Answers
Explanation:
All period have the same number of shells
Related Questions
Draw the structure of phosphatidylserine and discuss its components
Answers
Phosphatidylserine is a type of phospholipid that is mainly found in cell membranes. Its structure is made up of two fatty acid chains, a phosphate group, a serine molecule, and a glycerol molecule.
The fatty acid chains are hydrophobic, meaning they repel water, while the phosphate group and serine molecule are hydrophilic, meaning they attract water.
The glycerol molecule acts as a bridge that connects the two fatty acid chains to the phosphate group and serine molecule.
The structure of phosphatidylserine is important for its function in the cell membrane.
Because of the hydrophobic and hydrophilic components of its structure, phosphatidylserine is able to form a lipid bilayer, which is a barrier that separates the inside of the cell from the outside environment.
The hydrophilic heads of the phosphatidylserine molecules face outward and interact with water, while the hydrophobic tails face inward and repel water.
Phosphatidylserine also plays a role in cell signaling and apoptosis, which is programmed cell death.
It acts as a signaling molecule by binding to proteins that are involved in cellular pathways.
In addition, phosphatidylserine is translocated to the outer leaflet of the cell membrane during apoptosis, which signals to immune cells that the cell is ready to be removed.
In conclusion, the structure of phosphatidylserine is made up of two fatty acid chains, a phosphate group, a serine molecule, and a glycerol molecule. Its hydrophobic and hydrophilic components allow it to form a lipid bilayer in cell membranes, and it also plays a role in cell signaling and apoptosis.
For more such questions on Phosphatidylserine
https://brainly.com/question/16179573
#SPJ8
How does a hypothesis differ from a scientific theory? (1 point)
O Hypotheses can change with new evidence, but theories remain constant.
O Theories always lead to the development of new scientific ideas.
o Hypotheses are testable, while theories only exist conditionally.
o Theories are well established with lots of evidence to support their claims.
Answers
Answer:
the answer is actually D. (theories are well established with lots of evidence to support their claims)
Explanation:
i just took the quiz
A hypothesis is a presented reason for a phenomenon based on limited proof and evidence. A scientific theory is an elucidation of an element that has been constantly experimented with and confirmed per scientific approach.
Theories are well established with lots of proof to back their assertions.
What is the difference between the theory and hypothesis?Scientific theories are established based on the evidence and repetitive experimental procedures to prove and establish them correctly.Theories can change over time as new supporting evidence are found or new procedures are established to amend the old theory.A scientific theory recapitulates a hypothesis that has been reinforced with repeated experiments and observations.A hypothesis should be an instinctive justification or the potential correlation that lies between several phenomena and depends on the scientific theory.Therefore, option D is correct.
Learn more about theory and hypothesis here:
https://brainly.com/question/14691306
what would the result be of adding one proton to an atom
Answers
Answer:Protons carry a positive electrical charge and they alone determine the charge of the nucleus. Adding or removing protons from the nucleus changes the charge of the nucleus and changes that atom's atomic number. For example, adding a proton to the nucleus of an atom of hydrogen creates an atom of helium and thats your answer
Explanation:
yurrrrrrr
A student mixes 5.00 mL of 2.00 x 10-3 M Fe(NO3)3 with 5.00 mL of 2.00 x 10-3 M KSCN. She finds that in the equilibrium mixture the of concentration of FeSCN2+ is 1.40 x 10-4 M.
a) What is the initial concentration in solution of the Fe3+ and SCN- ?
b) What is the equilibrium constant for the reaction?
Answers
part a.)
The initial concentration in solution of the Fe3+ and SCN- is:
moles of Fe3+ = (2.00 x 10^-3 M) x (5.00 x 10^-3 L) = 1.00 x 10^-5 mol
moles of SCN- = (2.00 x 10^-3 M) x (5.00 x 10^-3 L) = 1.00 x 10^-5 mol
part b)
The equilibrium constant for the reaction is 1.54 x 10^10.
How do we calculate?Fe(NO3)3(aq) + 3KSCN(aq) ⇌ Fe(SCN)3(aq) + 3KNO3(aq)
b)
Therefore, the equilibrium concentration of Fe(SCN)2+ is also 1.40 x 10^-4 M.
Applying law of mass action, the equilibrium constant expression for the reaction is:
Kc = [Fe(SCN)2+] / ([Fe3+] [SCN-]3)
Kc = (1.40 x 10^-4) / [(1.00 x 10^-5) (1.00 x 10^-5)^3]
Kc = 1.54 x 10^10
Learn more about equilibrium constant at: https://brainly.com/question/26990521
#SPJ1
The complete photoelectron spectrum for an element is shown above. Which of the following observations would provide evidence that the spectrum is consistent with the atomic model of the element?
answer choices
A neutral atom of the element contains exactly two electrons.
The element does not react with other elements to form compounds.
In its compounds, the element tends to form ions with a charge of +1.
In its compounds, the element tends to form ions with a charge of +3.

Answers
Evidence that the spectrum is consistent with the atomic model is that the neutral atom of an element contains exactly two electrons.
What is an element?It is defined as a substance which cannot be broken down further into any other substance. Each element is made up of its own type of atom. Due to this reason all elements are different from one another.
Elements can be classified as metals and non-metals. Metals are shiny and conduct electricity and are all solids at room temperature except mercury. Non-metals do not conduct electricity and are mostly gases at room temperature except carbon and sulfur.
Learn more about element,here:
https://brainly.com/question/14347616
#SPJ1
What causes a change in the state of matter

Answers
Answer:
Temperature
Explanation:
This is because temperature interrupts the arrangement of molecules by increasing their kinetic energy and vibratory energy
\(.\)
mention the main applications of analytical chemistry in geology.
Answers
Answer:
geochemistry
Explanation:
geo and chemistry
The knowledge of Analytical methods has helped to determine about rocks and landforms of the earth.
Analytical chemistry is the branch of chemistry that deals with the obtaining, accessing and processing the nature and chemical composition of matter by relying on the thorough Qualitative and Quantitative analysis.
Also,
Geology is the science that studies everything about the earth from its structure, composition, to the processes that revolve around it and the most effective way to use the earth;s resources.
The role of Analytical Chemistry in Geology is very important because with the knowledge of Qualitative and Quantitative analysis fro Chemistry. Scientists are able decipher the composition together with the processes that occur in the Earth, Solar System,Planets , Rocks and the atmosphere,.
For example, with the knowlegde of Isotopes, Carbon dating and Analytivcal ethods ( X-ray Fluorescence (XRF),Laser Ablation (LA- ICP-MS), Inductively Coupled Plasma Mass Spectrometry (ICP-MS), carbon, hydrogen, moisture analysis and Atomic Absorption , Spectrometry (AA) has helped to determine about rocks and other landforms of the earth.
among the following which is the most stable?
a.Co2 b.C2 c.O2 d.No
Answers
NO is the most stable among the given compound. Thus, the most appropriate answer comes out to be option D.
The bond order is the number of bonds between two atoms. The higher the bond order the higher the stability of the compound.
It is calculated by (number of bonding electrons - Number of the anti-bonding electron) * 0.5
So, for C\(O_2\) the bond order is 2
For \(C_2\) ,
The number of electrons in the bonding orbital = 8.
Number of electrons in anti-bonding orbital = 4.
Bond order = (8- 4) * 0.5 = 2.
For \(O_2\) ,
The number of electrons in the bonding orbital = 10.
Number of electrons in anti-bonding orbital = 6.
Bond order = (10 - 6) * 0.5 = 2.
For NO,
The number of electrons in the bonding orbital = 10.
Number of electrons in anti-bonding orbital = 5.
Bond order = (10 - 5) * 0.5 = 2.5
Thus, the most stable compound is NO as it has a 2.5 bond order.
Learn more about Bond Order:
https://brainly.com/question/29974526
#SPJ1
Given that the molar mass of NAOH is 40.00G/MOL, what mass of NAOH is needed to make 2.500 L of a 2.000 M NaOH Solution?
Answers
Answer:
200g
Explanation:
n = CV
n = mass/molar mass
mass/molar mass = CV
mass/40 = 2 x 2.5
mass/40 = 5
mass = 5x 40
mass = 200g
Cu+2AgNO 3 Cu(NO 3 ) 2 +2Ag what are the reactants

Answers
Answer:
Cu + 2AgNo3 is the reactants
Which location is Georgia experiencing fall in the diagram above?
Location A
Location B
Location B
Location D
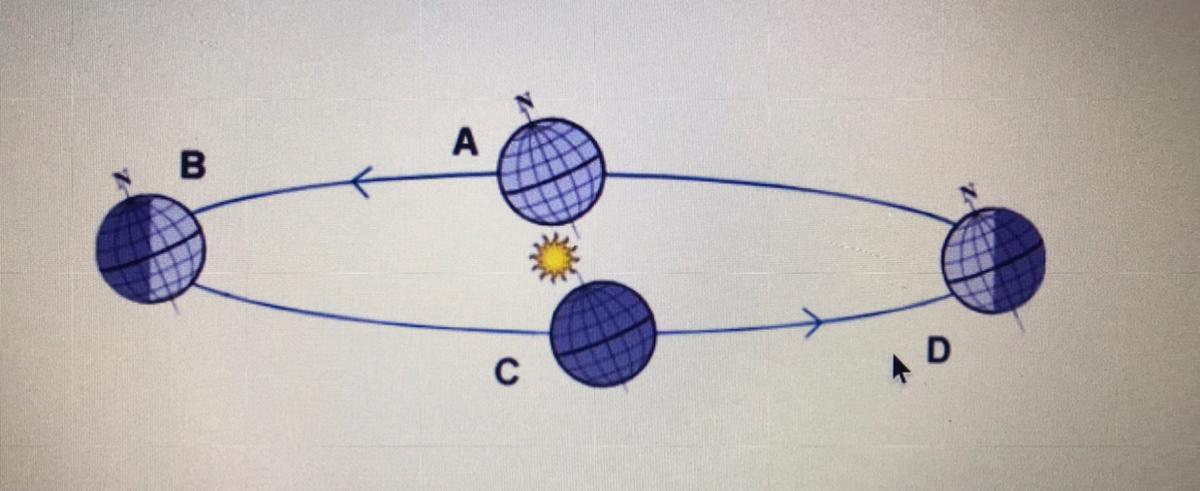
Answers
Answer:
Location A
Explanation:
Answer:
location d
Explanation:
A silver bar has a mass of 368g. What is the volume in cm³ of the bar? The density of silver is 19.5g/cm³.
Answers
Answer:
18.87 cm³Explanation:
The volume of a substance when given the density and mass can be found by using the formula
\(volume = \frac{mass}{density} \\\)
From the question we have
\(volume = \frac{368}{19.5} \\ = 18.871794..\)
We have the final answer as
18.87 cm³Hope this helps you
What is a hypothesis
A. An explanation of events that is most often right and is proven by experiments
B. A series of guesses based on experiments C. An idea based on reasoning that can be test often written in as an if then statement
D. An experiment to prove a law
Answers
Answer:
c
Explanation:
State collision theory
Answers
Collision theory states that when suitable particles of the reactant hit each other with correct orientation, only a certain amount of collisions result in a perceptible or notable change; these successful changes are called successful collisions.
Explanation:here your answer matehave a great day

the current wave mechanical model of the atom has electrons in clouds orbitals around the nucleus
Answers
The current wave mechanical model of the atom describes electrons in clouds called orbitals that surround the atomic nucleus is based on principles of quantum mechanics .
This model emphasizes the wave-like properties of electrons. In contrast to the earlier Bohr model, which proposed that electrons move in well-defined paths around the nucleus.
The wave mechanical model suggests that electrons do not possess precise trajectories but instead occupy regions of space with varying probabilities. These regions are mathematically represented by wave functions or orbitals.
This model provides a more precise depiction of electron behavior, facilitating a better understanding of phenomena like electron energy levels, electron-electron interactions, and chemical bonding.
learn more about wave mechanical model :
https://brainly.com/question/15660887
the current wave mechanical model of the atom has electrons in clouds orbitals around the nucleus, on which principle does this phenomenon is based .
ANSWER QUICK!!!! GIVING BRAINIEST!!!

Answers
Answer:
B.
Explanation:
Can you give me brainliest? i need to rank up
Answer:
2nd One is correct
Glucose, C6H12O6, is used to prepare intravenous feeding solutions. What volume of 5.0 % W/V glucose solution can be prepared using 125 g of glucose? Show your working.
Please if the answer is correct, ill give brainliest
Answers
250 L of 5.0% w/v glucose solution can be prepared using 125 g of glucose.
We use the below formula to solve our problem,w/v = [ mass of solute (g) / volume of solution (mL) ] × 100
Substitute the values from our problem,5.0 % w/v = [ 125 g / volume of solution (mL) ] × 100
Rearranging the formula, we havevolume of solution (mL) = [ 125 g / 5.0 % w/v ] x 100
Substitute further for w/v,volume of solution (mL) = [ 125 g / (5.0 / 100) ] x 100
Simplify the expression,volume of solution (mL) = [ 125 g / 0.05 ] x 100
Hence, the volume of solution (mL) = 250,000 mL or 250 LAnswer the following questions about the last lesson below. What did we figure out about chain reactions? What did we figure out about fission? Fission produce more energy than a typical fuel sources, chain reaction
Answers
Answer:
(Nothing)-But No answer
How many moles of calcium chloride are there when you have 55.5 grams of calcium chloride(CaCl2)
Answers
Answer:
0.5mol
Explanation
Please mark answer as brainliest

ou are a work study for the chemistry department. Your supervisor has just asked you to prepare 500 mL of 3 M HCl for tomorrow’s undergraduate experiment. In the stockroom explorer, you will find a cabinet called "stock solutions". Open this cabinet to find a 2.5 L bottled labeled "11.6 M HCl". The concentration of the HCl is 11.6 M. Please prepare a flask
Answers
Answer:
Add to a 500mL volumetric flask 300mL of water, the 129mL of the 11.6M HCl solution and then complete to volume with water
Explanation:
To make 500mL = 0.500L of a 3M HCl from the 11.6M HCl stock we need first to find the moles of HCl we need:
Moles HCl:
0.500L * (3mol / L) = 1.5 moles of HCl are needed
These moles are obtained from the 11.6M HCl solution. The volume required is:
1.5mol * (1L / 11.6moles HCl) = 0.129L = 129mL must be added to the solution.
That means to prepare the 500mL of the 3M HCl you need to:
Add to a 500mL volumetric flask 300mL of water, the 129mL of the 11.6M HCl solution and then complete to volume with water
Answer:
Calculation: 11.6 M × V = 3.0 M × 0.500 liters
V = 0.13 liters
Steps for dilution:
Measure out 0.13 liters of the concentrated solution of 11.6 M HCl using a volumetric pipet.
Transfer this into solution into a 500 milliliter volumetric flask.
Add water to the flask until it reaches a total volume of 500 milliliters.
Solution: V = 0.13 liters
Explanation:
If a soccer ball's mass is 5 kg and a player who weighs 50 kg kicks the soccer ball forward with a force of 5 N, how much
force does the ball exert on the player's foot and in what direction? (1 point)
O 10 kg/N backward
O 100 kg forward
O 5 N backward
O 5 N forward
Answers
If a soccer ball's mass is 5 kg and a player who weighs 50 kg kicks the soccer ball forward with a force of 5 N , 5 N force does the ball exert on the player's foot and in backward direction .
The four forces are gravity, normal force , friction force, and applied force. When the ball is kicked or kicked by player , when a player leaps into the air to avoid a tackle or grab the ball, and when a player leaps into the air to catch the ball , Force of Gravity is continuously at work in football .
to learn more about force please click here ,
https://brainly.com/question/13191643
#SPJ1
Answer:
Explanation:
1.) A car’s engine provides a forward force of 2000 N while the force of air resistance is 800 N in the opposite direction. With what force will the car move, and in which direction?
ANSWER: 1200 N forward
2.) Two movers are attempting to push a couch up a ramp. One mover applies a force of 80 N to the right, but the force of friction between the couch and the ramp is providing a force of 110 N in the opposite direction, and the couch slides down the ramp. At minimum, how much force will the second mover need to apply to help push the couch up the ramp?
ANSWER: 31 N
3.) A group of students conduct an experiment to study Newton’s second law of motion. They applied a force to a toy car and measure its acceleration. The table shows the results.
Force (N) Acceleration (m/s²)
2.0 5.0
3.0 7.5
6.0 15.0If the students graph the data points, which conclusion will they be able to make?
ANSWER: The data points will fall along a line. This shows that as the force increases, the acceleration increases.
4.) In the experimental setup shown, a car has one end of a string attached to it, and the other end is attached to a fixed number of metal discs. The car moves along the table and two probes sense the motion of the car. The probes send information to a computer that displays the acceleration and velocity of the experiment. When looking at these results, which quantity stays constant during the trials?
ANSWER: mass
5.) Imagine a scenario in which an animal's force is pushing itself forward 5 N, friction is pushing it 4 N backward, gravity is pushing the animal 10 N down, and the animal is pushing itself 10 N up. Describe the movement of the animal.
ANSWER: It only moves forward because there is a net force forward.
6.) Two rockets with the same mass are accelerated. Rocket A accelerates twice as quickly as rocket B. Which statement is correct?
ANSWER: The motor in rocket A is twice as powerful as the motor in rocket B.
7.) A model rocket has a mass of 0.2 kg, with a motor that can provide a force of 100 N. A second model rocket is being built with the same motor, but it is being designed to accelerate half as much as the first rocket. What kind of change can be made in the design to achieve this objective?
ANSWER: The mass of the second rocket should be 0.4 kg.
8.) Five motorboats are being tested to see which reaches the highest velocity in the same amount of time. After graphing the acceleration versus force for each motorboat, the graph is a sloped line with a y-intercept of zero. Which statement is correct about these motorboats?
ANSWER: All five motorboats have the same mass, which can be calculated from the graph.
9.) If a soccer ball’s mass is 5 kg and a player who weighs 50 kg kicks the soccer ball forward with a force of 5 N , how much force does the ball exert on the player’s foot and in what direction?
ANSWER: 5 N backward
10.)
A toy helicopter flies forward with a force of 15 N into an oncoming wind of 10 N. The force of gravity pulls the helicopter down with a force of 5 N, but the propeller is providing an upward force of 10 N. Which of the following accurately describes the helicopter’s force?
ANSWER: The toy helicopter is flying forward with a force of 5 N and upward with a force of 5 N.
11-15 are short answer questions but I hope this helps whoever needs it <3
SO42- + 2Cr(OH)3 + 4OH- → + 2CrO42- + 3SO32- + 5H2O
In the above redox reaction. Use oxidation numbers to identify the element oxidized, the element reduced, the oxidizing agent and the reducing agent.
Answers
Answer:
Reduced species and oxidizing agent: sulfur in the form of sulfate.
Oxidized species and reducing agent: chromium in the form of chromous hydroxide.
Explanation:
Hello!
In this case, for the reaction:
\(SO_4^{2-} + 2Cr(OH)_3 + 4OH^- \rightarrow 2CrO_4^{2-} + 3SO_3^{2-} + 5H_2O\)
We can see that the oxidation states of sulfur and chromium change from +6 to +4 and +3 to +6 respectively; in such a way, since the oxidized species is the same reducing agent because it undergoes an increase in the oxidation state, we infer that chromium is it as it goes from +3 to +6.
Moreover, since the reduced species is the same oxidizing agent because it undergoes a decrease in the oxidation state, we infer that sulfur is it as it goes from +6 to +4.
Best regards!
If 27.37ml of 0.2115M NaOH is able to neutralize 37.45 of HCl, what is the concentration of the acid?
Answers
You just gotta match the moles:
moles of NaOH = 0.02737 L * 0.2115 M = 0.005789 mol of NaOH. The amount of moles of HCl it can neutralize should be the same since they are in a 1:1 ratio. Therefore, you can do moles of NaOH divided by 0.003745L to get the answer. It turns out to be 0.1546M.
Write the equilibrium expression for the following reaction. CaO(s) + CH₄(g) + 2H₂O(g) —> CaCO₃(s) + 4H₂(g)
Answers
The equilibrium expression can be written as follows:
K = [CaCO₃] × [H₂]⁴
------------------
[CaO] × [CH₄] × [H₂O]²
In this equilibrium expression, the square brackets represent the concentrations of the species involved in the reaction, and the coefficients of the balanced equation indicate the stoichiometric coefficients of the corresponding species.
The concentration of a pure solid (CaCO₃ in this case) is not included in the equilibrium expression, as it remains constant throughout the reaction.
The equilibrium constant (K) represents the ratio of the product concentrations to the reactant concentrations at equilibrium, each raised to the power of their respective stoichiometric coefficients. The specific values of these concentrations depend on the initial conditions, and K remains constant as long as the temperature is unchanged.
It is important to note that the equilibrium constant expression is written based on the balanced chemical equation. The stoichiometric coefficients determine the relationship between the concentrations of reactants and products, allowing us to express the equilibrium state quantitatively using the equilibrium expression.
For more such question on equilibrium. visit :
https://brainly.com/question/30843966
#SPJ8
word equation for k2co3->k2o+co2
Answers
Answer:
potassium carbonate -> potassium oxide + carbon dioxide
Explanation:
remember to look at your periodic table, located each element so in this case you would locate k,c and o which are potassium, carbon and oxygen. From there you use nomenclature rules to get your answer.
The number 0.000402 expressed in scientific notation is
Answers
Answer:
hope its help you
Explanation:
4.02 x 10-4
This is how manganese appears in the periodic table.
What is the arrow is pointing to?
period symbol of manganese
isotope symbol of manganese
group symbol of manganese
atomic symbol of manganese
Answers
Answer: The atomic symbol of manganese or Aka D
Explanation: I did the test
Answer:
The atomic symbol of manganese or Aka D
Explanation:
Give the IUPAC name for the following structure

Answers
Answer:
6-metyl-2-heptyne
Explanation:
C-C-C-C-C-C-C hept
2
C-C≡C-C-C-C-C 2-heptyne
C
| 6
C-C≡C-C-C-C-C
6-metyl-2-heptyne
The IUPAC name for the above structure is 6 methyl, hept-2-yne.
What is IUPAC?IUPAC stands for international Union of pure and applied chemistry. It is the body in charge of naming organic chemical compounds.
The naming is is based on a molecule's longest chain of carbons connected by single/double/triple bonds, whether in a continuous chain or in a ring etc.
According to this question, a structure is given. The following applies;
The compound has a triple bond located on the second carbon, hence, belongs to alkyne group. It has seven carbon atoms, hence, is heptyne. The methyl group is on the sixth carbon.Learn more about IUPAC at: https://brainly.com/question/33646537
#SPJ6
• How does the name of the salt tell us that:
a) there is just one other element combined with the metal?
b) there is oxygen present in the salt?
Answers
The name of the salt tells us that:
a) there is just one other element combined with the metal by looking at the suffix of the salt's name.
b) the presence of oxygen in a salt can be indicated by the name of the salt.
a) The name of a salt can tell us that there is just one other element combined with the metal by looking at the suffix of the salt's name. If the salt name ends in "-ide," it indicates that the salt is composed of a metal and a single non-metal element.
For example, sodium chloride (NaCl) and potassium bromide (KBr) are salts where the metal (sodium and potassium) is combined with a single non-metal element (chlorine and bromine, respectively). The "-ide" suffix suggests the presence of only one other element in the salt.
b) The presence of oxygen in a salt can be indicated by the name of the salt. If the salt name includes the element oxygen, it suggests that oxygen is present in the salt compound.
For example, sodium carbonate (Na₂CO₃) and calcium sulfate (CaSO₄) contain the element oxygen in their chemical formulas. The presence of oxygen in the salt is implied by the name and the combination of elements in the compound.
Therefore, the name of salt tells us that there is just one other element combined with the metal and there is oxygen present in the salt
Learn more about salt here:
https://brainly.com/question/31814919
#SPJ 1
Calculate the ratio of imidazole to the imidazolium ion in a solution at pH 7.4.
Answers
2.5 to 1. Imidazolium ion will deprotonate to imidazole at pH 7.4 because it is more basic than pKa, which results in more imidazole being present overall.
What is imidazole used for?Imidazole becomes charged when it is protonated (i.e., in acid or HA+ form). It is uncharged when it is in its deprotonated (basin or A) form. Therefore, the uncharged:charged species ratio is [A][HA+], or 10:1.
The chromatography column's beads that are coated with nickel ions to which tagged proteins are bound are eluted using imidazole. In order to release the His-tagged proteins from nickel coordination, too much imidazole is delivered through the column. This causes the His-tag to be dislodged from nickel coordination.
2.5 to 1. Imidazolium ion will deprotonate to imidazole at pH 7.4 because it is more basic than pKa, which results in more imidazole being present overall.
To learn more about imidazolium refer to:
https://brainly.com/question/24260976
#SPJ4