For the following reaction, 8.71 grams of nitrogen gas are allowed to react with 6.34 grams of oxygen gas . Nitrogen (g) + oxygen (g) -> Nitrogen monoxide (g) What is the maximum amount of nitrogen monoxide that can be formed? What is the formula for the limiting reagent? What amount of the excess reagent remains after the reaction is complete?
Answers
Therefore, the maximum amount of nitrogen monoxide that can be formed is 0.396 mol or 22.1 g. The limiting reagent is O2, and 3.16 g of N2 remains as excess reagent after the reaction is complete
How nitrogen monoxide is formed?To find the maximum amount of nitrogen monoxide that can be formed, we need to determine which reactant is limiting and which is in excess.
First, we need to write and balance the equation for the reaction:
N2(g) + O2(g) → 2NO(g)
Next, we need to calculate the number of moles for each reactant, using their respective molecular weights:
moles of N2 = 8.71 g / 28.02 g/mol = 0.311 mol
moles of O2 = 6.34 g / 32.00 g/mol = 0.198 mol
Based on the balanced equation, the stoichiometric ratio of N2 to O2 is 1:1, meaning that we need equal moles of each to completely react. However, we have more moles of N2 than O2, so O2 is the limiting reagent.
To determine the maximum amount of NO that can be formed, we can use the number of moles of O2 as the basis for our calculation:
moles of NO = 0.198 mol O2 × (2 mol NO / 1 mol O2) = 0.396 mol NO
Finally, we can calculate the amount of excess reagent that remains after the reaction is complete. Since O2 is the limiting reagent, all of the N2 will not be used up. We can calculate the amount of N2 that is not consumed by subtracting the moles of N2 used from the initial moles of N2:
moles of N2 used = 0.198 mol O2 × (1 mol N2 / 1 mol O2) = 0.198 mol N2
moles of N2 remaining = 0.311 mol N2 - 0.198 mol N2 = 0.113 mol N2
To convert this to grams, we can use the molecular weight of N2:
mass of N2 remaining = 0.113 mol N2 × 28.02 g/mol = 3.16 g N2
To know more about limiting reagent visit:-
brainly.com/question/11848702
#SPJ1
Related Questions
c) Discuss precision and Accuracy as they relate to types of errors.
what is the answer
Answers
Precision relates to the consistency and reproducibility of measurements, while accuracy reflects how close measurements are to the true value.
Precision and accuracy are two important concepts in the context of errors in measurements. While they both pertain to the quality of data, they refer to different aspects.
Precision refers to the degree of consistency or reproducibility in a series of measurements. It reflects the scatter or spread of data points around the average value. If the measurements have low scatter and are tightly clustered, they are considered precise. On the other hand, if the measurements have a high scatter and are widely dispersed, they are considered imprecise.
Accuracy, on the other hand, refers to the closeness of measurements to the true or target value. It represents how well the measured values align with the actual value. Accuracy is achieved when measurements have a small systematic or constant error, which is the difference between the average measured value and the true value.
Errors in measurements can be classified into two types: random errors and systematic errors.
Random errors are associated with the inherent limitations of measurement instruments or fluctuations in the measurement process. They lead to imprecise data and affect the precision of measurements. Random errors can be reduced by repeating measurements and calculating the average to minimize the effect of individual errors.
Systematic errors, on the other hand, are caused by consistent biases or inaccuracies in the measurement process. They affect the accuracy of measurements and lead to a deviation from the true value. Systematic errors can arise from factors such as instrumental calibration issues, environmental conditions, or experimental techniques. These errors need to be identified and minimized to improve the accuracy of measurements.
In summary, precision refers to the degree of consistency or reproducibility of measurements, while accuracy refers to the closeness of measurements to the true value. Random errors affect precision, while systematic errors affect accuracy. To ensure high-quality measurements, both precision and accuracy need to be considered and appropriate techniques should be employed to minimize errors.
Know more about Precision here:
https://brainly.com/question/30461151
#SPJ8
How much time did it take Little Debbie to run 15 m if she was running 3 m/s?
Answers
Time did it take a little debbie to run 15 m if she was running 3 m/s is 5 sec.
debbie running speed = 3 m/s
so,
in one meter = 1/3 sec
we have to calculate the time taken by little debbie to run 15 meters ,
therefore,
Time = distance / speed
time take by debbie to run 15 m = 15 / 3 sec
= 5 sec.
so, time taken by little dubbie to cover the distance of 15 meter is 5 sec.
Hence, Time did it take a little debbie to run 15 m if she was running 3 m/s is 5 sec.
To learn more more distance and time here
https://brainly.com/question/13311948
#SPJ1
What is the name of PbS2
Answers
Answer:
Lead sulfide.
Explanation:
What is the mass of 1.0 × 10^9 molecules of aspartame?
Answers
Answer:
294.3 g/mol b.
The mass of 1.0*10^9 molecules of aspartame is 2.943*10^11 g/mol
ASPARTAME
Aspartame is used as an artificial non-saccharide sweetener 200 times sweeter than sucrose, and is commonly used as a sugar substitute in foods and beverages.
CALCULATION
mass of one molecule of aspartame = 294.3g/mol
mass of 1.0*10^9 molecules of aspartame =294.3*(1.0*10^9) g/mol
=2.943*10^11 g/mol
refer https://brainly.com/question/25225559
#SPJ2
Calculate the volume of 986 µg of a gas whose density is 4.8 x 10-4 g/mL. Express your answer in milliliters using the correct number of significant figures. Do not enter your answer using scientific notation
Answers
Answer:
2,050,000,000,000 mL
Explanation:
To find the volume in mL, you need to (1) convert the mass from µg to g and then (2) calculate the volume (by multiplying the mass by the density). It is important to arrange the conversion in a way that allows for the cancellation of units (the desired unit should be in the numerator). Discrediting the sig figs of the density (because official conversions do not affect sig figs), the final answer should have 3 sig figs like the given mass.
(Step 1)
1 x 10⁻⁶ µg = 1 g
986 µg 1 g
---------------- x -------------------- = 9.86 x 10⁸ g
1 x 10⁻⁶ µg
(Step 2)
9.86 x 10⁸ g 1 mL
----------------------- x ---------------------- = 2.05 x 10¹² mL
4.8 x 10⁻⁴ g
= 2,050,000,000,000 mL
The above graph shows the journey of squirrel for a certain interval. Which of the following describes the motion of the squirrel between 5 s and 8 s?
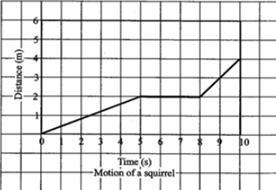
Answers
The statement that describes the motion of the squirrel between 5s and 8s is as follows: The squirrel's speed did not change (option C).
What is a graph?A graph in mathematics and statistics is a data chart (graphical representation of data) intended to illustrate the relationship between a set (or sets) of numbers (quantities, measurements or indicative numbers) and a reference set, whose elements are indexed to those of the former set(s) and may or may not be numbers.
According to this question, a graph is used to illustrate the relationship between the distance moved by a squirrel and the time it took it to move i.e. the journey of squirrel for a certain interval or period of time.
Based on the graph above, the speed increased with time up until 5 seconds, however, from 5seconds till 8 seconds, the speed of the squirrel remained constant i.e. did not change until it picked up again.
Therefore, option C is the correct description of the situation of the squirrel between 5s to 8s.
The options to the incomplete question are as follows:
A. The squirrel's speed increased
B. The squirrel's speed decreased
C. The squirrel's speed did not change
D. The squirrel moved backward
Learn more about graph at: https://brainly.com/question/17267403
#SPJ1
Which of the following is an observation of a chemical property?
Ozinc reacts with hydrochloric acid
density of wood is 0.51 g/cm³
water boils at 100°C.
sand paper is roughly textured
Answers
The right answer to the previous question and the correct observation of a chemical property is that zinc reacts with hydrochloric acid.
ZnCl2 +H2 = Zn +2HCL
Balanced equation:
ZnCl2 + H2 Zn + 2HCL
Zinc is a chemical element, designated by the symbol Zn and the atomic number 30. When the oxidation is removed, zinc turns shiny-greyish and at room temperature turns into a somewhat brittle metal.
All living things, including people, animals, plants, and microorganisms, depend on the trace metal zinc. It is the trace metal that is present in people in the second-highest concentration after iron. Zinc is an essential nutrient for development and a key cofactor for various enzymes. A lack of zinc can lead to a variety of diseases. Deficiency can lead to diarrhoea, infection susceptibility, and slower growth.
To know more about element, visit:
https://brainly.com/question/14347616
#SPJ1
How many moles of oxygen (02) are required to produce 40 liters of water (H20) if there is excess hydrogen (H2)?
Answers
According to the above-balanced reaction, 2 moles of hydrogen and 1 mol of oxygen combine to form 2 moles of water.
How much water is created when 2h2 O2 is combined with 2h2o?As long as there is one mole of O2, 2 H2 + O2 2 H2O produces the same amount of water as H2. Hence, if you start wit 3 mole of H2 and 1.5 moles with O2, you will end up with 3 mole of H2O.
12 mole of H2O are created when extra H2 reacts with how many of O2?Response and justification 6 moles more oxygen gas must be produced in order to make 12 moles of water. This is due to the fact that water and hydrogen mix according to the equation for balance: O2 (g) + 2H2 (g) = 2H2O (g) 2 H2 (g) with O2 (g) results in 2 H2 O (g).
To know more about reaction visit:
https://brainly.com/question/28984750
#SPJ1
A sample of carbon monoxide initially at 79.0 °C was heated to 158 °C. If the volume of the carbon monoxide sample is 990.4 mL at 158 °C , what was its volume at 79.0 °C?
Answers
Answer:
V₁ = 808.9mL
Explanation:
Based on Charles's law, the volume of a sample of gas is directely proportional to its absolute temperature. The formula is:
\(\frac{V_1}{T_1} =\frac{V_2}{T_2}\)
Where V is volume and T is absolute temperature of 1, initial state and 2, final state of the gas.
The initial state is:
V₁ = Our incognite
T₁ = 79°C + 273.15 = 352.15K
Final state is:
V₂ = 990.4mL
T₂ = 158°C + 273.15 = 431.15K
Replacing:
\(\frac{V_1}{352.15K} =\frac{990.4mL}{431.15K}\)
V₁ = 808.9mLConsidering the Charles's Law, the volume at 79 °C is 808.86 mL.
Charles's Law consists of the relationship that exists between the volume and the temperature of a certain quantity of ideal gas, which is maintained at a constant pressure.
This law says that for a given sum of gas at a constant pressure, as the temperature increases, the volume of the gas increases and as the temperature decreases, the volume of the gas decreases because the temperature is directly related to the energy of the movement they have the gas molecules.
In summary, Charles's law is a law that says that when the amount of gas and pressure are kept constant, the quotient that exists between the volume and the temperature will always have the same value:
\(\frac{V}{T}=k\)
Studying two different states, an initial state 1 and a final state 2, it is satisfied:
\(\frac{V1}{T1}=\frac{V2}{T2}\)
In this case, you know:
V1= ?T1=79 C= 352 K (being 0 C= 273 K)V2= 990.4 mLT2= 158 C= 431 KReplacing in Charles's law:
\(\frac{V1}{352 K}=\frac{990.4 mL}{431 K}\)
Solving:
\(V1=\frac{990.4 mL}{431 K}x352 K\)
V1=808.86 mL
Finally, the volume at 79 °C is 808.86 mL.
Learn more:
https://brainly.com/question/4147359?referrer=searchResultshttps://brainly.com/question/11442294Insert the term that correctly completes the paragraph.
Illustration of a rock showing that the layers are not stacked horizontally and look like a rainbow of layers.
Soledad studied rocks and how they help show the history of Earth. She knew that scientists use different ways to find out how old rock layers are and recognized one such example in the rock in the image. The image is an example of a/an
rock.
Answers
Gravity causes spacecraft such as stars, planets, moons, and other bodies to orbit one another. Revolution describes this style of movement.
What is Gravity ?Gravity causes spacecraft such as stars, planets, moons, and other bodies to orbit one another. Revolution describes this style of movement. Space is also home to several stationary things. Rotation describes this movement.We experience day and night because of how long it takes the Earth to complete one rotation. A year is the length of one Earth rotation around the sun. Due to their varying rates of rotation and revolving, other planets have days and years that differ from our own.In the same direction, every planet in our solar system orbits the sun. In addition, the majority of them rotate in the same direction (with the exception of Venus and Uranus). In the cosmos, approximately half of all galaxies rotate in a clockwise direction, and the other half in a counterclockwise direction. This may be due to the manner that the cosmos started, according to scientists.To Learn more About Gravity refer To:
https://brainly.com/question/557206
#SPJ1
Which species in the reaction below is being reduced?
H2O(g) + CO(g) → H2(g) + CO2(g)
Answers
Answer:
Therefore, H2O is being reduced, while CO is being oxidized.
Explanation:
In a chemical reaction, a species that gains electrons is being reduced, while a species that loses electrons is being oxidized.In the reaction given, H2O is gaining electrons, while CO is losing electrons.
En una práctica experimental, para la obtención de cloruro cobaltoso, se hacen reaccionar 120 g de sulfuro cobaltoso de 60% de pureza con 30 cm3 de ácido nítrico concentrado (densidad 1,142 g/cm3, 69,8% en peso de HNO3), en presencia de ácido clorhídrico concentrado (densidad 1,19 g/cm3, 37,33 % en peso de HCl). Calcular:
a) El volumen de ácido clorhídrico concentrado que se requiere para la reacción.
b) La cantidad máxima de cloruro de cobalto (II) que se puede preparar.
c) El número de moléculas de monóxido de nitrógeno que se deprenden.
d) El número de átomos de azufre que se forman.
e) El número de moles de agua que se obtiene.
CoS + HNO3 + HCl → CoCl2 + NO + S + H2O
Answers
Answer: D
Explanation:
Utilicé traductor de español para responder esta pregunta
Sodium hydroxide neutralises hydrochloric acid as shown in the equation:
NaOH(aq) + HCl(aq) i NaCl(aq) + H2O(l)
(4)
dm3
(3)
The student found that 27.20 cm3 of 0.100 moles per dm3 sodium hydroxide neutralised 5.00 cm3 of hydrochloric acid.
Calculate the concentration of the hydrochloric acid in moles per dm3.
Give your answer to three significant figures.
Answers
Explanation:
Mole ratio of NaOH : HCl = 1: 1
Moles of NaOH used = 0.1 mol/1000 cm3 × 27.20 cm3
= 2.72 × 10^-3 mol
Therefore moles of HCl used is also 2.72 × 10^-3 mol
So concentration of HCl can be found by dividing the no.of moles of HCl by the volume of HCl as follows
2.72 × 10^-3 mol/ 5cm3
1000cm3 = 1dm3
Therefore,
1cm3 = 1/1000 dm3
5cm3 = 5/1000 dm3
HCl conc. = 2.72 × 10^-3 mol/ 5×10^-3 dm3
= 0.544 moldm-3
How do the varying characteristics of Earth's atmospheric layers affect the types of wavelengths that are reflected back into space, absorbed or allowed to pass to Earth's surface?
Answers
The specific characteristics of the layers that make up the Earth's atmosphere affect the types of wavelengths that are reflected. Troposphere, stratosphere, and mesosphere are the three layers.
The stratosphere, which contains the ozone layer, is the next layer. The sun's dangerous UV light is absorbed by ozone, keeping it from reaching the surface of the Earth. Visible light and some infrared radiation can travel through this layer.
The mesosphere is located above the stratosphere, where the majority of meteoroids burn up as they enter the atmosphere of the Earth.
Learn more about stratosphere, here:
https://brainly.com/question/20888230
#SPJ1
the table shows the percentages of some gases in the exhaust from a petrol engine what is the name of the compound that makes up most of the other gases
Answers
The other compound that makes up most of the other gases in the table is water vapor.
What is water vapor?Water vapor is not listed in the table because it is not a pollutant. However, it is a significant component of exhaust gas, and it can contribute to smog formation. Water vapor is formed when the fuel in a petrol engine is burned. The combustion process produces water as a byproduct.
The amount of water vapor in the exhaust gas depends on the temperature of the combustion process. At higher temperatures, more water vapor is produced. Water vapor is not a pollutant in itself, but it can contribute to smog formation.
Find out more on water vapor here: https://brainly.com/question/6345787
#SPJ1
Complete question:
this table shows the percentages of some gases in the exhaust from a petrol engine
nitrogen 68
carbon dioxide 15
carbon monoxide 1
oxygen 0.75
nitrogen oxides 0.24
hydrocarbons 0.005
sulphur dioxide 0.005
other gases
what is the name of the other compound that makes up most of the other gases in the table?
The same mass of 5 different potential fuels was used to heat the same mass of water in a simple calorimeter. The results are shown below. Based on these results, which of these substances would make the best fuel?
Answers
We can see here that the best fuel is the one that produces the most heat per unit mass. In this case, the fuel that produces the most heat per unit mass is methanol.
What is fuel?Fuel is a substance that is used to produce energy through combustion or other chemical reactions. It is commonly utilized to power various forms of transportation, generate heat or electricity, and operate machinery and appliances.
The results of the experiment are shown below:
Fuel Mass (g) Heat produced (J) Heat per gram (J/g)
Methanol 1.0 350 350
Ethanol 1.0 250 250
Propane 1.0 200 200
Butane 1.0 150 150
Pentane 1.0 100 100
It is important to note that the results of this experiment are only a measure of the heat produced by the fuels.
Learn more about fuel on https://brainly.com/question/10172005
#SPJ1
How many formula units are in 50.0g of Pb02?
Answers
There are approximately \(1.258 x 10^2^3\) formula units in 50.0 g of PbO2.
To solve this problem
We must utilize the molar mass of PbO2 (lead dioxide) and the idea of Avogadro's number to calculate the number of formula units in a given mass of PbO2.
The molar mass of PbO2 is calculated as follows:
1 atom of Pb (lead) has a molar mass of approximately 207.2 g/mol.
2 atoms of O (oxygen) have a combined molar mass of approximately 32.0 g/mol (16.0 g/mol per oxygen atom).
Therefore, the molar mass of PbO2 is:
Molar mass of PbO2 = (1 * molar mass of Pb) + (2 * molar mass of O)
= (1 * 207.2 g/mol) + (2 * 16.0 g/mol)
= 207.2 g/mol + 32.0 g/mol
= 239.2 g/mol
Now, we can use the molar mass to determine the number of formula units in 50.0 g of PbO2.
Number of moles = Mass (in grams) / Molar mass
= 50.0 g / 239.2 g/mol
≈ 0.209 moles (rounded to three decimal places)
Since 1 mole of any substance contains Avogadro's number of particles \((approximately 6.022 x 10^2^3),\)we can calculate the number of formula units by multiplying the number of moles by Avogadro's number:
Number of formula units = Number of moles * Avogadro's number
\(= 0.209 moles * (6.022 x 10^2^3 formula units/mole)\)
≈\(1.258 x 10^2^3 formula units\)
Therefore, there are approximately\(1.258 x 10^2^3\) formula units in 50.0 g of PbO2.
Learn more about molar mass here : brainly.com/question/21334167
#SPJ1
reaction will be spontaneous at all temperatures if _____
Answers
If a reaction has a negative ΔG and a positive ΔS, the reaction will be spontaneous at all temperatures.
If a reaction is spontaneous at all temperatures, it implies that the reaction will occur without the need for any external intervention, such as the addition of energy. For a reaction to be spontaneous, it must satisfy the criteria of thermodynamic favorability, which is determined by the change in Gibbs free energy (ΔG) associated with the reaction.
The relationship between ΔG, temperature (T), and the equilibrium constant (K) of a reaction is described by the equation ΔG = ΔH - TΔS, where ΔH is the change in enthalpy and ΔS is the change in entropy.
To ensure spontaneity at all temperatures, two conditions must be met:
ΔG must be negative: A negative ΔG indicates a thermodynamically favorable reaction, meaning the products have a lower Gibbs free energy than the reactants. If ΔG is negative, the reaction will proceed spontaneously in the forward direction.
ΔS must be positive: A positive ΔS signifies an increase in the overall entropy of the system. Higher entropy means more disorder, and spontaneous reactions often involve an increase in randomness. When ΔS is positive, it can compensate for the enthalpic term, ΔH, allowing the reaction to proceed spontaneously.
For more such questions on spontaneous visit:
https://brainly.com/question/30127476
#SPJ8
A 10.00 mL sample of a solution containing formic acid (a weak acid) was placed in a 25 mL volumetric flask and diluted to the mark with water. A 10.00 mL sample of the diluted formic acid solution was then titrated with 0.1322 M sodium hydroxide. The titration required 15.80 mL of sodium hydroxide to reach the equivalence point. Calculate the molarity and the percentage (by mass) formic acid in the original solution. The density of the formic acid solution was found to be 1.02 g/mL.
Answers
Answer:
Molarity: 0.522M
Percentage by mass: 2.36 (w/w) %
Explanation:
Formic acid, HCOOH reacts with NaOH as follows:
HCOOH + NaOH → NaCOOH + H₂O
To solve this question we must find the moles of NaOH added = Moles formic acid. Taken into account the dilution that was made we can find the moles -And molarity of formic acid and its percentage by mass as follows:
Moles NaOH = Moles HCOOH:
0.01580L * (0.1322mol / L) =0.002089 moles HCOOH
Moles in the original solution:
0.002089 moles HCOOH * (25mL / 10mL) = 0.005222 moles HCOOH
Molarity of the solution:
0.005222 moles HCOOH / 0.01000L =
0.522MMass HCOOH in 1L -Molar mass: 46.03g/mol-
0.522moles * (46.03g / mol) = 24.04g HCOOH
Mass solution:
1L = 1000mL * (1.02g / mL) = 1020g solution
Mass percent:
24.04g HCOOH / 1020g solution * 100
2.36 (w/w) %
Which scatterplot shows a positive association between the variables
Answers
Answer:
there is no picture, but it will be a graph that shows a mostly straight line going up to the right
Explanation:
The partial pressure of F2 and a mixture of gases were the total pressure is one ATM. What is the mole fraction of F2?
Answers
The partial pressure of F2 and a mixture of gases where the total pressure is one atm. The mole fraction of F2 is 0.394736.
What is mole fraction ?
The term mole fraction is defined as the number of molecules of a component in a mixture is divided by the total number of moles in the given mixture.
Total pressure = 1 atm
= 760 torr
Then,
The partial pressure of F2 = 300 torr
The mol fraction of F2 = PF2/PT
= 300/760
= 0.394736
Thus, The partial pressure of F2 and a mixture of gases where the total pressure is one atm. The mole fraction of F2 is 0.394736.
To learn more about the mole fraction, follow the link;
https://brainly.com/question/8076655
#SPJ1
Explain how the following reaction demonstrates that matter is neither created or destroyed in a chemical reaction: Ca(OH)2 + 2HCI-> CaCl2 + 2H20
Answers
Answer:
In this reaction, Ca(OH)2 is a reducing agent. It reacts with hydrogen chloride to form calcium chloride and water. Therefore, the following reaction shows that matter is neither created nor destroyed in a chemical reaction: Ca(OH)2 + 2HCI -> CaCl2 + 2H20. The formation of calcium chloride and water from the hydrolysis of calcium hydroxide is not an example of matter being created or destroyed in a chemical reaction because it does not involve the breaking down of any bonds between atoms.
Explanation:
These building bricks are in the same container. What type of matter would it represent?
A. a mixture consisting of two compounds
B. a mixture consisting of two elements
C. a compound
D. a mixture of an element and a compound

Answers
The container's building bricks are a type of material known as a mixture since it consists of two different components.
What is being displayed?A mixture of two chemicals would be the kind of substance represented in the diagram. This is so because the first brick is made of two separate elements, one of which is yellow and the other of which is black. The other block has a red and blue color scheme to indicate that it is also a composite made up of two separate elements.
What are combinations and substances?Chemical reactions between two or more elements can result in the formation of compounds. Physical mixing of two or more substances results in the formation of mixtures. 2. Types. Three categories of compounds are possible.
To know more about Mixtures and compounds visit: brainly.com/question/13729453
#SPJ1
Who ever does this, get brainiest.

Answers
Answer:
C.
Explanation:
They will need to know the influence of gravitational force on objects because gravity can affect an objects weight.
A 550.0 mL sample of gas at 40.0 °C and 895 torr is transferred to a second vessel where the temperature is 0.0 °C and the pressure is 745 torr. What is the volume of the second vessel?
Answers
The volume of the second vessel is approximately 450 mL. In physics and chemistry, pressure is an important concept that is used to describe the behavior of gases, liquids, and solids under different conditions.
What is Pressure?
It is a scalar quantity and is expressed in units such as pascals (Pa), pounds per square inch (psi), atmospheres (atm), or torr. Pressure is created by the collision of particles (atoms or molecules) with the walls of a container, and it can be influenced by factors such as temperature, volume, and the number of particles present.
The first step is to use the combined gas law to relate the initial conditions to the final conditions:
(P1V1)/T1 = (P2V2)/T2
where P is pressure, V is volume, and T is temperature, with subscripts 1 and 2 representing the initial and final conditions, respectively.
Plugging in the given values, we get:
(895 torr)(550.0 mL)/(313.15 K) = (745 torr)(V2)/(273.15 K)
Solving for V2, we get:
V2 = (895 torr)(550.0 mL)/(313.15 K) * (273.15 K)/(745 torr) ≈ 450 mL
Therefore, the volume of the second vessel is approximately 450 mL.
Learn more about Pressure from given link
https://brainly.com/question/28012687
#SPJ1
help!! An atomic number of 4, an atomic mass of 8 and a charge of +1.
Answers
Answer:
berylium
Explanation:
atomic number 4 atomic mass 8 and charge +1
A solid is heated to its melting point and the resulting liquid is heated. Choose the graph below that shows these temperature changes.

Answers
I need help calculating the error % in molar mass
Answers
Error % = |(experimental - actual) / actual| x 100%
For example, let's say the actual molar mass of a compound is 100 g/mol, and the experimental molar mass determined in the lab is 95 g/mol. The error percentage would be:
Error % = |(95 - 100) / 100| x 100%
Error % = |-0.05| x 100%
Error % = 5%
Therefore, the error percentage in molar mass is 5%
Please explainnnnnnnnnn

Answers
The mass of a substance when given the density and volume can be found by using the formula
Mass = Density x Volume
From the question
Density of chloroform=1.5 g/ml
Volume=10mL
We have
Mass=1.5x10
We have the final answer as
15g
Volume=10ml
Density=1.5g/ml
\(\boxed{\sf Density=\dfrac{Mass}{Volume}}\)
\(\\ \sf\longmapsto Mass=Density\times Volume\)
\(\\ \sf\longmapsto Mass=1.5(10)\)
\(\\ \sf\longmapsto Mass=15g\)
Indicate the type and number of orbitals in each of the following energy levels or sublevels.
a. n = 1
b. 3p sublevel
Answers
Quantum numbers are a set of four numbers used to describe the quantum state of an electron in an atom. These numbers specify the energy, orbital shape, orientation, and spin of the electron.
What is Energy Sublevels?
Energy sublevels, also known as subshells, are regions within an energy level where electrons are likely to be found. Each energy sublevel is characterized by a unique shape and orientation in space, and can contain a specific number of electrons.
The energy sublevels within an energy level are arranged in order of increasing energy, with the s sublevel being the lowest in energy and the f sublevel being the highest in energy. The number and arrangement of electrons in an atom's energy sublevels determine its electronic configuration and its chemical and physical properties.
a. When n = 1, there is only one energy level and it contains one s orbital.
b. The 3p sublevel corresponds to the third energy level (n=3) and consists of three p orbitals. The p orbitals are oriented along the x, y, and z axes and can hold a total of 6 electrons (2 electrons per orbital). Therefore, the 3p sublevel contains three p orbitals.
Learn more about Energy Sublevels from given link
https://brainly.com/question/29344931
#SPJ1