Answers
Answer:
The empirical formula for the compound is C3H4O3
Explanation:
The following data were obtained from the question:
Carbon (C) = 40.92%
Hydrogen (H) = 4.58%
Oxygen (O) = 54.50%
The empirical formula for the compound can be obtained as follow:
C = 40.92%
H = 4.58%
O = 54.50%
Divide by their molar mass
C = 40.92/12 = 3.41
H = 4.58/1 = 4.58
O = 54.50/16 = 3.41
Divide by the smallest i.e 3.41
C = 3.41/3.41 = 1
H = 4.58/3.41 = 1.3
O = 3.41/3.41 = 1
Multiply through by 3 to express in whole number
C = 1 x 3 = 3
H = 1.3 x 3 = 4
O = 1 x 3 = 3
The empirical formula for the compound is C3H4O3
Related Questions
Select the statement that best describes how energy is passed from a herbivore to a carnivore. (2 points)
Group of answer choices
Energy from the food sources that both herbivores and carnivores eat is passed directly from them to plants.
Energy from the foods carnivores eat is passed directly to an herbivore.
When an herbivore eats meat, and a carnivore eats the herbivore, energy from the eaten meat is passed indirectly to the carnivore.
When an herbivore eats plants, and a carnivore eats the herbivore, energy from the eaten plants is passed indirectly to the carnivore.
Answers
answer:when an herbivore eats meat and a carnivore eats the herbivore energy from the eatin meat is passed indirectly to the carnivore.
What is the IUPAC name for Fe203?
Answers
What happened to the substances in the test tube during Step 2?
A. The heat started a chemical reaction between the iron and sulfur, making the compound
iron sulfide.
B. The heat started a chemical reaction between the iron and sulfur, making the element iron
sulfide.
C.
D.
The heat started a physical reaction between the iron and sulfur, making the solution iron
sulfide.
The heat started a physical reaction between the iron and sulfur, making the mixture iron
sulfide.

Answers
Answer:
A the heat started a chemical reactions
The heat started a chemical reaction between the iron and sulfur, making the compound iron sulfide. So, the correct option is (A).
What is Chemical Reaction?
A chemical reaction is defined as the process that leads to a chemical change from one set of chemical substances to another.
In this process, a substance or substance undergoes a chemical change to produce a new substance or substances, with whole new properties, in a process known as chemical reactions. The nature and identity of the products is completely different from that of the reactants.
For the information above, the chemical reaction caused by the heat, the iron and the suphur mixed with each other are heated on a burner forming a yellow color, which turns into iron sulphide, a black colored compound having totally different properties.
Thus, the heat started a chemical reaction between the iron and sulfur, making the compound iron sulfide. So, the correct option is (A).
Learn more about Chemical Reactions, here:
https://brainly.com/question/29039149
#SPJ2
Your little sister asks you a scientific question: "Does chocolate milk come from brown cows?" In order to answer the question, you decide to form a hypothesis.
Explain whether or not the following statements are effective hypotheses.
i. Brown cows produce chocolate milk.
ii. Brown cows never produce chocolate milk.
iii. Brown cows produce white milk.
Answers
A hypothesis is a proposed explanation or prediction based on limited evidence or observations, which can be tested through further investigation or experimentation. It should be specific, testable, and based on existing knowledge.
Now, let's evaluate each statement as a hypothesis:Brown cows produce chocolate milk.This statement can be considered an effective hypothesis as it proposes a relationship between the color of cows and the color of milk they produce. It is specific and testable, as one could observe and analyze the milk produced by brown cows to see if it is indeed chocolate milk. However, based on existing knowledge, we can confidently say that this hypothesis is not accurate, as the color of a cow does not determine the color of the milk it produces.Brown cows never produce chocolate milk.This statement can also be considered an effective hypothesis because it makes a specific claim that can be tested. However, based on existing knowledge, we can say that this hypothesis is not accurate. While the color of a cow does not determine the color of the milk, it is possible for chocolate milk to be produced by adding chocolate syrup or cocoa powder to regular white milk.Brown cows produce white milk.This statement is not an effective hypothesis as it is a general statement that aligns with existing knowledge. It does not propose any specific relationship or prediction to be tested. In the context of this question, the statement is not accurate as milk produced by cows is typically white, regardless of their coat color.For such more question on hypothesis
https://brainly.com/question/606806
#SPJ8
URGENT
A chemical equilibrium between gaseous reactants and products is shown.
N2(g) + 3H2(g) ⇌ 2NH3(g)
How will the reaction be affected if the pressure on the system is increased?
It will shift toward the reactant side as there is lower pressure on the reactant side.
It will shift toward the product side as there is higher pressure on the product side.
It will shift toward the reactant side as there are a greater number of moles of gas on the reactant side.
It will shift toward the product side as there are a fewer number of moles of gas on the product side.
Answers
Answer:
Explanation:
Discussion
When Pressure increases equilibrium shifts to the side with the smallest number of moles. But which side is that?
N2(g) + 3H2(g) ⇌ 2NH3(g)
The left side has 1 mol of nitrogen (N2) and 3 moles of Hydrogen = 4 mols
on the left side.
The right side has 2 mols of NH3 = 2 mols on the right.
Conclusion: You tell the number of mols by the Balance numbers to the left of each chemical in an equation.
Since the left side N2 + 3H2 = 4 mols, the equilibrium does NOT shift left.
2NH3 is only two mols.
The equilibrium shifts Right
Answer
D
Which of the following statements is true?a) energy is released when bonds are broken because bond breaking is an endothermic process b) energy is required to break bonds because bond breaking is an endothermic process c) energy is released when bonds are broken because bond breaking is an exothermic processd) energy is required to break bonds because bond breaking is an exothermic process
Answers
Answer
b) energy is required to break bonds because bond breaking is an endothermic process.
Explanation
In endothermic reactions, more energy is absorbed when the bonds in the reactants are broken than is released when new bonds are formed in the products.
Hence, energy is required to break bonds. Energy is released when chemical bonds are formed because atoms become more stable. However, bond-forming is an exothermic process, because it releases energy.
Please help me with this
If somebody posts b.u.l.l.s.h.i.t. answers, please report them!!
Answers
Answer:
where is the question
Explanation:
F and G react together. When the concentration of F is tripled and the concentration of G remains constant, there is a nine-fold increase in the rate of the reaction. What is the order of the reaction with respect to F?
Answers
There is a nine-fold rise in the pace of the reaction when the concentration of F is tripled but the concentration of G stays the same. The response is in the second order relative to F.
What happens to rate of reaction if concentration is tripled?If you increase one reactant's concentration in a third order reaction involving two reactants by three times, the rate rises by a factor of three. If a reactant is first order, its concentration will cause a doubling in the reaction's rate; a tripling in the reaction's rate, etc. If a reactant is third order, its rate of reaction will increase by a factor of 8 when its concentration is doubled (23 = 8), etc.
The concentration of a reactant does not impact the rate. The rate remains unchanged even if the concentration is doubled. The given rate law needs to be written first. The concentrations of A and B will be increased by three times each after that.
To learn more about order refer to :
https://brainly.com/question/28179168
#SPJ4
what is the PH scale of 0.02m of hydrochloric acid
Answers
Answer:
Explanation:
The pH of 0.02 M hydrochloric acid is approximately 1.7.
THANKS
IF THE ANSWER IS CORRECT , THEN MARK ME AS BRAINLIST
To determine the pH of a hydrochloric acid solution, we need to know its concentration. You mentioned a concentration of 0.02 M (molar), which refers to 0.02 moles of hydrochloric acid dissolved in 1 liter of solution.
Hydrochloric acid (HCl) is a strong acid that dissociates completely in water, meaning all HCl molecules release their hydrogen ions (H+) into the solution. Since the concentration is given as 0.02 M, it means there are 0.02 moles of H+ ions in 1 liter of the solution.
To calculate the pH, we can use the formula:
pH = -log[H+]
In this case, [H+] represents the concentration of hydrogen ions in moles per liter. Since hydrochloric acid is a strong acid and it dissociates completely, the concentration of hydrogen ions is equal to the concentration of HCl, which is 0.02 M.
pH = -log(0.02) ≈ 1.70
Therefore, a hydrochloric acid solution with a concentration of 0.02 M would have a pH of approximately 1.70, indicating it is strongly acidic.
cl-+peg=hcl+peg rate law, rate constant k
Answers
a. The rate law for this reaction is: Rate = k[Cl] [H₂]. This means that the rate of the reaction is directly proportional to the concentrations of both Cl and H₂ molecules.
What is rate law?Rate law is an equation that describes the rate of a chemical reaction as a function of the concentrations of reactants. The rate law allows us to describe how the rate of a reaction changes when the concentrations of reactants are changed. It is derived from the rate equation, which is a mathematical expression that can be used to calculate the rate of a reaction from the concentrations of the reactants and the rate constant.
b. The rate law for this reaction is: Rate = k[O] [Os]. This means that the rate of the reaction is directly proportional to the concentrations of both O and Os molecules.
c. The rate law for this reaction is: Rate = k[NO₂]₂. This means that the rate of the reaction is directly proportional to the square of the concentration of NO₂ molecules.
To learn more about rate law
https://brainly.com/question/16981791
#SPJ1
Complete Question:

Electroplating is a way to coat a complex metal object with a very thin (and hence inexpensive) layer of a precious metal, such as silver or gold. In essence the metal object is made the cathode of an electrolytic cell in which the precious metal cations are dissolved in aqueous solution. Suppose a current of 0.270 A is passed through an electroplating cell with an aqueous solution of Ag_2 SO_4 in the cathode compartment for 72.0 seconds. Calculate the mass of pure silver deposited on a metal object made into the cathode of the cell. Round your answer to 3 significant digits. Also, be sure your answer contains a unit symbol.
Answers
Mass of the pure silver deposited on a metal object made into the cathode of the cell is calculated to be 0.0217 gm.
What is electroplating?The process of using electrodeposition to coat an object in a layer of metal is called electroplating .
As we know that, Q = I * t
=0.270 * 72
= 19.44 C
Here Q is quantity of electricity , I is current in amperes = 0.270 A (given)
t is time in seconds (72.0 sec)
As 96500 Coulomb of electricity electrolyzes 1 mole of Ag
then,19.44 C of electricity deposits,
=1/96500 * 19.44
= 0.000201 moles of Ag
Mass of Ag is = number of moles * molar mass
= 0.000201 * 108
= 0.0217 gm
Thus, mass of pure silver deposited on a metal object made into the cathode of the cell is 0.0217 gm.
To know more about electroplating, refer
https://brainly.com/question/16266707
#SPJ4
Explain what an ion is.
Answers
An ion is an atom or a group of atoms that carries an electric charge.
What is an ion?An atom becomes an ion when it gains or loses one or more electrons, resulting in an unequal number of protons and electrons. This imbalance of positive and negative charges gives the ion a net charge, either positive or negative.
When an atom loses one or more electrons, it becomes a positively charged ion, called a cation. Cations have fewer electrons than protons, resulting in a net positive charge.
When an atom gains one or more electrons, it becomes a negatively charged ion, called an anion. Anions have more electrons than protons, resulting in a net negative charge.
Learn more about ions at.
https://brainly.com/question/22277121
#SPJ1
Describe the cause and effect relationship between density and ocean currents.
Answers
Answer:
Differences in water density affect vertical ocean currents. Denser water tends to sink, while less dense water tends to rise. Other causes of currents include tides, rain, runoff, and ocean bottom topography. Topography is the surface features of a place. Ocean topography includes slopes, ridges, valleys, and mountains! All these things are found at the bottom of the ocean, and can influence currents.
The cause-and-effect relationship between density and ocean currents is the mixing and circulation are influenced by the density differences between the various layers of the water column.
What are ocean currents?The continuous, predictable, and directional movement of seawater known as ocean currents is caused by gravity and wind.
Ocean vertical currents are influenced by variations in water density. Less dense water tends to rise, while denser water sinks. Tides, rainfall, runoff, and the topography of the ocean bottom are additional causes of currents.
Thus, the mixing and circulation are influenced by the differences in densities between the various layers of the water column, which is the cause-and-effect relationship between density and ocean currents.
To learn more about ocean currents, refer to the below link:
https://brainly.com/question/1543125
#SPJ2
Which of the following compounds is likely to make the best conductor when
dissolved in water?
a. SO2
b. C6H1206
c. KBr
d. CO
Answers
Answer:
c. KBr .
Explanation:
Hello!
In this case, since electrolytes are substances that are able to carry electric current in the form of electrons via ions, those that are ionic are said to have the greatest capacity to conduct the electricity; in such a way, since SO2, C6H12O6 and CO are non-ionic molecules but covalent, they are not good conductor, therefore the best conductor would be c. KBr as it is an ionic compound due to the electronegativity of the K-Br bond.
Best regards!
Write the balanced net ionic equation for the reaction of aqueous sodium sulfate with aqueous lead(II) nitrate. Include phases.
net ionic equation:
Answers
Answer:
SO₄²⁻ (aq) + Pb²⁺ (aq) → PbSO₄ (s)
General Formulas and Concepts:
Solubility RulesExplanation:
Step 1: RxN
Na₂SO₄ (aq) + Pb(NO₃)₂ (aq) → PbSO₄ (s) + NaNO₃ (aq)
Step 2: Balance RxN
Na₂SO₄ (aq) + Pb(NO₃)₂ (aq) → PbSO₄ (s) + 2NaNO₃ (aq)
Step 3: Ionic Equations
Total Ionic Equation:
2Na⁺ (aq) + SO₄²⁻ (aq) + Pb²⁺ (aq) + 2NO₃⁻ → PbSO₄ (s) + 2Na⁺ (aq) + 2NO₃⁻ (aq)
Cancel out spectator ions.
Net Ionic Equation:
SO₄²⁻ (aq) + Pb²⁺ (aq) → PbSO₄ (s)
100 Points! PLZ HELP
Which statement best describes the model below?

Answers
the image shows the moles of gas increasing as well as volume increasing
Answer:
Option D
Explanation:
Because we know the relationship between no of moles and volume
Volume is directly proportional to no of molesSo if moles is increased volume would be increased too .Option D
Please help me! I am a bit stuck on this.

Answers
What occurs during a solar elcipse
Answers
Answer:
the moon gets infront of the sun
Answer:
A solar eclipse happens when the moon moves in front of the Sun as seen from a location on Earth. During a solar eclipse, it gets dimer and dimmer outside as more and more of the Sun is covered by the Moon. During a total eclipse, the entire Sun is covered for a few minutes and it becomes very dark outside. The temperature outside also drops.
Once you pour the boiling water into the beaker, why is it important to wait a while before you measure the reading on the thermometer?
Answers
Drawing were everything goes will be much appreciated it thank you

Answers
Following is the classification of given substances:
Atomic element: Ag
Molecular element: F₂
Molecular compound: CO
Ionic compound: PbI₄
What is a molecule?Molecules, groups of two or more atoms that form the smallest identifiable unit that can divide a pure substance and that retain the composition and chemical properties of that substance.
An atom consists of a single positively charged nucleus surrounded by a cloud of negatively charged electrons. As the atoms approach, the electron clouds interact with each other and with the nucleus. This interaction causes atoms to combine to form molecules when the energy of the entire system is reduced. From a structural point of view, a molecule consists of a collection of atoms joined by valences. A diatomic molecule contains two atoms chemically bonded together. When two atoms are identical, like oxygen (O2) molecules, they form a homonuclear diatomic molecule, but when the atoms are different, like carbon monoxide (CO) molecules, they are heteronuclear. Forms a diatomic molecule. Molecules containing two or more atoms are called polyatomic molecules. B. Carbon dioxide (CO2) and water (H2O). A polymer molecule can contain thousands of constituent atoms.
To know more about molecules, visit:
https://brainly.com/question/28069497
#SPJ1
Question 3 (2 points)
Using the following equation how many grams of water you would get from 846 g of
glucose:
C6H12O6 + 602 + 6CO2 + 6H20 + energy
Your Answer:
Answer
units g)
Answers
Answer: The mass of water produced is 507.6 g
Explanation:
The number of moles is defined as the ratio of the mass of a substance to its molar mass. The equation used is:
\(\text{Number of moles}=\frac{\text{Given mass}}{\text{Molar mass}}\) ......(1)
Given mass of glucose = 846 g
Molar mass of glucose = 180 g/mol
Plugging values in equation 1:
\(\text{Moles of glucose}=\frac{846g}{180g/mol}=4.7 mol\)
The given chemical equation follows:
\(C_6H_{12}O_6+6O_2\rightarrow 6CO_2+6H_2O+energy\)
By the stoichiometry of the reaction:
If 1 mole of glucose produces 6 moles of water
So, 4.7 moles of glucose will produce = \(\frac{6}{1}\times 4.7=28.2mol\) of water
Molar mass of water = 18 g/mol
Plugging values in equation 1:
\(\text{Mass of water}=(28.2mol\times 18g/mol)=507.6g\)
Hence, the mass of water produced is 507.6 g
7) How many molecules of CO2 are in 2.5 L at STP?
Answers
By using the ideal gas law and Avogadro's number, we find that there are approximately 6.72 × 10^22 molecules of CO2 in 2.5 L at STP.
To determine the number of molecules of CO2 in 2.5 L at STP (Standard Temperature and Pressure), we can use the ideal gas law and Avogadro's number.
Avogadro's number (N_A) is a fundamental constant representing the number of particles (atoms, molecules, ions) in one mole of substance. Its value is approximately 6.022 × 10^23 particles/mol.
STP conditions are defined as a temperature of 273.15 K (0 °C) and a pressure of 1 atmosphere (1 atm).
First, we need to convert the volume from liters to moles of CO2. To do this, we use the ideal gas law equation:
PV = nRT,
where P is the pressure, V is the volume, n is the number of moles, R is the ideal gas constant, and T is the temperature in Kelvin.
Since we have STP conditions, we can substitute the values:
(1 atm) × (2.5 L) = n × (0.0821 L·atm/(mol·K)) × (273.15 K).
Simplifying the equation:
2.5 = n × 22.4149.
Solving for n (the number of moles):
n = 2.5 / 22.4149 ≈ 0.1116 moles.
Next, we can calculate the number of molecules using Avogadro's number:
Number of molecules = n × N_A.
Number of molecules = 0.1116 moles × (6.022 × 10^23 particles/mol).
Number of molecules ≈ 6.72 × 10^22 molecules.
Therefore, there are approximately 6.72 × 10^22 molecules of CO2 in 2.5 L at STP.
For more such questions on ideal gas law visit:
https://brainly.com/question/27870704
#SPJ8
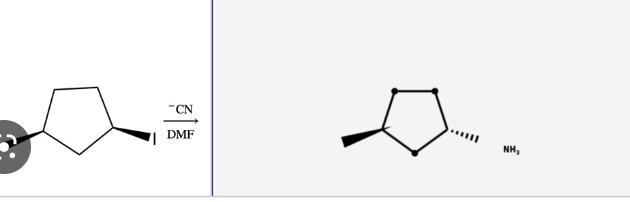
check your work. does your synthesis strategy give a substitution reaction with the expected regiochemistry and stereochemistry? draw the expected product of the forward reaction.
Answers
Yes, the synthesis strategy can give a substitution reaction with the expected regiochemistry and stereochemistry.
The expected product of the forward reaction would be a single enantiomer of the substituted product, depending upon the starting materials used. For example, if the starting material is an unsubstituted chiral compound, then the product would be a single enantiomer of the desired product. If the starting material is a racemic mixture, then the product would be a racemic mixture of the desired product. In detail, a substitution reaction involves the replacement of a functional group in a molecule with a different functional group. This process can be catalyzed by a transition metal catalyst, such as a palladium or nickel complex. The reaction is usually carried out in the presence of a base, such as sodium hydroxide or potassium hydroxide, and a nucleophile, such as an alcohol or an amine.
To learn more about regiochemistry click here https://brainly.com/question/14957697
#SPJ4
What is the difference between agar and pectin?
Answers
Answer:
Both come from vegetable sources; pectin is a soluble fiber found in plants, while agar comes from various species of algae. Chemically, they're closely related, and both consist of long strands of sugar molecules.
Explanation:
Am nevoie rapid de aceste exercitii

Answers
Answer:
I will find the answer for you
Explanation:
Just a minute
A sample of an unknown compound is vaporized at 160 c . The gas produced has a volume of 2330 ml at a pressure of 1.00 atm ,and it weighs 2.10 g
Round answer to 3 significants digits

Answers
The molar mass is 3230.8 g/mol
How to determine the valueFirst, we need to know that the formula for the general gas law is represented as;
PV = nRT
such that the parameters are;
P is the pressureV is the volumen is the number of molesR is the gas constantT is the temperatureSubstitute the values
1 × 2.33 = n × 8.314 × 433.15
Multiply the values, we get;
n = 2.33/ 8.314 × 433.15
Divide the values
n = 6.5 × 10⁻⁴ moles
But, number of moles = mass/molar mass
Molar mass = 2.10/ 6.5 × 10⁻⁴
Molar mass = 3230.8 g/mol
Learn about ideal gas law at: https://brainly.com/question/25290815
#SPJ1
GIVING BRAINLY AND 20 POINTS
A sound wave in air has the wavelength of 1.36 m. Calculate its frequency? Assume the speed is 340 m/s.
Answers
Answer: the answer is 0.074Hz
Explanation:
Given, (In air)
Velocity V=340m/s
Frequency f=20,000Hz
Wavelegth λ=?
V=f.λ
λ=
F
V
=
20,000
340
=0.017Hz
Also, Given (in Water)
Velocity, V=1480m/s
Frequencyf=20,000Hz
wavelength, λ=?
V=F.λ
λ=
F
V
=
20,000
1480
=0.074Hz
Answer:
frequency
Explanation:
frequency is velocity/ wavelength
340/1.36
250
Name the type of chemical reaction that occurs when calcium hydroxide (Ca(OH)2) reacts with nitric acid (HNO3).
Answers
To solve such this we must know the concept of Neutralization reaction. Therefore, neutralization is the type of chemical reaction that occurs when calcium hydroxide (Ca(OH)\(_2\)) reacts with nitric acid (HNO\(_3\)).
What is chemical reaction?Chemical reaction is a process in which two or more than two molecules collide in right orientation and energy to form a new chemical compound. The mass of the overall reaction should be conserved. There are so many types of chemical reaction reaction like combination reaction, double displacement reaction.
Neutralization is a reaction that occur between acid and base. The products of neutralization reaction is salt and water. Neutralization is the type of chemical reaction that occurs when calcium hydroxide (Ca(OH)\(_2\)) reacts with nitric acid (HNO\(_3\)).
Therefore, neutralization is the type of chemical reaction that occurs when calcium hydroxide (Ca(OH)\(_2\)) reacts with nitric acid (HNO\(_3\)).
Learn more about the chemical reactions, here:
brainly.com/question/3461108
#SPJ1
The water treatment plant adds chlorine gas to the municipal water supply to
control microorganisms. If a technician adds Cl2 at a rate of 1 mg per liter of water,
what is the molar concentration of chlorine in the drinking water?
Answers
Answer:
1 × 10⁻⁵ M
Explanation:
Step 1: Given data
Concentration of chlorine: 1 mg/LStep 2: Convert the concentration of chlorine from mg/L to g/L
We will use the conversion factor 1 g = 10³ mg.
1 mg/L × 1 g/10³ mg = 1 × 10⁻³ g/L
Step 3: Convert the concentration of chlorine from g/L to mol/L (molar)
We will use the molar mass of chlorine: 70.91 g/mol.
1 × 10⁻³ g/L × 1 mol/70.91 g = 1 × 10⁻⁵ mol/L = 1 × 10⁻⁵ M
On a recent car ride, a student traveled the first 20 kilometers in 5 minutes, the next 60 kilometers in 50 minutes, and the last 10 kilometers in 5 minutes. What was the average speed of the car over the student's entire ride?
1.5 km/min
5 km/min
2 km/min
4 km/min
Answers
Answer:
it is 90 km in one hour it is 90km/h
90/60=1,5