Give an example of elastic potential energy
Answers
Answer:
An archer's stretched bow.
Explanation:
Elastic potential energy is energy stored as a result of applying a force to deform an elastic object.
Related Questions
I need help with Molar Ratio
______SO2(g) +_____O2(g) ------> _____SO3(g)
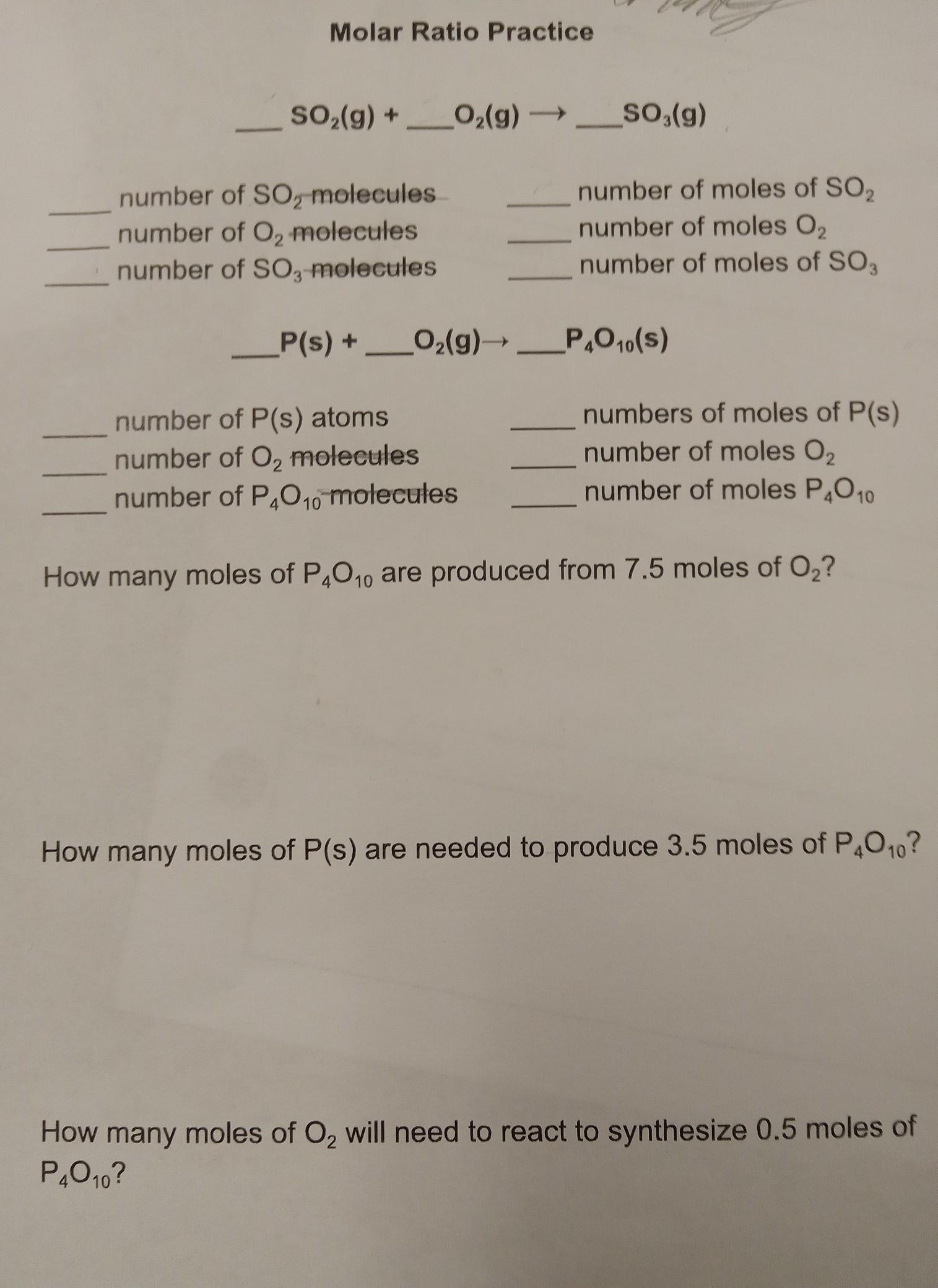
Answers
2 SO₂ (g) + O₂ (g) ---> 2 SO₃ (g)
1.204 * 10²⁴ number of SO₂ molecules = 2 number of moles of SO₂
6.02 * 10²³ number of O₂ molecules = 1 number of moles O₂
1.204 * 10²⁴ number of SO₃ molecules = 2 number of moles of SO,
4 P(s) + 5 O₂ (g) ----> P₄O₁₀ (S)
2.408 * 10²⁴ number of P(s) atoms = 4 numbers of moles of P(s)
3.01 * 10²⁴ number of O₂ molecules = 5 number of moles O₂
6.02 * 10²³ number of moles P₄O₁₀ = number of P₄O₁₀ molecules
1.5 moles of P₄O₁₀ are produced from 7.5 moles of O₂.
14 moles of P(s) are needed to produce 3.5 moles of P₄O₁₀.
2.5 moles of O₂ will need to react to synthesize 0.5 moles of P₄O₁₀.
What is the mole ratio of the given reactions?The mole ratio of the given reactions is obtained from their equations of reaction.
1. 2 SO₂ (g) + O₂ (g) ---> 2 SO₃ (g)
The mole ratio is 2 : 1 : 2
1 mole of atoms or molecules contains 6.02 * 10²³ particles.
Hence, the number of particles is obtained by multiplying the number of moles by 6.02 * 10²³.
4 P(s) + 5 O₂ (g) ----> P₄O₁₀ (S)
7.5 moles of O₂ will produce 7.5/5 moles of P₄O₁₀ = 1.5 moles of P₄O₁₀
3.5 moles of P₄O₁₀ will be produced by 3.5 * 4 moles of + = 14 moles of P(s)
0.5 moles of P₄O₁₀ will be produced by 0.5 * 5 moles of O₂ = 2.5 moles of O₂
Learn more about mole ratio at: https://brainly.com/question/30632038
#SPJ1
What molecular geometry would be expected for BF3 and NH3?
Answers
Answer:
Boron trifluoride would have a trigonal planar geometry.Ammonia would have a trigonal pyramidal geometry.Explanation:
There are three valence electrons in a boron atom.
In boron trifluoride, the central boron atom did not achieve an octet with eight valence electrons. Rather, that boron atom would be electron deficient with only six valence electrons.
Each of the three fluoride atoms would have shared one valence electron with that boron atom, with a total of three boron-fluorine single bonds. On the other hand, all three of the valence electrons of that boron atom would be involved in bonding. Hence, there would be no extra valence electrons to act as lone pairs on that boron atom.
Hence, the central boron atom would have three electron domains (one for each boron-fluorine bond) with none of the electron domains coming from lone pairs. By the VSEPR theory, the geometry of the molecule would be trigonal planar. All four atoms in this molecule would be in the same plane.
There are five valence electrons in a nitrogen atom.
In ammonia, the central nitrogen atom is indeed able to achieve an octet (with eight valence electrons in total.) Three of the five valence electrons of nitrogen would form a total of three hydrogen-nitrogen bonds. The other two valence electrons would form a lone pair.
Hence the central nitrogen atom would have four electron domains (one for each of the three hydrogen-nitrogen bond, and one for the lone pair.) Hence, by the VSEPR theory, the geometry of this molecule would be trigonal pyramidal.
4. What part of the Amino Acid structure is affected by heat? (1 point)
Answers
Heat primarily affects the non-covalent interactions that stabilize the secondary, tertiary, and quaternary structures of proteins, leading to denaturation and loss of their functional conformation.
Heat can affect various aspects of the amino acid structure, including conformational changes and chemical modifications. The specific region affected by heat depends on the temperature and duration of exposure. Here, we will primarily focus on the heat-induced denaturation of proteins, which are composed of amino acids
Protein denaturation occurs when the heat disrupts the weak non-covalent interactions that stabilize the folded structure of the protein. These interactions include hydrogen bonds, ionic bonds, van der Waals forces, and hydrophobic interactions. As the temperature rises, the increased kinetic energy causes these interactions to weaken and eventually break, leading to the unfolding of the protein.
The primary structure of an amino acid, which consists of a central carbon atom (the alpha carbon) bonded to an amino group, a carboxyl group, a hydrogen atom, and a side chain (R-group), is generally not directly affected by heat. The covalent bonds within the amino acid molecule, including the peptide bonds between adjacent amino acids in a protein chain, are relatively stable and require higher temperatures or other harsh conditions to break.
However, the disruption of non-covalent interactions by heat can lead to changes in the secondary, tertiary, and quaternary structures of proteins. The secondary structure, such as alpha helices and beta sheets, are formed by hydrogen bonding between the backbone atoms of the amino acids. Heat can cause these hydrogen bonds to break, leading to the unfolding of the secondary structure.
Additionally, the tertiary structure, which involves the overall three-dimensional folding of a protein, can be affected by heat. The hydrophobic interactions and other non-covalent bonds responsible for stabilizing the folded conformation can be disrupted, resulting in the protein unfolding into a more extended and disordered state.
In summary, heat primarily affects the non-covalent interactions that stabilize the secondary, tertiary, and quaternary structures of proteins, leading to denaturation and loss of their functional conformation. The primary structure of amino acids, which is determined by the covalent bonds, is generally more resistant to the effects of heat.
for more questions on covalent
https://brainly.com/question/30396627
#SPJ8
Choose the best tool for each scenario.
Identifying a trend in temperature change over time:
graph
Measuring the mass of a product of a chemical reaction:
Answers
Answer:
a) Graph
b) Weight balance or gas syringe or upside-down measuring cylinder
Explanation:
a) Identifying a trend in temperature change over time - The best tool for this scenario is to represents the temperature daily, weekly, monthly or annually on graph to interpret the fluctuation in temperature owing to local seasonal changes and weather conditions
b) Measuring the mass of a product of a chemical reaction - If the product is solid or liquid then the balance is used to measure the mass. If the product is a gas, then gas syringe or upside-down measuring cylinder is used.
what needs to be done to the products in order to reduce the amount of reactants that are formed
Answers
To reduce the amount of reactants formed, one or more of the following strategies like adjusting reaction conditions, catalyst usage, separating products, optimizing reaction mechanism and maintaining stoichiometry can be employed:
1. Adjust reaction conditions: By altering factors such as temperature, pressure, or concentration, the reaction conditions can be optimized to favor the formation of products over reactants. For example, lowering the temperature or increasing the pressure can shift the equilibrium towards the desired products.
2. Use a catalyst: Introducing a catalyst can enhance the reaction rate and selectively promote the formation of products. Catalysts provide an alternative reaction pathway with lower activation energy, facilitating the conversion of reactants into products.
3. Remove or separate the products: Continuously or periodically removing the products from the reaction mixture can help drive the equilibrium towards product formation. This can be achieved through techniques such as distillation, extraction, or adsorption.
4. Adjust stoichiometry: Modifying the stoichiometry of the reactants can impact the extent of product formation. By carefully balancing the amounts of reactants used, it is possible to minimize the formation of undesired reactants and maximize the production of desired products.
5. Optimize reaction conditions or reaction mechanism: Through detailed kinetic studies and understanding of the reaction mechanism, it may be possible to identify specific steps or conditions that lead to the formation of unwanted reactants. By modifying these aspects, it is possible to reduce the amount of undesired reactants formed.
It is important to note that the specific approach to reduce reactant formation depends on the nature of the reaction and the desired products. Different reactions may require tailored strategies for maximizing product formation while minimizing the formation of undesired reactants.
Learn more about stoichiometry at: https://brainly.com/question/14935523
#SPJ11
Two materials A and B are heated separately in air. The product formed is dissolved in water. How will you identify which one is metal?
Answers
In which situation is it better to have low friction?
Choose the correct answer.
a skydiver uses a parachute
a student walks down a hallway
a ladder leans against the wall
a hockey puck slides toward the goal
Answers
Answer: D- a hockey puck slides toward the goal
Explanation:
It needs to be able to slide easily and thats the correct answer on the test
The temperature of some air is minus 20 degrees C at 95kPa of pressure. What is the potential temperature, assuming a reference pressure at sea level (101.3kPa) ? Give your answer in degrees C, to the nearest degree.
Answers
The potential temperature is 15°C.
Given,The temperature of some air is minus 20 degrees C at 95 kPa of pressure.
Reference pressure at sea level = 101.3 kPa
The potential temperature (θ) is the temperature a parcel of dry air would have if it were adiabatically brought to a standard reference pressure, typically 1000 millibars (100 kPa).
Potential temperature is directly proportional to the absolute temperature and inversely proportional to the pressure in a system.
In order to find the potential temperature of the given air, we can use the formula below:
θ = T × (P0 / P)^(R/cp)
where,θ = potential temperature (in Kelvin)
T = temperature (in Kelvin)
P0 = reference pressure (in Pa)
P = actual pressure (in Pa)
R = gas constant for dry air (287 J/(kg·K))
cp = specific heat of dry air at constant pressure (1004 J/(kg·K))
Converting the given temperature in Celsius to Kelvin:
T = -20°C + 273.15K= 253.15K
The formula can be written as:
θ = T × (P0 / P)^(R/cp)θ
= 253.15 × (101300/95000)^(287/1004)θ
= 288.5 K
Converting the potential temperature from Kelvin to Celsius:
θ = 288.5 K - 273.15
= 15.35°C (to the nearest degree)'
= 15°C (rounded off to the nearest degree).
Therefore, the potential temperature is 15°C.
Learn more about the potential temperature from the given link-
https://brainly.com/question/4735135
#SPJ11
How many atoms are there in one molecule of C2RS
Answers
Answer: 6.022×1023
Explanation: Chemists generally use the mole as the unit for the number of atoms or molecules of a material. One mole is equal to 6.022×1023 molecular entities and each element has a different molar mass depending on the weight of 6.022×1023 of its atoms (1 mole)
1h magnetic resonance spectroscopy of 2h-to-1h exchange quantifies the dynamics of cellular metabolism in vivo
Answers
In vivo, the dynamics of cellular metabolism can be quantified using 2H-to-1H exchange in magnetic resonance spectroscopy of 1H nuclei.
To understand this concept, let's break it down step by step:
1. Magnetic resonance spectroscopy (MRS) is a non-invasive imaging technique that uses magnetic fields and radio waves to measure the chemical composition of tissues in the body.
2. In this case, 1H refers to the hydrogen nucleus (proton) in the molecules being studied. Protons are commonly used in MRS because they are abundant in the body and have strong magnetic properties.
3. 2H-to-1H exchange refers to the replacement of deuterium (2H) atoms with hydrogen (1H) atoms in metabolic processes. Deuterium is a stable isotope of hydrogen that can be tracked using MRS.
4. By quantifying the exchange of deuterium with hydrogen, MRS can provide information about the dynamics of cellular metabolism. This means it can reveal how metabolic processes, such as the breakdown of glucose or the synthesis of neurotransmitters, occur in living tissues.
5. The "in vivo" part of the question indicates that this technique is performed on living organisms, allowing researchers to study metabolism in real-time and in its natural environment.
So, in summary, 1H magnetic resonance spectroscopy of 2H-to-1H exchange is a method that uses MRS to measure the dynamics of cellular metabolism in living organisms.
Learn more about Magnetic resonance spectroscopy (MRS): https://brainly.com/question/30216189
#SPJ11
If 18 grams of oxygen reacts completely with 4 grams of hydrogen, we would expect how many grams of water?
A.) Less than 22 grams because some mass is lost in the reaction
B.) More than 22 grams because the oxygen gains mass during the reaction
C.) 22 grams because mass cannot be created or destroyed
D.) There is no way to tell from this information.
Answers
Answer: 22 grams because mass cannot be created or destroyed
Explanation: I just took the test
Answer:
C.) 22 grams because mass cannot be created or destroyed
Explanation:
Small children are occasionally injured when they try to inhale helium from a compressed helium tank. If a small child tries to transfer the contents of a 5.0 L tank of helium at a pressure of 125 atm and a temperature of 20⁰C into its lungs, how many moles of gas would the child inhale?
Answers
Answer:
26.0 moles
Explanation:
Given the formula;
PV =nRT
P= pressure of the gas
V = volume of the gas
n = number of moles of the gas
R = gas constant
T = temperature
n = PV/RT
n = 125 * 5/0.082 * (20 + 273)
n = 625/24.026
n = 26.0 moles
Your start-up company has invented a new water filter. If the filter can process 10 gallons of water per minute, how many gallons of water can it process in one 24-hour day?.
Answers
14,400 gallons of water can be filtered by the new water filter invented by the company.
It is given that the filter can process 10 gallons of water per minute.
So In an hour:
10 gallons per minute x 60 minutes = 600 gallons in 1 hour.
And in a day:
24 hours x 600 gallons per hour = 14,400 gallons in 24 hours.
Hence, 14,400 gallons of water can be filtered.
To know more about this gallons click here,
https://brainly.com/question/9560744
#SPJ4
Which gas does not bind to the porphyrin ring Fe(II) ion in myoglobin? A) NO B) CO C) CO2 D) O2 E) H2S
Answers
The gas that does not bind to the porphyrin ring Fe (II) ion in myoglobin is option E) H2S. Myoglobin is a protein that is found in muscles and plays a crucial role in storing and transporting oxygen.
The heme group present in myoglobin contains an iron ion that is surrounded by a porphyrin ring. This iron ion is responsible for binding to oxygen and facilitating its transport. However, some gases like CO, NO, and O2 can also bind to this iron ion, which can have adverse effects on the body. Carbon monoxide CO and nitric oxide NO have a higher affinity for binding to the iron ion than oxygen, which can lead to oxygen deprivation in the body. In contrast, carbon dioxide CO2 can bind to a different site on the protein and assist in the release of oxygen. However, hydrogen sulfide H2S does not bind to the porphyrin ring Fe (II) ion in myoglobin and therefore does not interfere with oxygen transport.
learn more about myoglobin here.
https://brainly.com/question/13599444
#SPJ11
You need to make an aqueous solution of 0.197 M barium nitrate for an experiment in lab, using a 500 mL volumetric flask. How many grams of solid barium nitrate should you add?
Answers
Answer:
Mass of barium nitrate needed = 25.74 g
Explanation:
Given data:
Molarity of solution = 0.197 M
Volume of solution = 500 mL (500 mL× 1 L /1000 mL = 0.5 L)
Mass of barium nitrate needed = ?
Solution:
Molarity = number of moles of solute / volume in L
0.197 M = number of moles of solute / 0.5 L
Number of moles of solute = 0.197 mol/L ×0.5 L
Number of moles of solute = 0.0985 mol
Mass of barium nitrate:
Mass = number of moles × molar mass
Mass = 0.0985 mol × 261.32 g/mol
Mass = 25.74 g
In a unit circle, ø = pi radians What is the terminal point? A. (1,0) B. (0,-1) C. (0,1) D. (-1,0)
Answers
Answer:
(-1,0)
Explanation:
If the same large amount of heat is added to a 250 g piece of aluminum and a 150 g piece of aluminum, what will happen?
Answers
please vote me brainliest i really need it for i can do my work
If magnesium nitrate and sodium hydroxide are reacted together. What is the resultant colorless jelly-like precipitate that forms?
Answers
If magnesium nitrate and sodium hydroxide are reacted together the product formed are magnesium hydroxide and sodium nitrate.
Sodium nitrate is the chemical compound having the formula NaNO₃. This alkali metal nitrate salt is also known as Chile saltpeter to distinguish it from ordinary saltpeter, potassium nitrate. It is used as preservative agents in cured meats such as bacon, sausage and in some cheeses. It appears as a white crystalline solid. It is Noncombustible but accelerates the burning of combustible materials. It May explode under prolonged exposure to heat or fire. The toxic oxides of nitrogen are produced in fires. It is used in solid propellants, explosives, fertilizers, and for many other uses.
To learn more about Sodium nitrate please visit:
https://brainly.com/question/14348213
#SPJ4
2) 2,047m2 + 42m = ?
Answers
Answer: It's 2,089m2
Explanation:
when the puck is dropped the potential energy will be
Answers
When the puck is dropped, the potential energy will be transformed into kinetic energy.
Potential energy is the energy stored by an object as a result of its position or configuration. It is the energy that is stored in an object due to its position with respect to its surroundings. Potential energy is an important concept in the study of energy since it is related to kinetic energy.
When an object is lifted to a certain height above the ground, it has potential energy. The potential energy of an object can be calculated using the formula PE = mgh, where m is the mass of the object, g is the acceleration due to gravity, and h is the height above the ground.
Potential energy is measured in joules.When the puck is dropped, it loses its potential energy and gains kinetic energy. The kinetic energy of an object is the energy that it possesses due to its motion. It is defined as the energy that is required to accelerate an object of a given mass to a given velocity.
The kinetic energy of an object can be calculated using the formula KE = 1/2mv², where m is the mass of the object and v is its velocity. Kinetic energy is also measured in joules.
Learn more about Potential energy
brainly.com/question/24284560
#SPJ11
2.F is a solution containing O.122mol/dm3 of HCl.G contains 7.0g of Y0H per dm3.Assuming at the end of titration exercise,28.00cm3 of the acid neutralized 25.00cm3 of the base.Calculate the, i.Concentration of G in mol/dm3 ii.Molar mass of YOH iii.Percentage by mass of Y in YOH The equation for the reaction HCl + YOH YCl + H20
Answers
i) The concentration of G in mol/dm3 is also 0.003416 mol/dm³.
ii) Molar mass of YOH is 204.49 g/mol
iii) The concentration of G is 0.003416 mol/dm³, the molar mass of YOH is 204.49 g/mol, and the percentage by mass of Y in YOH is 91.63%.
To solve this problem, we'll use the given information and the equation for the reaction: HCl + YOH → YCl + H2O
i. Concentration of G in mol/dm³:
From the given information, we know that 28.00 cm³ of the acid (F) neutralizes 25.00 cm³ of the base (G). This means the stoichiometric ratio between HCl and YOH is 1:1. Therefore, the number of moles of HCl neutralized by 28.00 cm³ of F is:
n(HCl) = concentration of F × volume of F in dm³
= 0.122 mol/dm³ × 28.00 cm³ / 1000 cm3/dm³
= 0.003416 mol
Since the stoichiometric ratio is 1:1, the concentration of G in mol/dm³ is also 0.003416 mol/dm3.
ii. Molar mass of YOH:
To calculate the molar mass of YOH, we need to know the mass of YOH used in the reaction. From the given information, we know that 7.0 g of YOH is present in 1 dm3 of G. Therefore, the molar mass of YOH can be calculated as:
Molar mass of YOH = Mass of YOH / Number of moles of YOH
= 7.0 g / 0.003416 mol
= 204.49 g/mol (rounded to two decimal places)
iii. Percentage by mass of Y in YOH:
The molar mass of Y in YOH can be calculated by subtracting the molar mass of OH from the molar mass of YOH:
Molar mass of Y = Molar mass of YOH - Molar mass of OH
= 204.49 g/mol - 17.01 g/mol
= 187.48 g/mol
The percentage by mass of Y in YOH can be calculated as:
Percentage by mass of Y = (Molar mass of Y / Molar mass of YOH) × 100%
= (187.48 g/mol / 204.49 g/mol) × 100%
= 91.63% (rounded to two decimal places)
Therefore, the concentration of G is 0.003416 mol/dm3, the molar mass of YOH is 204.49 g/mol, and the percentage by mass of Y in YOH is 91.63%.
for more questions on concentration
https://brainly.com/question/28564792
#SPJ8
The picture above is a diagram of what earth-sun-moon phenomena?
A.Solar System
B.Solar Eclipse
C.Lunar Eclipse
D.Earth-Sun-Moon
If you don't know plz don't answer

Answers
The recipe above yields 24 cookies. Please use the above recipe to determine how many cookies each of the following list of ingredients will make: 1 dozen eggs 24 teaspoons of vanilla 1 lb. (82 tsp) of salt 1 lb (84 tsp) of baking soda 3 cups of chocolate chips 5 lb (11 cups) of sugar 2 lb (4 cups) of brown sugar 1 lb (4 sticks) of butter 4 lb of all purpose flour Please show your calculations for all ingredients. Which of the above ingredients will be the limiting reactant? What is the maximum about of cookies that can be made with the new quantity of ingredients? Please make sure that your discussion is written in complete sentences. (Hint: you will need to calculate how many cookies can be made with the original recipe and the new quantities).
Answers
(A). From eggs -288, from vanilla -576, from salt-3969, from baking soda -4032, from chocolate chips -54, from sugar -528, from brown sugar -192, from butter -72, from flour- 78.
(B). The ingredient that is the limiting reactant is chocolate chips because it yields the minimum amount of cookies.
(C). The maximum amount of cookies made from the new quantity of ingredients is, 4032 from baking soda.
What are cookies?Cookies are sweet baked desserts made from all-purpose flour and butter and sugar.
They are made in many flavors and are very versatile to prepare.
A. Calculation from the new ingredients:
From eggs-
\(\bold{12\;eggs\times\dfrac{ 24\; cookies}{1\;egg}=288\; cookies}\)
From vanilla-
\(\bold{24\times\dfrac{ 24\; cookies}{1}=576\; cookies}\)
From salt-
\(\bold{82\times\dfrac{24\; cookies}{0.5}=3936\; cookies}\)
From baking soda-
\(\bold{84\times\dfrac{ 24\; cookies}{0.5}=3936\; cookies}\)
From chocolate chips-
\(\bold{3\; cups\times\dfrac{ 24\; cookies}{1.33}=4032\; cookies}\)
From sugar-
\(\bold{11\; cups\times\dfrac{ 24\; cookies}{0.5 cup}=54\; cookies}\)
From brown sugar-
\(\bold{4\; cups\times\dfrac{ 24\; cookies}{0.5\; cup}=528\; cookies}\)
From butter-
\(\bold{4\; sticks\times\dfrac{ 24\; cookies}{1.3\; cup}=72\; cookies}\)
From flour-
\(\bold{4\; lb\times\dfrac{ 24\; cookies}{1.2\; cup}=78\; cookies}\)
B.) chocolate chips
C.) baking soda (4032)
Thus, these are the calculations.
Learn more about cookies, here:
https://brainly.com/question/13219686
Does protecting animals so they won’t go extinct help with climate change?
Answers
Answer:
Yes
Explanation: Yes it depends on what kind of animal but yes it can help
Answer:
locations with local extinctions had larger and faster changes in hottest yearly temperatures than those without
How much energy is given off by the following reaction, if 162. 5 g of oxygen reacts with
216. 7 g of ammonia (NH3)?
4 NH3 + 502 → 4 NO + 6H2O H = -1225. 6 kJ
Answers
4974.9 kJ of energy are released during the interaction between 162.5 g of O2 and 216.7 g of NH3.
The given chemical equation shows the reaction between ammonia (NH3) and oxygen (O2) to form nitrogen monoxide (NO) and water (H2O). The enthalpy change (ΔH) for this reaction is -1225.6 kJ per mole of O2 consumed.
To determine the energy given off by the reaction between 162.5 g of O2 and 216.7 g of NH3, we need to first determine the limiting reactant. This is the reactant that is completely consumed in the reaction and limits the amount of product formed.
To find the limiting reactant, we need to calculate the number of moles of each reactant. The molar mass of O2 is 32.00 g/mol, so 162.5 g of O2 is equivalent to 5.078 moles of O2. The molar mass of NH3 is 17.03 g/mol, so 216.7 g of NH3 is equivalent to 12.71 moles of NH3.
The stoichiometric ratio of O2 to NH3 is 5:4, meaning that for every 5 moles of O2 consumed, 4 moles of NH3 are required. From the above calculations, we can see that there is excess NH3 in this reaction since only 4.063 moles of O2 are required to react with 3.250 moles of NH3.
Therefore, the amount of O2 that reacts is 4.063 moles, and the energy given off by the reaction is:
ΔH = (-1225.6 kJ/mol) x (4.063 mol) = -4974.9 kJ
Therefore, the reaction between 162.5 g of O2 and 216.7 g of NH3 gives off 4974.9 kJ of energy.
To learn more about ammonia refer to:
brainly.com/question/14672082
#SPJ4
I NEED HELP ASAP IM ON TIMER!!!!!!
Which of these refers to the strength of an acid?
a measure of the quantity of acid dissolved in water designated by molarity
an intrinsic characteristic of a particular acid, not related to concentration
the amount of hydrogen ions present in the acid
the amount of hydroxide ions present in a solution
Answers
Answer:
an intrinsic characteristic of a particular acid, not related to concentration
Explanation:
this is easy
An intrinsic characteristic of a particular acid is not related to concentration. Hence, option B is correct.
What is an acid?An acid is any substance that tastes sour, changes blue litmus paper to red, reacts with some metals to liberate hydrogen, and reacts with bases to form salts.
Whereas pH measures the concentration of solvated protons and does not tell us about the intrinsic acidity of chemical species present in solution, electrode potentials tell us about the intrinsic oxidizing power of the species present but provide no information on their concentration.
By the addition of a sufficient amount of redox indicator to a solution, its redox potential can be measured using redox electrodes.
Hence, an intrinsic characteristic of a particular acid is not related to concentration.
Learn more about acid here:
https://brainly.com/question/3700851
#SPJ5
A beaker contains a 25 ml solution of an unknown monoprotic acid that reacts in a 1:1 stochiometric ratio with naoh. Titrate the solution with naoh to determine the concentration of the acid.
Answers
The molarity of the acid is : 0.82 Molar
Calculate the molarity of acid?
Volume of acid = 25 ml
Molarity of base = 0.5 M
Volume of base = 41.00 ml
Since, acid and base reacts in 1:1 stoichiometric ratio, we can use the molar equivalence formula as:
M(acid) × V(acid) = M(Base) × V(Base)
M(acid) = 0.82 M
What is Molarity?
Molarity (M) is the amount of a substance in a given volume of solution. Molarity is defined as the number of moles of solute per liter of solution. Molarity is also called the molarity of a solution.
To know more about Molarity, check out:
https://brainly.com/question/26873446
#SPJ4
to find the ph of a buffer composed of h2po−4(aq)h2po4−(aq) and hpo2−4(aq)hpo42−(aq) , which p Kaka value should be used in the henderson–hasselbalch equation?
Answers
The pH of a buffer composed of H2PO−4(aq)/H2PO4−(aq) and HPO2−4(aq)/HPO42−(aq), we need to use the pKa value of the specific acid-base pair involved.
In this case, the Henderson-Hasselbalch equation can be written as:
pH = pKa + log([A-]/[HA])
Here, [A-] represents the concentration of the conjugate base (H2PO4− or HPO42−) and [HA] represents the concentration of the acid (H2PO−4 or HPO2−4).
For H2PO−4(aq)/H2PO4−(aq) pair, the pKa value of H2PO−4(aq) should be used.
For HPO2−4(aq)/HPO42−(aq) pair, the pKa value of HPO2−4(aq) should be used.
It is important to note that the pKa values for these acid-base pairs can vary depending on the temperature and ionic strength of the solution. It is recommended to refer to reliable sources, such as chemistry textbooks or scientific literature, to obtain the accurate pKa values for these species at the specific conditions you are working with.
To know more about acid-base pair visit:-
https://brainly.com/question/27756480
#SPJ11
Please help. I will also award brainlyest or whatever its called.
I don't want to have to do the experiment because I can't afford to leave water running for that long.
I have a bar of soap that weighs 135 grams. If I leave a sink dripping on the same spot on the bar of soap for 45 minutes how much of the soap will be eroded and how much will the soap weigh. The sink is dripping at 60 drips per minute.
Answers
If you leave a sink dripping on the same spot on a bar of soap for 45 minutes, about 54 grams of soap will be eroded and the soap will weigh about 81 grams.
Which of the following radiactive elements was often put in beauty products?
Francium
Curium
Radium
Uranium
Answers
Answer:
Radium
Explanation:
Radium is a radioactive element which was often put in beauty products.