For some reason my bff think's people also use this website for dating is that true?
Answers
For some reason my bff think's people also use this website for dating that is true.
What is mean by dating ?to spend time with someone you are in a love connection with on a frequent basis: Before getting married, they were together for five years.A stage of romantic relationships known as dating involves two people participating in an activity together with the goal of determining if they would make a good companion for an intimate relationship down the road.Dating: The 3 Types
Service Dating Duty Dating is practicing your dating techniques; it is not dependent on chemistry.actual dating Real dating occurs when two people are attracted to one other and go on dates, whereas courting occurs when both people are actively seeking a partner.Courtship.To learn more about dating refer to:
https://brainly.com/question/868048
#SPJ1
Related Questions
If earths glaciers were to melt what would be the most likely effect in florida
Answers
Answer:
Well if glaciers were to most likley melt wit would have a big affect on the world. But on Florida the affect would be as everybody would expect, a flash flood, with high water's and everything under water. AND there would most lilkley be something in the glacier. Not highly sure about it though
Explanation:
Hope this helps!
Pls mark brainlest!
Which pair of compounds represents one Arrhenius acid and one Arrhenius base?
1. CH3OH and NaOH
2. HNO3 and NaOH
3. CH3OH and HCI
4. HNO3 and HCI
Answers
Answer: C HNO3 and NaOh
Explanation:
What is the molar concentration a a 12 % sodium chloride solution (MW 58.5)
Answers
The molar concentration of a 12% sodium chloride solution is approximately 2.05 M.
To determine the molar concentration of a 12% sodium chloride solution, we need to convert the given percentage concentration into molarity.
First, we need to understand that the percentage concentration refers to the mass of the solute (sodium chloride) relative to the total mass of the solution.
In this case, a 12% sodium chloride solution means that there are 12 grams of sodium chloride in 100 grams of the solution.
To convert this into molar concentration, we need to consider the molar mass of sodium chloride, which is 58.5 g/mol.
We can start by calculating the number of moles of sodium chloride in 12 grams:
Moles of sodium chloride = mass of sodium chloride / molar mass of sodium chloride
Moles of sodium chloride = 12 g / 58.5 g/mol = 0.205 moles
Next, we calculate the volume of the solution in liters using the density of the solution. Since the density is not provided, we assume a density of 1 g/mL for simplicity:
Volume of solution = mass of solution / density
Volume of solution = 100 g / 1 g/mL = 100 mL = 0.1 L
Finally, we calculate the molar concentration (Molarity) by dividing the number of moles by the volume in liters:
Molar concentration = moles of solute / volume of solution
Molar concentration = 0.205 moles / 0.1 L = 2.05 M
Therefore, the molar concentration of a 12% sodium chloride solution is approximately 2.05 M.
To learn more about molarity click here: brainly.com/question/31545539
#SPJ11
Why are pure liquids and solids not included in the reaction quotient or the equilibrium constant expression for a given heterogeneous reaction
Answers
Pure liquids and solids at the equilibrium stage:
Pure solids and pure liquids are not included in expressions for the equilibrium constant of heterogeneous equilibria since their concentrations are constant across time. Therefore, including it in our equilibrium expression is not significant.
Equilibrium expression:
When a certain chemical reaction is in equilibrium, the rates of the forward and backward reactions are equal, which means that there is no net change in the concentrations of the reactants and products and that the reaction mixture's composition does not change.
The concentration of solid and liquid:
Solid or liquid concentration,
= No. of Moles/ Volume in L
= Mass/(Volume × molar mass)
= Density/ Molar mass
Pure solids and liquids have constant densities and molar masses at a constant temperature.
Because of this, they have constant molar concentrations, which means that they are not considered in the equilibrium constant for a heterogeneous reaction.
Learn more about the pure solids, liquids, and heterogenous mixture here,
https://brainly.com/question/1811148
#SPJ4
Help what’s the answer?
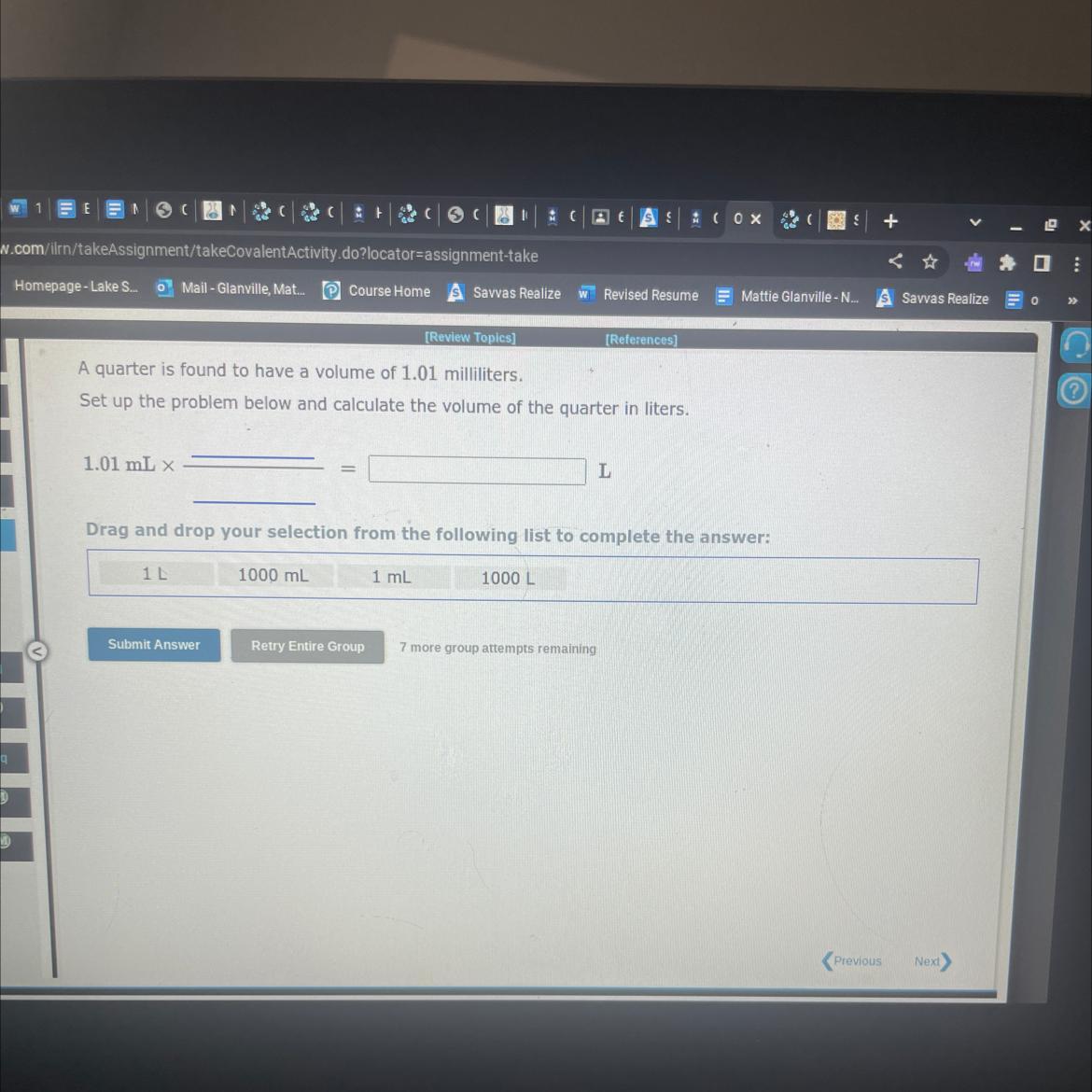
Answers
The supplied solution will have a volume of 1.01 mi, or 0.00101 Liters.
How much is a liter?The metric unit of volume known as the liter is mostly used to measure liquids. Liters are denoted by the letters "L" or "l". Milliliters are the unit of measurement for smaller liquid amounts (mL). 1000 milliliters make up one liter.
What is equivalent to 1 liter?The liter is a metric unit of volume that can be written with the letters L or l in the SI. Its American English equivalent is the liter. It is equivalent to one cubic decimeter (dm3), one thousand cubic centimeters (cm3), or one thousandth of a cubic metre (m3).
To know more about solution visit:-
https://brainly.com/question/7932885
#SPJ1
How many grams of barium chloride are produced when 1.25 x 1023 molecules of HCl reacts with barium?
Answers
Considering the reaction stoichiometry and Avogadro's Number, the mass of barium chloride produced is 21.6 grams.
Balanced reaction
The balanced reaction is
2 HCl + Ba → BaCl₂ + H₂
Moles of HCl that reactAvogadro's Number is called the number of particles that make up a substance (usually atoms or molecules) and that can be found in the amount of one mole of said substance. Its value is 6.023×10²³ particles per mole. Avogadro's number applies to any substance.
Then you can apply the following rule of three: if 6.023×10²³ molecules are contained in 1 mole of HCl, then 1.25×10²³ molecules are contained in how many moles of HCl?
amount of moles of HCl= (1.25×10²³ molecules × 1 mole)÷ 6.023×10²³ atoms
amount of moles of HCl= 0.2075 moles
Then, 0.2075 moles of HCl react.
Reaction stoichiometryBy reaction stoichiometry (that is, the relationship between the amount of reagents and products in a chemical reaction), the following amounts of moles of each compound participate in the reaction:
HCl: 2 moles Ba: 1 mole BaCl₂: 1 mole H₂: 1 moleMass of barium chloride producedThen you can apply the following rule of three: if by stoichiometry 2 moles of HCl produce 1 mole of BaCl₂, 0.2075 moles of HCl will produce how many moles of BaCl₂?
\(amount of moles of BaCl_{2} =\frac{0.2075 moles of HCl x 1 mole of BaCl_{2} }{ 2moles of HCl}\)
amount of moles of BaCl₂= 0.10375 moles
Being the molar mass of BaCl₂ 208.24 g/mole, then the mass of barium chloride produced is calculated as:
\(0.10375 molesx\frac{208.24 grams}{1 mole} =21.6 grams\)
Finally, the mass of barium chloride produced is 21.6 grams.
Learn more about:
Avogadro's Numberbrainly.com/question/11907018?referrer=searchResults brainly.com/question/1445383?referrer=searchResults brainly.com/question/1528951?referrer=searchResultsReaction stoichiometry brainly.com/question/16487206?referrer=searchResults brainly.com/question/14446695?referrer=searchResults brainly.com/question/11564309?referrer=searchResults brainly.com/question/4025026?referrer=searchResults brainly.com/question/18650135?referrer=searchResultsA gas sample with a mass of 0.250g is collected at 150.0°C and 720 mmHg. The volume is 85.0mL. What is the molar mass of the gas?
Answers
The molar mass of the gas, given that 0.250 g of the gas is collected at 150.0 °C and 720 mmHg is 108.7 g/mol
How do i determine the molar mass of the gas?First, we shall obtain the mole of the gas collected. This is shown below:
Volume of gas (V) = 85 mL = 85 / 1000 = 0.085 LTemperature (T) = 150.0 °C = 150 + 273 = 423 KPressure (P) = 720 mmHg = 720 / 760 = 0.947 atmGas constant (R) = 0.0821 atm.L/mol KNumber of mole (n) =?PV = nRT
0.947 × 0.085 = n × 0.0821 × 423
Divide both sides by (0.0821 × 423)
n = (0.947 × 0.085) / (0.0821 × 423)
n = 0.0023 mole
Finally, we shall obtain the molar mass of the gas. This is shown below:
Mass of gas = 0.250 gNumber of mole of gas = 0.0023 mole Molar mass of gas = ?Molar mass = mass / mole
Molar mass of gas = 0.250 / 0.0023
Molar mass of gas = 108.7 g/mol
Thus, the molar mass of the gas collected is 108.7 g/mol
Learn more about molar mass:
https://brainly.com/question/18983376
#SPJ1
help picture down below thanks

Answers
The vacuum layer of a vacuum-insulated thermos prevents conduction and convection heat transmission.
A vacuum-insulated thermos is made to reduce heat transfer and maintain the temperature of its contents for extended periods. It is made up of many layers and parts that combine to provide this insulation. The hoover acts as an insulator, blocking both conduction of heat and convection of hot particles through direct contact. Although heat loss is greatly reduced by the vacuum layer, it is not completely stopped as some heat transfer through radiation still occurs.
Therefore, the correct option is C.
Learn more about Conduction, here:
https://brainly.com/question/31201773
#SPJ1
A compound containing a functional group with a C-Z σ bond is often polar because the heteroatom Z is ______ electronegative than carbon. The atom Z has one or more lone pairs of electrons, allowing it to act as both a nucleophile and a _______
Answers
A functional group is a group of atoms within a molecule that is responsible for the characteristic chemical reactions of that molecule. When a functional group contains a C-Z σ bond, the compound is often polar because the heteroatom Z is more electronegative than carbon.
Electronegativity refers to the tendency of an atom to attract electrons towards itself when it is part of a chemical bond. Since the electronegativity of Z is higher than carbon, the electron density in the C-Z bond is shifted towards the Z atom, creating a partial positive charge on the carbon and a partial negative charge on the Z atom.
The atom Z in a functional group with a C-Z σ bond typically has one or more lone pairs of electrons. This makes it a nucleophile, meaning it is attracted to positively charged atoms or molecules and can donate its lone pair of electrons to form a new bond. The Z atom can also act as a leaving group, meaning it can dissociate from the molecule and take its lone pair of electrons with it.
Examples of functional groups with a C-Z σ bond include carbonyl groups (C=O), carboxylic acid groups (COOH), and amine groups (NH2). In each case, the Z atom is more electronegative than carbon, resulting in a polar molecule. The presence of lone pairs on the Z atom also allows it to participate in chemical reactions as both a nucleophile and a leaving group.
To know more about nucleophile
https://brainly.com/question/14052597
#SPJ11
There are 2 gasses, A, B. They weigh 2.46g and 0.5g respectively, and the Volume of A is 3 times the volume of B. A has a molecular mass of 28. What could A be?
(A) NO2
(B) N2O
(C) N2O4
(D) N2O5
Answers
Answer:
B
Explanation:
molecular mass of B is 28
Answer:
(B) N2O is the answer.....................
Really need help with the rest of them, please it's due in 20 minutes

Answers
Answer:
chlorine is for pool
Explanation:
hope is help
1SnO2 + 2H2 = 1Sn + 2 H2O. What is the theoretical yield of Sn that you
will produce if you start with 6.5 grams H2?
Answers
Answer: 193 grams
Explanation:
The gram-formula mass of elemental hydrogen is about 2 g/mol, meaning that if you consume 6.5 grams of elemental hydrogen, you will consume 6.5/2 = 3.25 moles.
This means that since for every 2 moles of hydrogen consumed, 1 mole of tin is produced, we need to find the mass of 3.25/2 = 1.625 moles of tin.
Since tin has an atomic mass of 118.71 g/mol, the theoretical yield of Sn is (118.71)(1.625) = 193 grams.
Spooky, scary skeletons
Send shivers down your spine
Shrieking skulls will shock your soul
Seal your doom tonight
Spooky, scary skeletons
Speak with such a screech
You'll shake and shudder in surprise
When you hear these zombies shriek
We're sorry skeletons, you're so misunderstood
You only want to socialize, but I don't think we should
'Cause spooky, scary skeletons
Shout startling, shrilly screams
They'll sneak from their sarcophagus
And just won't leave you be
Answers
Answer:
I LOVE THIS OMG
help me !! Using the solubility curve, what is the effect of increased temperature on the solubility of KBr in 100 grams of water?

Answers
According to the graph, increasing the temperature of the solution will increase the solubility of KBr.
What is the relation between solubility and temperature?There is direct relationship between solubility and temperature because if one increases the other decreases whereas if one decreases the other is also decreases. The higher temperature of the solution is, the easier a solid will be able to dissolve in that solution while on the other hand, the lower is the temperature of the solution, the solid will not dissolve easily. In the graph, we can see that the line of KBr moves in the upward direction which clearly shows that with increasing temperature, the solubility of KBr is also increases.
So we can conclude that According to the graph, increasing the temperature of the solution will increase the solubility of KBr.
Learn more about solubility here: https://brainly.com/question/9098308
#SPJ1
Which of the following liquids would turn
blue litmus paper red?
1. orange juice
2. milk of magnesia
3.
distilled water mixed with a little baking soda
4. oven cleaner
Answers
Answer:
orange juice because it a type of acid.
A light pulse travels over a 50 km of step-index fiber whose n₁ is 1.4870 and n2 1.4613. How much will a light pulse spread? Ats/= (L x NA2)/(2 cn ₁) OA.4.238 μs OB. 4.328 ns OC 4.238 ns OD.423.8 ms OE. 4.275 s
Answers
To determine how much a light pulse will spread in a step-index fiber, we can use the formula:
Δt = (L * NA^2) / (2 * c * n₁)
where:
Δt is the pulse spread,
L is the length of the fiber (50 km),
NA is the numerical aperture of the fiber,
c is the speed of light in a vacuum, and
n₁ is the refractive index of the fiber (1.4870).
First, let's calculate the numerical aperture (NA) using the refractive indices (n₁ and n₂):
NA = √(n₁^2 - n₂^2)
NA = √(1.4870^2 - 1.4613^2)
NA ≈ 0.206
Next, we can substitute the given values into the formula:
Δt = (50 km * (0.206)^2) / (2 * (3 x 10^8 m/s) * 1.4870)
Δt ≈ 4.238 ns
Therefore, the light pulse will spread approximately 4.238 ns in the step-index fiber. The correct answer is option OC.
To know more about numerical aperture .
https://brainly.com/question/31563574
#SPJ11
If the same test, given at different points in time to the same test takers, yields different scores, then the method typically used to assess this source of error is_________.
Answers
If the same test, given at different points in time to the same test takers, yields different scores, then the method typically used to assess this source of error is alternate form.
What is alternate forms test?
An alternate form or parallel form test is a test which is conducted at different time on the same set of test takers in order to test the reliability of the results obtained.
In conclusion, alternate or parallel form tests is a test of reliability.
Learn more about alternate forms tests at: https://brainly.com/question/14745567
#SPJ1
A solution of aluminum chloride is mixed with a solution of potassium hydroxide to produce a precipitate of aluminum hydroxide and aqueous potassium chloride.
Find Balanced chemical equation
Find complete ionic equation
Answers
Balanced equation:
1 AlCl3 (aq) + 3 KOH (aq) -> 1 Al(OH)3 (s) + 3 KCl (aq)
Ionic equation:
1 Al + 1 Cl3 + 3 K + 3 OH -> 1 Al(OH)3 + 3 K + 3 Cl
Perform the following
mathematical operation, and
report the answer to the
appropriate number of
significant figures.
1204.2 +4.79613 = [ ? ]

Answers
This problem is providing a mathematical expression which the result should be expressed with the correct significant figures. At the end, the result is 1209.0 because of the following:
Significant figures:In science, the use of significant figures is crucial as long numbers are not necessarily required when reporting a numerical value, for that reason the importance of reporting measurements with the correct number of significant figures.
In the case of additions, we perform the normal operation as the first step:
1204.2 +4.79613 = 1208.99613
Next, we round the result to the least number of decimal places, in this case one because 1204.2 has just one decimal place, unlike the 4.79613 which has five, so that we round the 8 up to 9 and leave a 0 as the only decimal place:
1209.0
Learn more about significant figures: https://brainly.com/question/11904364
plz help this is due today........

Answers
The energy released in the reaction F(g) + e⁻ → F(g) is known as theA) Ionization energy B) Electron affinity C) Enthalpy of ionization D) Electronegativity E) Enthalpy of electronegativity
Answers
The energy released in the reaction F(g) + e⁻ → F(g) is known as the Ionization energy. Hence option A is correct.
The ionization energy measures an element's ability to participate in chemical processes that call for the creation of ions or the donation of electrons. It is often connected to the type of chemical bonds that exist between the components in the compounds they form.
The quantity of energy required to eject an electron from an atom is referred to as ionization energy. As we move down a group, ionization energy diminishes. On the periodic table, ionization energy rises from left to right.
The quantity of electrons in the inner shells determines the ionization energy. Ionization energy reduces as the inner electron count rises. Because they serve as a barrier between the electrons in the outermost shell and the nucleus. The screening effect is a name for this phenomena.
To know about ionization energy
https://brainly.com/question/28385102
#SPJ4
How many atoms are in 3.2 moles of N2
Answers
Answer:
38.54 x 10^23 atoms of N2
Explanation:
Each mole contains Avagadro's Number ( 6.022 x10^23) of particles
3.2 x 6.022 x 10^23 = 19.27 x 10^23 MOLECULES
N2 is TWO atoms soooo 2 x 19.27 x 10^23 = 38.54 x 10^23 atoms
In the solar system, most asteroids are ____. (A). Beyond Neptune (B). Orbiting Saturn (C). Between Mars and Jupiter (D). Next to the sun True of false: An object made of ice, dust, and rock that orbits the sun is called an asteroid.
Answers
Answer:
Part 1
In the solar system, most asteroids are;
(C) Between Mars and Jupiter
Part 2
False: An object made of ice, dust, and rock that orbits the Sun is called a Comet
Explanation:
Part 1
The main asteroid belt which is between Mars and Jupiter is the path of the orbit of about 1.9 million asteroids which make up the majority of the known asteroids
Part 2
An asteroids are composed of mainly silicate rocks, and clay for C-type asteroids, nickel-iron, silicate material for the S-type asteroid and nickel-iron, iridium, gold, platinum, palladium, and magnesium for the M-type asteroids
Asteroids are also known to contain olivine and pyroxene minerals, and some asteroids contain water-ice within their interiors
However, a Comet is primarily composed of ice, dust and and rock that orbit the Sun which are as big as a small town in their frozen state.
Therefore, an object made of ice, dust, and rock that orbits the Sun is called a Comet.
Answer:
Its C between Mars and Jupiter
Explanation:
Most asteroids are found in the main asteroid belt located between Mars and Jupiter
The molecular mass of methyl ethanoate is 75. 1 amu. Calculate the molecular mass of propanoic acid, an isomer of methyl ethanoate.
Answers
Molecular mass of propanoic acid an isomer of methyl ethanoate. is 74.1 amu or 74.1 g/mol.
What does isomer mean?Isomers can be defined as molecules with the same number of atoms of the same element but with different structural arrangements and properties.
Since the molecular formulas are the same for isomers, they have the same mass. Methyl ethanoate is an isomer of propionic acid so it has the same mass.
there's two type of isomer, geometric isomers and structural isomers
Geometric isomers are molecules that have the same chemical formula but different atomic arrangements. Geometric isomers have different physical and chemical properties.
An interesting property of geometric isomers is that their groups cannot switch positions independently.
Structural isomers are substances with the same chemical formula but different atomic bonds. Examples of structural isomers are the already mentioned chemicals n-butane and isobutane.
Since the molecular formulas are the same for isomers, they have the same mass.
Methyl ethanoate is an isomer of propionic acid so it has the same mass.
learn more about isomer at https://brainly.com/question/28188244
#SPJ4

Which of the following statements about electron position in an atom is true? PLEASE HELP!
Answers
Answer:
An electron has a negative electrical charge
Explanation:
Copper (II) metal reacts with liquid iodine to form solid copper (II) iodide as the only product.
1. Write a balanced chemical equation for this reaction.
2. When 57.8 g of iodine reacts with excess copper, 49.2 g of copper (II) iodine is produced. Calculate the percentage yield for this reaction.
Answers
1. The balanced equation for the reaction is:
Cu + I₂ -> CuI₂
2. The percentage yield of the reaction is 68%
1. The balanced equation for the reaction between copper and iodine to produce copper(II) iodide is:
Cu + I₂ -> CuI₂
2. How do I determine the percentage yield?
First, we shall obtain the theoretical yield. This can be obataned as follow:
Cu + I₂ -> CuI₂
Molar mass of I₂ = 126.9 × 2 = 253.8 g/molMass of I₂ from the balanced equation = 1 × 253.8 = 253.8 gMolar mass of CuI₂ = 63.55 + (126.9 × 2) = 317.35 g/mol Mass of H₂SO₄ from the balanced equation = 1 × 317.35 = 317.35 gFrom the balanced equation above,
253.8 g of I₂ reacted to produce 317.35 g of CuI₂
Therefore,
57.8 g of I₂ will react to produce = (57.8 × 317.35) / 253.8 = 72.3 g of CuI₂
Now, we shall determine the percentage yield of the reaction. Details below
Actual yield of recation = 49.2 gTheoretical yield of reaction = 72.3 gPercentage yield of reaction =?Percentage yield = (Actual /Theoretical) × 100
Percentage yield of reaction = (49.2 / 72.3) × 100
Percentage yield of reaction = 68%
Learn more about percentage yield:
https://brainly.com/question/16978406
#SPJ1
The nucleus of the radioactive carbon isotope from the passage contains how many neutrons and protons, respectively, before it undergoes any decay?
Answers
The nucleus of the radioactive carbon isotope before any decay contains 6 protons and 8 neutrons.
The radioactive carbon isotope mentioned in the passage is most likely carbon-14 (C-14). Carbon-14 has an atomic number of 6, which means it has 6 protons in its nucleus. Protons are positively charged particles found in the nucleus of an atom.
To determine the number of neutrons in the nucleus of carbon-14, we need to subtract the atomic number (protons) from the atomic mass. Carbon-14 has a mass number of 14, so subtracting the atomic number of 6 from the mass number gives us 8 neutrons.
Therefore, the nucleus of the radioactive carbon isotope before any decay contains 6 protons and 8 neutrons.
In summary:
- Atomic number of carbon-14: 6
- Protons in the nucleus: 6
- Mass number of carbon-14: 14
- Neutrons in the nucleus: 14 - 6 = 8
Learn more about radioactive carbon isotope here:-
https://brainly.com/question/1291732
#SPJ11
Which of the following are subatomic particles? Choose all that apply.
- electron
- proton
- ion
neutron
Answers
Answer:
proton, electron, and neutron
Explanation:
Help. Why are state symbols used in chemical equations?
O A. They tell which reactions will happen and which won't.
B. They identify how much product will be made.
C. They identify what phase the substances are in.
O D. They tell how the atoms are arranged in the substances.
Answers
Answer:
The answer is option C.
They identify what phase the substances are in.
Hope this helps
How can sound waves be used to put out fire