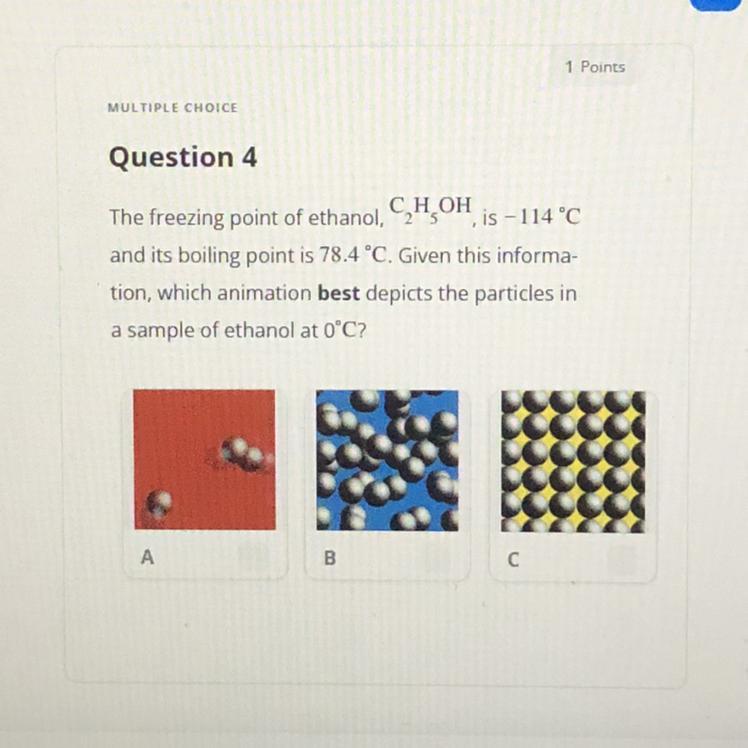
Answers
B because 0°c is higher than freezing point and lower than boiling point so it is in between
A victim’s body was found at a crime scene at 6:00AM with a body temperature of 90°F, at an outside temperature of 65°F. What was the likely Time of death?
Answers
To estimate the time of death, we can use the formula for determining the time since death based on the body temperature:
Hours since death = (initial body temperature - measured body temperature) / cooling rate
The cooling rate for a body in a moderate environment is generally assumed to be about 1.5°F per hour. Using this information, we can calculate the time of death as follows:
1. First, we need to calculate the number of degrees the victim's body temperature needs to drop to reach the outside temperature.
Initial body temperature - outside temperature = 90°F - 65°F = 25°F
2. Next, we can calculate the number of hours that have passed since the victim died:
Hours since death = (initial body temperature - measured body temperature) / cooling rate
Hours since death = (90°F - measured body temperature) / 1.5°F per hour
3. We can solve for the unknown variable (measured body temperature) by substituting in the information we have:
Hours since death = (90°F - 90°F) / 1.5°F per hour
Hours since death = 0 hours
This means that the victim likely died around 6:00AM, which is when the body was found. However, it's important to note that this is just an estimate, and there are many factors that can affect the cooling rate of a body, such as clothing, humidity, and ambient temperature. Therefore, the actual time of death may be different.
To know more about cooling rate refer here
https://brainly.com/question/6100283#
#SPJ11
Calculate the percent water in ZnSO4.7 H20. Show your work.
Answers
Answer:
43.8%
Explanation:
Find the sum of the relative atomic mass of all the elements constituting the compound, which is known as the relative formula mass;
\(M_{r} = Relative \ formula \ mass \\\\M_{r} \ of \ ZnSO_{4} = 65.4 + 32.1 + 4(16) \\\\M_{r} \ of \ ZnSO_{4} = 97.5 + 64 \\\\M_{r} \ of \ ZnSO_{4} = 161.5 \\\\M_{r} \ of \ H_{2}O = 18 \\\\M_{r} \ of \ ZnSO_{4}.7H_{2}O = 161.5 + 7(18) \\\\M_{r} \ of \ ZnSO_{4}.7H_{2}O = 287.5\)
Then, divide the relative formula mass of all the water by that of the whole compound
w = % water
\(w = \frac{7(18)}{287.5} * 100 \\\\w = 0.43826... * 100\\\\w = 43.826...\)
w = 43.8%
Why do you think people cannot drink seawater?
Answers
Answer:
Its contaminated
Explanation:
Sodium azide NaN3 decomposes when heated according to the equation 2 NaN3 (s) → 2 Na(s) + 3 N₂ (g) The reaction is used to fill balloons, called air bags, inside motor vehicles to protect passengers during accidents. Calculate the mass of sodium azide needed to fill a 48dm³ air bag with gas, measured at r.t.p.
Answers
Answer:
Explanation:
To calculate the mass of sodium azide needed to fill a 48dm³ air bag with gas at r.t.p., you need to use the ideal gas law. The ideal gas law states that the pressure of a gas is directly proportional to its temperature (in Kelvins) and the number of moles of gas present, and inversely proportional to its volume. The ideal gas law can be expressed as:
PV = nRT
Where:
- P is the pressure of the gas
- V is the volume of the gas
- n is the number of moles of the gas
- R is the ideal gas constant
- T is the temperature of the gas
You can rearrange the ideal gas law to solve for the number of moles of gas present:
n = PV / RT
To solve this problem, you need to plug in the known values. At r.t.p., the pressure is 1 atm, the temperature is 273 K, and the volume is 48 dm³. The ideal gas constant is 8.31 J/mol*K. Plugging in these values, you get:
n = (1 atm * 48 dm³) / (8.31 J/mol*K * 273 K)
Solving for n, you get:
n = 0.0174 moles
Now that you have the number of moles of gas present, you can use the balanced chemical equation for the decomposition of sodium azide to calculate the number of moles of sodium azide needed. The balanced chemical equation is:
2 NaN3 (s) → 2 Na(s) + 3 N₂ (g)
This equation tells you that for every 2 moles of sodium azide that decompose, you get 3 moles of nitrogen gas. Since you need 0.0174 moles of nitrogen gas, you can divide that number by 3 to get the number of moles of sodium azide needed:
0.0174 moles N₂ / 3 moles N₂/2 moles NaN3 = 0.0087 moles NaN3
To convert the number of moles to mass, you need to know the molar mass of sodium azide. The molar mass of sodium azide is 65.01 g/mol. Plugging in the number of moles and the molar mass, you get:
0.0087 moles NaN3 * 65.01 g/mol = 0.5686 g NaN3
So, the mass of sodium azide needed to fill a 48dm³ air bag with gas at r.t.p. is approximately 0.5686 grams.
Using dimensional analysis
10) Lake Michigan holds 1.3 x 1015 gallons of water. If just Chicago removed water from the
lake and it never rained again, how many days would the water last? Chicago uses 1.2 x 109
gallons of water /day
1,300,000,000,000,000 gallons
Answers
Answer:
the answer is diensional 12:2
Explanation:
Which statement about the physical change of liquid water boiling into steam is true?
The heat added represents an energy change.
The action cannot be reversed.
O The steam cannot conserve mass.
O The weight lost represents a mass change.
Answers
Answer:
A: The heat added represents an energy change.
Explanation:
Correct on EDG test
Explain how magnesium is produced at the negative electrode from molten magnesium chloride
Answers
Answer:
being cation it migerates to cathode to be reduced to elemental magnesium
Explanation:
Mg+2. +2e -----------Mg
Answer the following questions in complete sentences.
A. Identify club soda as an element, compound, or mixture.
B. Explain the classification of club soda identified in Part A.

Answers
Answer:
a) club soda is a compound
b) Club soda is a manufactured form of carbonated water, commonly used as a drink mixer. Sodium bicarbonate, potassium sulfate, potassium bicarbonate, potassium citrate, or sodium citrate is artificially added to replicate constituents commonly found in natural mineral waters
A liquid that occupies a volume of 8.2L has a mass of 5.6kg. What is the density of the liquid in kg/L
Answers
Answer:
0.68
Explanation:
This is because the formula for finding density is mass/volume. Therefore the equation becomes 5.6/8.2 giving you 0.68
how many atoms are there in 6.2 grams of silver