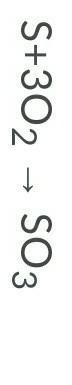For two nondegenerate energy levels separated by an amount of energy ?/k = 850K , at what temperature will the population in the higher-energy state be 1/2 that of the lower-energy state?T= ?
Answers
The temperature at which the population in the higher-energy state will be 1/2 that of the lower-energy state is approximately 567 K.
To find the temperature (T), we can use the Boltzmann distribution formula:
n₂/n₁ = e^(-ΔE/kT)
Where n₁ and n₂ are the populations of the lower-energy and higher-energy states, respectively, ΔE is the energy difference between the two states, k is the Boltzmann constant, and T is the temperature. Since the population of the higher-energy state is 1/2 that of the lower-energy state, we can write the equation as:
1/2 = e^(-ΔE/kT)
Given the energy difference ?/k = 850 K, we can substitute ΔE/k = 850 in the equation:
1/2 = e^(-850/T)
To solve for T, we can take the natural logarithm of both sides:
ln(1/2) = -850/T
Now, we can isolate T:
T = -850/ln(1/2) ≈ 567 K
At a temperature of approximately 567 K, the population in the higher-energy state will be 1/2 that of the lower-energy state.
To know more about higher-energy state, click here
https://brainly.com/question/15331654
#SPJ11
Related Questions
Which of the following BEST accounts for the charge in potential energy going from Point B to Point A?As the atoms get closer together, their nuclei begin to repel one another,As the atoms get closer together, the attraction of the nucleus of each atom for the electron(s) of the other gets stronger and stronger,As the molecules get closer, they slow down, converting kinetic to potential energy,The bond is becoming stronger as the atoms approach each other.
Answers
The statement which accounts for the change in potential energy going from Point B to Point A is that as the atoms get closer together, the attraction of the nucleus of each atom for the electron(s) of the other gets stronger and stronger and is denoted as option B.
What is Potential energy?This is referred to as the energy possessed by a body by virtue of its position.
The change in the potential energy is as a result of the atoms getting closer which leads to the attraction of the nucleus of the electron of the other getting stronger.
Read more about Potential energy here https://brainly.com/question/14427111
#SPJ1
What is the density of an object (g/cm) that has a mass of 2.78 g & a volume of 10.0
cm3?
Answers
Answer:
The answer is 0.28 g/cm³Explanation:
The density of a substance can be found by using the formula
\(density = \frac{mass}{volume} \\ \)
From the question we have
\(density = \frac{2.78}{10} \\ = 0.278\)
We have the final answer as
0.28 g/cm³Hope this helps you
Answer:
p = 0.278 g/cm3
Explanation:
5HCl what are the number of elements that 5HCl has ?
Answers
Answer:
Penta hydrogen chlorine
Explanation:
they are ploy atomic elements
did the electron move into a region of higher potential or lower potential?
Answers
Since there is an electrostatic force, electrons go from a location of lower potential to one of greater potential.
What is an electron example?The electron, the smallest constituent part of an atom, does indeed have a negative charge. Both electrons and protons are present in an equal proportion in a negative ion. The subatomic particle only contains one proton and one particle.
What are three facts about electrons?The outside borders of the nucleus are circled by the negatively charged electrons. They rotate so fast that it can be difficult for researchers to keep track of them. The smallest atom particles—you can fit 2000 of them inside a hydrogen atom—are pulled to the neutrons' ions with positive charges.
To know more about electrons visit:
https://brainly.com/question/15054861
#SPJ4
The Earth's axis changes direction (but the angle does NOT change).
is called what ?
Answers
Answer:
Axial precession
Explanation:
It happens ever ~26'000 years.
using the letters on the image, identify each component of the liquid waste set-up.A-- funnelB-- primary containerC-- waste labelD-- secondary container
Answers
According to the given information the correct answer is to identify each component of the liquid waste set-up using the letters on the image:
A-- Funnel: This is a device that is used to guide the liquid waste into the primary container without spilling.
B-- Primary container: This is the first container that collects the liquid waste. It can be a glass or plastic bottle, a jug, or any other appropriate container that is labeled for hazardous waste.
C-- Waste label: This is a label that indicates the contents of the primary container. It should include information such as the name of the chemical, the date it was collected, and the person who collected it.
D-- Secondary container: This is a larger container that is used to collect multiple primary containers. It should be labeled with the same information as the primary container, as well as a warning label indicating that it contains hazardous waste.
To know more about lipid set up visit:
https://brainly.com/question/28481240
#SPJ11
the electron configuration for nitrogen is 1s22s22p31s22s22p3 . according to hund’s rule, how are the electrons distributed in the three 2p2p -orbitals?
Answers
According to Hund's rule, electrons will fill the orbitals in a way that maximizes the number of unpaired electrons.
In the case of nitrogen, the electron configuration is 1s²2s²2p³. The 2p subshell has three orbitals: 2px, 2py, and 2pz. The two electrons in each orbital will have opposite spins (antiparallel spin) in order to minimize the energy and achieve the lowest possible energy state.
For nitrogen, the distribution of the three 2p electrons would be as follows:
- One electron in the 2px orbital (↑)
- One electron in the 2py orbital (↑)
- One electron in the 2pz orbital (↑)
Each electron occupies a separate 2p orbital with parallel spins, maximizing the number of unpaired electrons, as stated by Hund's rule.
learn more about Hund's rule Refer: https://brainly.com/question/12646067
#SPJ11
Calculate the wavelength of radiation with a frequency of 7.13 x 1020 s-1
Answers
Answer:
λ = 4.2x10^-13 m
Explanation:
c = λν
c is speed of light 3x10^8 m/s
λ is wavelength
ν is frequency
3.10^8 = λ . 7.13 × 10^20
λ = 4.2x10^-13 m
A 0. 150-mole quantity of CoCl2 is added to a liter of 1. 20 M NH3 solution. What is the concentration of Co2 ions at equilibrium? Assume the formation constant* of Co(NH3)62 is 5. 0 × 1031 M–6
Answers
The concentration of Co²⁺ ions at equilibrium is 0.150 M.
To determine the concentration of Co²⁺ ions at equilibrium, we need to consider the reaction between CoCl₂ and NH₃ to form Co(NH₃)₆²⁺.
The balanced equation for the reaction is:
CoCl₂ + 6NH₃ ⇌ Co(NH₃)₆²⁺ + 2Cl⁻
We can set up an ICE (Initial, Change, Equilibrium) table to solve for the concentration of Co²⁺ ions at equilibrium.
Initially, we have 0.150 moles of CoCl₂ and 1 liter of 1.20 M NH₃ solution.
Using the stoichiometry of the reaction, we can see that for every mole of CoCl₂, we form 1 mole of Co(NH₃)₆²⁺ ions. Therefore, the concentration of Co(NH₃)₆²⁺ ions at equilibrium is equal to the initial concentration of CoCl₂.
Therefore, the concentration of Co²⁺ ions at equilibrium is 0.150 M.
learn more about ions here
https://brainly.com/question/30663970
#SPJ11
Which describes one way that Aristarchus's explanation of the movement of objects in the sky was different from other scientists' earlier explanations? He discovered Venus’s phases. He argued that Earth rotates on its axis. He explained the concept of retrograde motion. He described the epicycles of the planets in the sky.
Answers
Answer:
The correct option is;
He argued that Earth rotates on its axis
Explanation:
The hypothesis that the Sun is at the center of the universe and the Earth, the other planets and other heavenly bodies are revolving around the Sun was first proposed by Aristarchus of Samos who also believed that the motion of the stars where actually due to the rotation of the Earth about its axis
However, the propositions where rejected at the time because it was contrary to perception and senses such that a revolving Earth has to have a moving "great wind" in the air around us
Aristarchus's explanation of the movement of objects in the sky was different from that of other scientists because he argued that Earth rotates on its axis.
The solar system was deeply studied by the early astronomers. Each of them offered an explanation for the movement of objects in the sky. Some of these postulations were inconsistent with scientific evidence.
However, Aristarchus postulated that the sun is at the center of the solar system and that the earth and other planets move round the sun in orbits.
Hence, he suggested that the sun and not the earth was at the center of the solar system.
Learn more; https://brainly.com/question/12075871
PLEASE HELP ASAP !!! WILL GIVE BRAINLIEST
What were the changes to the atomic model over time?
Answers
Answer:
The atomic model started out as a large single ball, then with electrons attached to it. It then became "planetary model" with electrons orbiting at specific levels, and then later one with a nucleus or multiple different particles like protons and neutrons, and electrons orbiting at general (but not specifically predictable) areas.
Explanation:
thank u :)
Los 4 números cuánticos del oxígeno
Answers
ACELLUS LAB-ACID BASE TITRATION
a student overshot the equivalence point when he was using NaOh to determine the concentration of an unknown acid, HA. What ions are present in the purple solution in the flask?
Answers
Answer: A-, NA+ And OH-
Explanation: Thank me later
how many resonance structures can be drawn for ozone, o3 ? express your answer numerically as an integer view available hint(s)
Answers
The actual bond lengths in ozone are intermediate between a single bond and a double bond.
Why will be resonance structures can be drawn for ozone, o3?There are three resonance structures that can be drawn for ozone, O3. The Lewis structure of ozone shows that it has one double bond and one single bond between the three oxygen atoms. The resonance structures involve moving the double bond to different positions around the molecule, while maintaining the overall charge distribution and number of valence electrons.
The three resonance structures for ozone are:
O = O - O+O - O = O+O+ - O = OEach of these resonance structures has a partial double bond between one of the oxygen atoms and the central oxygen atom
Learn more about ozone.
brainly.com/question/14330630
#SPJ11
Jasmine's friend was rollerblading at 12 meters per second. To try to catch up with her friend, Jasmine ran 98meters down the street at a constant velocity. She ran that distance in 35seconds. What was Jasmine's velocity?
Answers
Jasmine's velocity was 2.8 meters per second. However, we don't know if this was enough to catch up with her friend who was rollerblading at 12 meters per second.
What is Velocity?
The pace at which the position of an object changes with respect to time is known as its velocity. Due to the fact that it is a vector quantity, it possesses both magnitude and direction.
Mathematically, velocity is expressed as:
velocity = displacement / time
where displacement is the change in an object's position from one point to another, and time is the duration of the motion. Velocity is measured in units of distance per time, such as meters per second (m/s) or kilometers per hour (km/h).
To calculate Jasmine's velocity, we need to use the formula:
velocity = distance / time
Jasmine ran a distance of 98 meters in 35 seconds, so her velocity is:
velocity = 98 meters / 35 seconds
velocity = 2.8 meters per second
Therefore, Jasmine's velocity was 2.8 meters per second. However, we don't know if this was enough to catch up with her friend who was rollerblading at 12 meters per second.
Learn more about Velocity from given link
https://brainly.com/question/80295
#SPJ1
plz help this was due yesterday butttttt i didn’t want to do it...

Answers
Answer:
It is the same it has cycles related to it (that's what I could think of)
What are the conditions that are required for electrical energy to be present in an electrical circuit?
Answers
Answer:
a supply of electric charges which are free to flow, some form of push to move the charges through the circuit and a pathway to carry the charges.
Explanation:
There's your answer have a good day
At 25oc, enough distilled water is added to a 30.0 ml sample of hno3(aq) with a ph of 4.20 so that the final ph of the diluted solution is 5.20. the volume of distilled water added to the original solution is closest to
Answers
The volume of distilled water added to the original solution is closest to 300.0 ml.
What is pH?The potential of hydrogen is a measure of the acidity or alkalinity of a solution equal to the common logarithm of the reciprocal of the concentration of hydrogen ions in moles per cubic decimetre of solution.
Concentration of 30.0 ml sample of \(HNO_3\) solution is = 6.31 X \(10^{-5}\) M
pH of diluted \(HNO_3\) solution = 5.20
We know that,
\(pH= -Log [H^+]\)
\(5.20= -Log [H^+]\)
\([H^+] = 10^{-5.2} = 6.31 X 10^{-6} M\)
Hence, concentration of unknown diluted \(HNO_3\) solution is 6.31 X \(10^{-5}\) M.
By the use of the equation:
\(HNO_3\) Concentration = \(HNO_3\) diluted
\(V_{dil} = \frac{(C_conc X V_conc)}{(C_{dil})}\)
\(V_{dil}\) = (6.31 X \(10^{-5}\) M X 30.0 ml) ÷ (6.31 X 10^{-6} M)
\(V_{dil} = 300.0 ml\)
Hence, the volume of distilled water added to the original solution is closest to 300.0 ml.
Learn more about pH here:
https://brainly.com/question/15289741
#SPJ1
Select the correct answer from each drop-down menu. sarah recently learned that o2 is found in the earth’s water bodies. she was amazed to know the proportion of o2 found in the ocean differs based on the water temperature. what fact did she find out? sarah recently learned that o2 is found in the earth’s water bodies. she was amazed to know the proportion of o2 found in the ocean differs based on the water temperature. she found out that parts of the oceans have a higher quantity of dissolved o2, whereas parts of the ocean have a lesser quantity of it.
Answers
Sarah discovered that the proportion of O2 in the ocean differs based on the water temperature. Some parts of the ocean have more dissolved O2, while other parts have less.
Sarah recently learned that the proportion of O2 found in the ocean differs based on the water temperature. Specifically, she found out that parts of the ocean have a higher quantity of dissolved O2, while other parts have a lesser quantity of it. This means that the amount of O2 present in the ocean can vary depending on the temperature of the water.
To know more about ocean visit:-
https://brainly.com/question/11803537
#SPJ11
Write a Balanced Equation
H2 + Cl2 --> __HCl
Answers
Answer:
2HCL
Explanation:
Both the elements have 2 so when placing the 2 infront ,it'll distribute/ apply to both
consider chlorine, which occurs as a mixture of two isotopes: chlorine-35, with a natural abundance of 75.8% and an exact mass of 34.97 amu and chlorine-37, with a natural abundance of 24.2% and an exact mass of 36.97 amu. what is the average atomic mass of chlorine? report to two decimal places.
Answers
If the natural abundance of chlorine-35 is 75.8% and that of chlorine-37 is 24.2%, then the average atomic mass of chlorine will be 35.44 amu.
Chlorine exists in two isomeric forms which are the chlorine-35 and chlorine-37. The atomic mass of the chlorine elements is basically the average atomic mass of its isotopes depending upon their abundance in nature.
The atomic mass of chlorine-35 is 34.97amu and a natural abundance equal to 75.8 %. The atomic mass of chlorine-37 is equal to 36.97 amu with an abundance of 24.2%.
To calculate the average atomic mass of chlorine,
The abundance of chlorine-35 = 75.8% = 0.758
The abundance of chlorine-37 = 24.2% = 0.242
⇒ Average atomic mass = 34.97 × 0.758 + 36.97 × 0.242
⇒ Average atomic mass = 26.50 + 8.94
⇒ Average atomic mass = 35.44 amu
To know more about chlorine here
https://brainly.com/question/11008051
#SPJ4
_____ is used in formal laboratories to put out fires or douse students or instructors who may have caught fire or suffered a large chemical spill.
Answers
Fire extinguishers are used in formal laboratories to put out fires or douse students or instructors who may have caught fire or suffered a large chemical spill.
Fire extinguishers are essential safety devices found in formal laboratory settings. They are designed to combat different types of fires, including those caused by flammable materials, electrical equipment, or combustible liquids.
In the event of a fire, using a fire extinguisher can help prevent the spread of flames and minimize potential damage or injuries. Fire extinguishers typically contain substances that can rapidly extinguish fires by removing one or more elements of the fire triangle: fuel, oxygen, or heat.
In laboratories, fire extinguishers are strategically placed in easily accessible locations to ensure quick response in case of an emergency. Different types of fire extinguishers are used, depending on the potential fire hazards present in the laboratory environment.
Learn more about Fire extinguishers from the link given below.
https://brainly.com/question/3905469
#SPJ4
write 5.5 x 104 in standard form
Answers
Answer: 55,000
Explanation:
Scientific Notation: 5.5 x 10⁴
Standard form: move the decimal 4 places to the right
5 5 0 0 0 0.
= 55,000
Answer:
5.72×10^2
Explanation:
5.5×104
=55×104
=5720÷10
=572
=5.72×10^2
why don't birds get electrocuted when they stand on the light post wire
Answers
Answer:
Because they have scaled feet
Answer:
Because they have scaled feet that prevents them from getting electrocuted
Lithium is not nearly as abundant as sodium. If sodium ion batteries that function as lithium ion ones were developed, do you think sodium cobalt oxide would still work as the electrode material?
Answers
Answer:
No, sodium cobalt oxide would not work as the electrode material
Explanation:
Sodium is a much larger cation than lithium. Remember that ion size is a very important consideration when developing a battery. The ions to be substituted must be nearly of the same size if one ion is to replace the other in a crystal.
Since sodium ions are larger than lithium ions, sodium cobalt oxide would not work as the electrode material in Lithium ion batteries.
2Ni(OH) → Ni2O + H2O
How many moles of water forms from 4.20 mol of nickel (I) hydroxide?
Answers
Answer:
2.1 moles of water formed.
Explanation:
Given data:
Moles of water formed = ?
Moles of Ni(OH) = 4.20 mol
Solution:
Chemical equation:
2Ni(OH) → Ni₂O + H₂O
Now we will compare the moles of Ni(OH) with water.
Ni(OH) : H₂O
2 : 1
4.20 : 1/2×4.20 = 2.1 mol
2.1 moles of water formed.
What is the correct electron configuration for Si atomic number
O A. 1s22s22p 3s23p²
OB. 1s22s22p 3s²3p³
O C. 1s22s22p63s²2d³
O D. 1s22s22p63s¹3p³
Answers
1s22s22p63s^13p^3 is correct electron configuration for SI atomic number.
What is electron configuration?
The distribution of electrons in an element's atomic orbitals is described by the element's electron configuration. Atomic electron configurations adhere to a standard notation in which all atomic subshells that contain electrons are arranged in a sequence with the number of electrons they each hold written in superscript. For instance, sodium's electron configuration is 1s22s22p63s1. Long electron configurations are frequently produced by the standard notation. In these circumstances, a shortened or condensed version of the standard notation may be used. The sequence of fully filled subshells that represent the electronic configuration of a noble gas are replaced in the abbreviated notation by the symbol for that noble gas enclosed in square brackets.
To know more about configuration click below
https://brainly.com/question/26084288
#SPJ1
How many grams of Sulfuric Acid are needed to produce 57.18 g of Lead (IV) Sulfate when being neutralized by a sufficient amount of Lead (IV) Hydroxide? *
Answers
Answer:
40.72g of sulfuric acid are needed
Explanation:
When sulfuric acid, H₂SO₄, is neutralized by lead (IV) hydroxide, Pb(OH)₄, Lead (IV) sulfate, Pb(SO₄)₂ and water as follows:
2 H₂SO₄ + Pb(OH)₄ → Pb(SO₄)₂ + 4H₂O
To solve this question we must find the moles of 57.18g of Pb(SO₄)₂. As 2 moles of H₂SO₄ produce 1mol Pb(SO₄)₂ we can find the moles of H₂SO₄ and its mass as follows:
Moles Pb(SO₄)₂ -Molar mass: 275.23 g/mol-
57.18g * (1mol / 275.23g) = 0.2078 moles Pb(SO₄)₂
Moles H₂SO₄:
0.2078 moles Pb(SO₄)₂ * (2mol H₂SO₄ / 1mol Pb(SO₄)₂) = 0.4155 moles H₂SO₄
Mass H₂SO₄ -Molar mass: 98g/mol-
0.4155 moles H₂SO₄ * (98g / mol) =
40.72g of sulfuric acid are needed20.0 ml of 0.200 m sodium hydroxide is used to completely titrate a vinegar sample. how many grams of acetic acid are present? (the molar mass of acetic acid is 60.0 g/mol.)
Answers
When 20.0 ml of 0.200 M sodium hydroxide is used to completely titrate a vinegar sample, the amount of acetic acid present is 0.24 grams.
In order to determine the grams of acetic acid present in the vinegar sample, we need to calculate the number of moles of acetic acid using the given information.
The volume of sodium hydroxide used in the titration is 20.0 ml, and its concentration is 0.200 M (molar). From this, we can determine the number of moles of sodium hydroxide used:
moles of NaOH = volume (in liters) x concentration
= 0.020 L x 0.200 mol/L
= 0.004 mol
Since acetic acid and sodium hydroxide react in a 1:1 ratio, the number of moles of acetic acid is also 0.004 mol.
The molar mass of acetic acid is given as 60.0 g/mol. Therefore, we can calculate the mass of acetic acid using the equation:
mass = moles x molar mass
= 0.004 mol x 60.0 g/mol
= 0.24 g
Therefore, there are 0.24 grams of acetic acid present in the vinegar sample.
Know more about Vinegar here:
https://brainly.com/question/23700611
#SPJ11
Is this equation equal? Why or Why not?