For each of the following substances below, identify the type of bonding between the atoms. Justify your
reasoning

Answers
Answer:
SF4 -Covalent bond
BaBr2 - Ionic bond
Ca - Metallic bond
Explanation:
Sulphur and fluorine are non metals. Nonmetal atoms combine by the sharing of electrons. This is also called a covalent combination(bond).
In BaBr2, a metal is combined with a nonmetal. This combination involves a transfer of electrons from the metal to the nonmetal forming an ion pair. This is otherwise called an ionic bond.
Atoms of a metal are held together by strong metallic bonds that involves positively charged cations and a cloud of electrons.
Related Questions
The simulation shows current in milliamps. Why was this size unit-with the prefix milli-used in this simulatantion
Answers
The prefix "milli-" is used in the simulation to represent the unit of current as milliamps (mA).
The prefix "milli-" is derived from the metric system and represents a factor of one-thousandth (1/1000). In the context of current, using milliamps allows for more convenient and practical measurements in many electrical and electronic applications.
Current is the flow of electric charge, and in most cases, the currents encountered in everyday situations are relatively small. Using milliamps as the unit of current allows for better resolution and ease of measurement compared to using amps, which is the base unit of electric current in the International System of Units (SI).
By using milliamps, the simulation can represent currents that are more commonly encountered in various electrical circuits and devices, making the measurements more practical and relevant to real-world scenarios.
To know more about milliamps click this link -
brainly.com/question/30459530
#SPJ11
physically, a 2d array is stored as a rectangular grid of columns and rows. T/F.
Answers
The statement "physically, a 2D array is stored as a rectangular grid of columns and rows" is true. In computer memory, a 2D array is typically stored as a contiguous block of memory arranged in a rectangular grid of columns and rows.
Each element of the array occupies a specific memory location determined by its row and column indices. The elements are stored in a sequential manner, with consecutive elements belonging to the same row or column being stored next to each other.
This arrangement allows for efficient memory access and indexing based on the row and column coordinates.
By representing the 2D array as a rectangular grid, the physical storage reflects the logical structure of the array, facilitating easy manipulation and retrieval of data in a systematic manner.
To know more about 2D array the refer here :
https://brainly.com/question/32139151#
#SPJ11
Plssss helppp quick it’s for science and I don’t get it

Answers
Question 4 of 10
The ACT science test takes______ minutes.
O A. 60
B. 30
O C. 35
OD. 45
Answers
how many unpaired electrons are there in the complex [co(oh2)4(oh)2]+? 1. 0 (diamagnetic) 2.) 5 3.) 4 4.) 3 5.)1 6.) 2
Answers
The [Co(OH2)4(OH)2]+ complex has four unpaired electrons, which makes it paramagnetic (option 3). Therefore, the correct answer is 3 i.e 4. To determine the number of unpaired electrons in the complex [Co(OH2)4(OH)2]+, we need to first determine the electronic configuration of the complex ion.
The central cobalt atom has a +3 oxidation state, which means it has lost three electrons. The atomic configuration of Co is 1s2 2s2 2p6 3s2 3p6 3d7 4s2. In the complex, the four water molecules (OH2) and two hydroxide ions (OH) are ligands, which donate electron pairs to the central metal atom.
The electronic configuration of the complex ion can be determined using crystal field theory, which predicts that the d-orbitals of the metal are split into two sets of energy levels in the presence of ligands. The d-orbitals that are closest to the ligands have higher energy and are referred to as the "eg" set, while the d-orbitals that are farther away from the ligands have lower energy and are referred to as the "t2g" set.
In an octahedral complex like [Co(OH2)4(OH)2]+, the d-orbitals split into two sets of three orbitals each: the eg set (dx2-y2 and dz2) and the t2g set (dxy, dxz, and dyz). The electrons in the t2g set are lower in energy than those in the eg set, and so the electrons will first fill up the t2g orbitals before occupying the eg orbitals.
The four water molecules (OH2) are neutral ligands and donate electron pairs to the cobalt atom via coordination bonds. Therefore, the electrons from the t2g orbitals will pair up with the electrons from the water molecules to form four coordination bonds. The two hydroxide ions (OH) are anionic ligands and also donate electrons to the cobalt atom. The remaining electrons in the d-orbitals will pair up with the electrons from the hydroxide ions.
For more questions like electrons visit the link below:
https://brainly.com/question/25301188
#SPJ11
Calculate the mass of water produced when 1.89 g of butane reacts with excess oxygen.
Express your answer to three significant figures and include the appropriate units.
Answers
This problem is providing the mass of butane (1.89 g) that undergoes combustion in excess oxygen to produce water and carbon dioxide, so the mass of water product is required and found to be 2.93 g water after the calculations.
Stoichiometry:In chemistry, stoichiometry is used for us to deal with chemical amounts and calculations, based on the balanced chemical equations, molar masses and mole ratios. In such a way, we first write the balanced equation for the combustion of butane as shown below:
\(C_4H_{10}+\frac{13}{2} O_2\rightarrow 4CO_2+5H_2O\)
Where we can see a 1:5 mole ratio of butane to water, which means we can write our stoichiometric setup as follows:
\(1.89gC_4H_{10}*\frac{1molC_4H_{10}}{58.12gC_4H_{10}} *\frac{5molH_2O}{1molC_4H_{10}} *\frac{18.02gH_2O}{1molH_2O} \)
Where the 58.12 g/mol is the molar mass of butane and 18.02 g/mol that of water, so they are used to convert from grams to moles of butane and from moles to grams of water respectively. Hence, the result turns out to be:
\(2.93 g H_2O\)
Learn more about mole ratios: https://brainly.com/question/15288923
An instrument plays a frequency of 266 Hz. Another identical instrument plays a frequency of 400 Hz. How does the wavelength of each of these sound waves compare?
Multiple choice question.
A)
The wavelength of 266 Hz is more than wavelength of 400 Hz.
B)
The wavelength of 266 Hz is less than wavelength of 400 Hz.
C)
The wavelength of 266 Hz is same as wavelength of 400 Hz.
D)
There is no relation between wavelength and frequency.
Answers
Answer:
The wavelength of 266 Hz is more than wavelength of 400 Hz.
Explanation:
In physics, the relationship between frequency and wavelength is an inverse relationship. This means that the higher the frequency, the lower the wavelength. High frequency waves do not travel too far.
The implication of this is that the wavelength of a wave of frequency 266 Hz is more than the wavelength of a wave of frequency 400 Hz. Since longer wavelength corresponds to lesser frequency and shorter wavelength corresponds to higher frequency.
6th grade science plz help Major grade Need help with 4,5, and 6

Answers
at what temperature (in k) would 0.0134650.013465 moles of ch4 in a container with a volume of 972.9972.9 ml have a pressure of 0.9220.922 atm?
Answers
At a temperature of 68.07 K, a container with a volume of 972.9 ml containing 0.013 moles of CH₄ would have a pressure of 0.922 atm.
To find the temperature in Kelvin (K) at which 0.013 moles of CH₄ in a container with a volume of 972.9 ml have a pressure of 0.922 atm, we can use the ideal gas law equation:
PV = nRT
Where:
P = Pressure (in atm)
V = Volume (in liters)
n = Number of moles
R = Ideal gas constant (0.0821 L·atm/(mol·K))
T = Temperature (in Kelvin)
First, we need to convert the volume from milliliters to liters:
V = 972.9 ml * (1 L / 1000 ml) = 0.9729 L
Now we can rearrange the ideal gas law equation to solve for temperature:
T = PV / (nR)
T = (0.922 atm) * (0.9729 L) / (0.013 moles * 0.0821 L·atm/(mol·K))
T = 68.07 K
Therefore, at a temperature of 68.07 K, the given amount of CH₄ in the specified container would have a pressure of 0.922 atm.
To know more about the CH₄ refer here :
https://brainly.com/question/29479680#
#SPJ11
What field of science study earths climates
Answers
Answer:
Climatology
Explanation:
Climatology is the study of climate and how it changes over time. This science helps people better understand the atmospheric conditions that cause weather patterns and temperature changes over time.
How would I solve this problem? How would I find the Atomic Mass for each isotope?
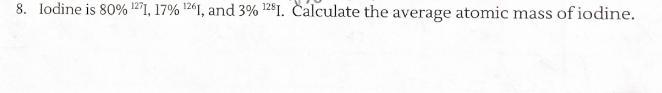
Answers
Answer:
126.86 amu
Explanation:
You divide each percent abundance by 100 and multiply it by the atomic masses and then add them all up to get the average atomic mass.
(.80 x 127) + (.17 x 126) + (.03 x 128) = 126.86 amu
what citric acid intermediates woudl accumulate in the presence of malonate?
Answers
The mitochondrial inner membrane contains succinate dehydrogenase, a respiratory enzyme involved in the citric acid cycle and electron transport chain. Succinate dehydrogenase catalyzes the dehydration of succinate, converting it to fumarate in step 6 of the cycle. Two hydrogens are transferred to FADH2 to reduce FAD.
In the citric acid cycle, α-ketoglutarate is a molecule that is converted to succinate, fumarate, malate and oxaloacetate in the following processes.
Succinate binding of the succinate dehydrogenase reaction is competitively inhibited by malonic acid. Citric acid cycle intermediates such as succinate, succinyl-CoA and α-ketoglutarate accumulate in the presence of malonic acid.
Learn more about the citric acid cycle. Please visit this link
Brainly.com/question/13934424
#SPJ4
Suppose a teaspoon of magnesium filings and a teaspoon of powdered sulfur are placed together in a metal beaker. Would this constitute a mixture or a pure substance? Suppose the magnesium filings and sulfur are heated so that they react with each other, forming magnesium sulfide. Would this still be a “mixture”? Why or why not?
Answers
1) When the powdered sulfur are placed together in a metal beaker, the both are examples of pure substances
2) When the both substances are heated such that magnesium sulfide is formed, it is no more a mixture but a compound.
What is a mixture?The term mixture has to do with a combination of two or more substances that are not chemically combined together. Let us note that a mixture would always retain the properties of the individual substances that do make up the mixture as it were.
When the components of a mixture react chemically then it is no more a mixture because a chemicals bond now exists between the substances and they can no more be separated by means of a physical method.
Learn more about mixture:https://brainly.com/question/24898889
#SPJ1
The volume of container 2 i 27. 32 L. How many mole of the ga are in container 2?
Answers
The number of moles in container 2 is 33.3moles when the container has 27.32L of gas inside it
The number of moles of gas in container 2 can be calculated using the Ideal Gas Law:
n = PV/RT
where n is the number of moles of the gas with known volume,
P is the pressure (assumed to be 1 atm for ideal gases),
V is the volume (27.32 liters),
R is the ideal gas constant (0.0821 L·atm/mol·K) and
T is the temperature (assumed to be 273.15 K).
Plugging in the values, we get:
n = (1 atm)(27.32 L)/(0.0821 L·atm/mol·K)(273.15 K)
n = 33.3 mol
Therefore, there are 33.3 moles of gas in container 2.
To know more about gas laws in chemistry, click below:
https://brainly.com/question/25290815
#SPJ4
In the chloroplast, energy in sunlight is passed around different chlorophyll molecules until it reaches a specific chlorophyll molecule that can transfer energy in sunlight to an energized electron. This chlorophyll molecule is called the accessory pigment. electron-carrier molecule. nucleus. photoelectric point. reaction center.
Answers
Answer:In the chloroplast, energy in sunlight is passed around different chlorophyll molecules until it reaches a specific chlorophyll molecule
Explanation:
Answer: In the chloroplast, energy in sunlight is passed around different chlorophyll molecules until it reaches a specific chlorophyll molecule
a mixture of gases contains 12.0 grams of n2 and 15.0 grams of ar and has a total pressure of 1.32 atm. what is the partial pressure of n2?
Answers
12.0 grams od n2 & 15.0 gram of ar are present in a gas mixture with a pressure head of 1.32 atm. 0.704 atm is the pressure drop of n2.
How does pressure work?Another of the fundamental observable characteristics of the this phase of matter, pressure (P) is understood to be the force of all gas particulate interactions divided by the size of the wall.
What connections does pressure have to chemistry?Pressure has a major impact on every aspect of our life, from of the weather to flying. It is particularly crucial during chemical reactions. Chemists can compel chemical reactions to happen and hasten the transitions among solids, fluids, or gases by adjusting pressure.
Briefing:N=12.0, Ar=15.0
Total pressure=1.32
Partial pressure= n2
WN₂=12.0
WAr =15.0
nN₂=12/28 =0.42
nAr=15/40 =0.37
Xn₂=0.42/0.42+0.37 =0.53
Pn=Xn2×Ptotal
=0.53×1.32=0.69atm that ie nearly equal to 0.7atm.
To know more about pressure visit:
https://brainly.com/question/14760196
#SPJ4
I need help answer with right answer

Answers
Answer:
I think it would be D
Explanation:
How many electron can be found in a p orbital?
Answers
Dating once living organisms is an example of a beneficial use of
Answers
All of the several forms of the same chemical element with various masses and unstable nuclei are referred to as radioactive isotopes. Dating once living organisms is an example of a beneficial use of radioactive isotopes.
What is radioactive isotope ?A chemical element in an unstable state that emits radiation as it decomposes and becomes more stable. Radioisotopes can be created in a lab or in the natural world. They are utilized in imaging studies and therapy in medicine. likewise known as radionuclide.
Isotopes are identical elemental atoms with differing quantities of neutrons. Numerous elements have one or more radioactive isotopes. Due to the instability of their nuclei, they decay and release radiation
Thus, the dating once living organisms is an example of a beneficial use of radioactive isotopes.
To learn more about radioactive isotope follow the link below;
https://brainly.com/question/1907960
#SPJ1
40. 0% carbon, 6. 7% hydrogen, and 53. 3% oxygen with a molecular mass of 60. 0 g/mol. What is the molecular formula of the unknown compound?
Answers
The molecular formula of the unknown compound is C2H2O2.
To determine the molecular formula of the unknown compound, we need to calculate the empirical formula first and then find the multiple of its subscripts to obtain the molecular formula.
Given:
Percentage of carbon = 40.0%
Percentage of hydrogen = 6.7%
Percentage of oxygen = 53.3%
Molecular mass = 60.0 g/mol
Step 1: Convert the percentages to grams.
Assuming we have 100 grams of the compound:
Mass of carbon = 40.0 g
Mass of hydrogen = 6.7 g
Mass of oxygen = 53.3 g
Step 2: Convert the masses to moles using the molar masses of the elements.
Molar mass of carbon = 12.01 g/mol
Molar mass of hydrogen = 1.008 g/mol
Molar mass of oxygen = 16.00 g/mol
Number of moles of carbon = Mass of carbon / Molar mass of carbon
= 40.0 g / 12.01 g/mol
= 3.332 mol
Number of moles of hydrogen = Mass of hydrogen / Molar mass of hydrogen
= 6.7 g / 1.008 g/mol
= 6.648 mol
Number of moles of oxygen = Mass of oxygen / Molar mass of oxygen
= 53.3 g / 16.00 g/mol
= 3.331 mol
Step 3: Determine the empirical formula by dividing the moles by the smallest value.
Dividing the moles of carbon, hydrogen, and oxygen by 3.331 gives approximately 1 for each element.
So, the empirical formula of the compound is CHO.
Step 4: Determine the multiple of the subscripts to obtain the molecular formula.
To find the multiple, we divide the molecular mass by the empirical formula mass.
Molecular mass = 60.0 g/mol
Empirical formula mass = (12.01 g/mol) + (1.008 g/mol) + (16.00 g/mol) = 29.018 g/mol
Multiple = Molecular mass / Empirical formula mass
= 60.0 g/mol / 29.018 g/mol
= 2.07
Rounding to the nearest whole number, we get 2.
Therefore, the molecular formula of the unknown compound is C2H2O2.
learn more about molecular here
https://brainly.com/question/30640129
#SPJ11
A substance X reacts with ammonium nitrate to produce a gas that turns damp red litmus paper blue. X must therefore be:
a) A salt
b) A catalyst
c) An alkali
d) An acid
Answers
Answer:
option C is correct
Explanation:
litmus paper turns red in acidic solution and turns blue in alkaline solution and turns purple in neutral solution
This is not considered to be a Galilean moon.
Europa
Callisto
Titan
Io
Answers
Select the correct answer. which atom or ion is the largest? a. k b. k c. ca d. ca2 e. li
Answers
Answer:
A
Explanation:
*HELP ASAP QUESTION IS LINKED IN PICTURE*

Answers
Answer:
C) FeExplanation:
The element which is oxidized is the element that losses electrons and its oxidation state be more positive.The element which is reduced is the element that gain electrons and its oxidation state be more negative.Fe goes from + 2 to +3, so, it is the element that is oxidized.Hope this helpsidentify the conditions for a standard electrochemical cell. select one or more: pressure of 1 atm temperature of 298 k solution concentrations of 1 m pressure of 5 atm solute masses of 1 g temperature of 273 k
Answers
The conditions for a standard electrochemical cell. select one or more : pressure of 1 atm temperature of 298 k solution concentrations of 1 M.
The electrochemical cell is the cell that is capable of generating the electrical energy from the chemical reactions or by the use of the electrical energy to cause the chemical reaction. The conditions for a standard electrochemical cell. select one or more : pressure of 1 atm temperature of 298 k solution concentrations of 1 M.
There are the two types of the electrochemical cells is as follows : the galvanic called the electrolytic cells. the galvanic cell is also called as the voltaic cell.
To learn more about temperature here
https://brainly.com/question/14995282
#SPJ4
Hydrogen gas was collected by water displacement. What was pressure of the H2 collected if the temperature was 26°C?
How do I solve this type of problem? Thanks!
Answers
The pressure of the H2 collected if the temperature was 26°C is 2.2195 x 10⁵ Pa
What is pressure?
Pressure is described as the force applied perpendicular to the surface of an object per unit area over which that force is distributed.
The ideal gas law may be written as
P = pRT/M
where
p = pressure
ρ =density
T = temperature
M = molar mass
R = 8.314 J/(mol-K)
For the given scenario,
ρ = 0.09 g/L = 0.09 kg/m³
T = 26°C = 26+273 K = 299 K
M = 1.008 g/mol = 1.008 x 10⁻³ kg/mol
Substituting into the equation of P = pRT/M, we have
P = 2.2195 x 10^5 pa.
we know that 1 atm = 101325 Pa
Therefore
p = 2.2195 x 10⁵ Pa
= 221.95 kPa
= (2.295 x 10⁵)/101325 atm
= 2.19 atm
Learn more about pressure at: https://brainly.com/question/28012687
#SPJ1
What is meant by the term compound? Provide an example
Answers
Answer:
I hope it helps
Explanation:
When two or more elements chemically combine in a fixed ratio by mass, the obtained product is known as a compound. Compounds can be defined as substances consisting of 2 or more different types of elements in a fixed ratio of its atoms. When the elements combine, some individual property of the elements is lost and the newly formed compound has new properties.Chemical Formula: Compounds are represented by their chemical formula. A chemical formula is a symbolic representation of the proportions of atoms that constitute a particular chemical compound.The chemical formula of water is H2O which shows two atoms of hydrogen and one atom of oxygen have combined to form one molecule of H2O. The chemical formula for common salt is NaCl which shows one atom of sodium and one atom of chlorine combine to form one molecule of NaCl.CAN SOMEONE PLEASE HELP ME WITH THIS I REALLY NEED HELP

Answers
Answer:
B
Explanation:
There's a three in front of the CH3COOH. But let's figure it out without that 3.
1 mol of CH3COOH has 3 H from CH3 and 1 hydrogen at the end. In all 4 hydrogens.
Now put the 3 in front 3CH3COOH multiplies everything by 3. So you had 4 before and with the 3 in front you must have 12.
Try it. Draw it out.
CH3COOH has 4
CH3COOH has 4
CH3COOH has 4
Total 12
That's B
Study the reaction. X(s) + 2B(aq)→ X+(aq) + B2(g) Under which circumstances will the reaction rate be highest? A) the solid X is ground to a fine powder B) the solid X is in the form of a few smooth spheres C) the solid X is densely packed at the bottom of the container D) the solid X is in the form of a single, large cube.
Answers
Answer:
Option (A) the solid X is ground to a fine powder.
Explanation:
X(s) + 2B(aq) → X+(aq) + B2(g)
In the reaction above, the rate of the reaction will be highest, when X being a solid is ground to fine powder.
Grounding X to fine powder simply means increasing the surface area of X.
An increase in surface area of reactants will definitely increase the rate of reaction because the particles of the solid will collide with the right orientation and hence speed up the reaction rate.
Question what is the mass of 4.5 moles of

Answers
Answer:
198g
have a great day my friend <333