Find the percent composition of a sample containing 1.29 grams of carbon and
1.71 grams of oxygen.
Answers
The percent composition of the sample containing 1.29 grams of carbon and 1.71 grams of oxygen is 43% carbon and 57% oxygen.
The percent composition of a sample can be calculated by dividing the mass of each element in the sample by the total mass of the sample and then multiplying by 100%.
To find the percent composition of a sample containing 1.29 grams of carbon and 1.71 grams of oxygen, we need to calculate the total mass of the sample first.
Total mass of the sample = mass of carbon + mass of oxygen
= 1.29 grams + 1.71 grams
= 3 grams
Now, we can calculate the percent composition of carbon and oxygen in the sample:
Percent composition of oxygen = (mass of oxygen / total mass of the sample) x 100%
= (1.71 grams / 3 grams) x 100%
= 57%
Percent composition of carbon = (mass of carbon / total mass of the sample) x 100%
=(1.29 grams / 3 grams) x 100%
= 43%
Therefore, the sample contains 43% carbon and 57% oxygen by mass.
To learn more about percent composition visit:
https://brainly.com/question/20393858
#SPJ11
Related Questions
what is a common use of bases?as a component of vinegaras a component of car batteriesto make foods tartto reduce indigestion
Answers
Bases are frequently used to alleviate dyspepsia. Antacids and other bases are frequently used to reduce acid reflux, heartburn, and indigestion symptoms by neutralizing excess stomach acid.
These bases reduce acidity by increasing the pH level in the stomach. This facilitates digestion and eases discomfort. The other options are not common ways to use bases. Not a base, vinegar is an acidic material. A sulfuric acid-based electrolyte solution, which is an acid and not a base, is used in car batteries. Foods can be made tart without bases; instead, acidic ingredients like lemon juice or vinegar are frequently employed in this capacity.
To know more about Bases, here
brainly.com/question/14291917
#SPJ4
Answer: D) To reduce indigestion
Explanation: because thats what it does :)
The titration of an impure sample of KHP found that 36.00 mL of 0.100 M NaOH was required to react completely with o.758 g of sample. What is the percentage of KHP in this sample?
Answers
The weight percentage of the solute in a solution can be calculated from the total weight of the solution determined. The weight percentage of KHP in the sample is 84.03 %.
What is weight percent?Weight percent of a component of a sample is the weight fraction of that component in the total weight of the sample. Thus it is the ratio of weight of the solute to the total weight of the solvent.
Here the molarity of the solution is 0.100 M and volume is 36.0mL, from this the number of moles of NaOH, is 0.036 L × 0.100 M = 0.0036 moles. The molar mass of NaOH is 40 g/mol. Thus the weight of 0.0036 moles is 40 × 0.0036 = 0.902 g.
Thus, the percent of KHP in the sample is calculated as follows:
percent = 0.758 /(0.902 +0.758 ) ×100
= 84.03%.
Hence, percent of KHP in the sample is 84.03.
To find more about mass percent, refer the link below:
https://brainly.com/question/14990953
#SPJ1
Write the net ionic equations that occur in the following cells:
1. Pb/Pb2+ // Ag+/Ag
2. Zn/Zn2+ // Pb2+/Pb
3. Al/Al3+ // Cd2+/Cd
Answers
The net ionic equation is; Pb(s) + 2Ag⁺(aq) → Pb²⁺(aq) + 2Ag(s), Zn(s) + Pb²⁺(aq) → Zn²⁺(aq) + Pb(s), and 2Al(s) + 3Cd²⁺(aq) → 2Al³⁺(aq) + 3Cd(s)
A net ionic equation is a chemical equation that only includes the species that are involved in the reaction and excludes the spectator ions. Spectator ions are ions that do not participate in the reaction and do not undergo any chemical changes.
Anode; Pb(s) → Pb²⁺(aq) + 2e⁻
Cathode; 2Ag⁺(aq) + 2e⁻ → 2Ag(s)
Overall; Pb(s) + 2Ag⁺(aq) → Pb²⁺(aq) + 2Ag(s)
Net ionic equation; Pb(s) + 2Ag⁺(aq) → Pb²⁺(aq) + 2Ag(s)
Anode; Zn(s) → Zn²⁺(aq) + 2e⁻
Cathode; Pb²⁺(aq) + 2e⁻ → Pb(s)
Overall; Zn(s) + Pb²⁺(aq) → Zn²⁺(aq) + Pb(s)
Net ionic equation; Zn(s) + Pb²⁺(aq) → Zn²⁺(aq) + Pb(s)
Anode; Al(s) → Al³⁺(aq) + 3e⁻
Cathode; Cd²⁺(aq) + 2e⁻ → Cd(s)
Overall; 2Al(s) + 3Cd²⁺(aq) →2Al³⁺(aq) + 3Cd(s)
Net ionic equation; 2Al(s) + 3Cd²⁺(aq) → 2Al³⁺(aq) + 3Cd(s)
To know more about net ionic equation here
https://brainly.com/question/22885959
#SPJ4
ct.
the
2.
16 Calculate For a more difficult training session, the
mass to be pushed is increased to 160 kg. If the
players still push with a force of 150 N, what is the
acceleration of the object?
Use Newton's law:
F = ma
150 N =
HWHO () ……..
Answers
The acceleration of the objection is 0.9375 m/s² if the players still push with a force of 150 N.
From the formula:
F = ma
a = F/m
a = 150/160
a = 0.9375 m/s²
Thus, the acceleration of the object is 0.9375 m/s² if the players still push with a force of 150 N.
How would you define Force?The word 'force' has a very precise meaning. Force can be described as push or a pull. A force is not something that an object contains or has in it. A force is exerted on one object by another. The idea of a force is not confined to living things or non-living things.
What is the SI unit of Force?The SI unit of force is the newton, symbolised as N. The base units suitable to force are: metre, unit of length — symbol m. The kilogram, unit of mass — symbol kg. The second, unit of time — symbol s.
To know more about Force, visit:
https://brainly.com/question/13191643
#SPJ4
The acceleration of the objection is 0.9375 m/s² if the players still push with a force of 150 N.
How would you define Force?The word 'force' has a very precise meaning. Force can be described as push or a pull. A force is not something that an object contains or has in it. A force is exerted on one object by another. The idea of a force is not confined to living things or non-living things.
From the formula:
F = ma
a = F/m
a = 150/160
a = 0.9375 m/s²
Thus, the acceleration of the object is 0.9375 m/s² if the players still push with a force of 150 N.
To know more about acceleration visit :-
https://brainly.com/question/3046924
#SPJ1
fuerza que ejerce el planeta tierra sobre los cuerpos que en ella se encuentran. Fuerza de distancia Fuerza de Contacto
Answers
Answer:
Ver explicacion
Explanation:
La gravedad es una fuerza que actúa a distancia. La fuerza que actúa entre la tierra y los cuerpos en ella es la fuerza gravitacional.
Es un ejemplo típico de una fuerza de acción a distancia.
Por lo tanto, la fuerza tat que existe entre la tierra y los cuerpos que se encuentran en ella es una fuerza de distancia.
To calculate the work that an object does when it moves covering a distance of 3000 meters when it is propelled by a force of 65 Newton, one of the principles of mechanics must be considered.
Physical principle of mechanicsA force does work when there is a displacement of the center of mass of the body on which the force is applied, in the direction of said force.
The work of the force on that body will be equivalent to the energy necessary to displace it, so that:
W = F.d
Force CalculationSubstituting the corresponding values in said formula:
F = 65N
d = 3000m
W = 65 N. 3000 m
W = 195000 W
Learn more about force calculations at https://brainly.com/question/23775339 #SPJ4

The equation below represents a chemical reaction that occurs in living cells. How many
atoms are represented in the reactants of this equation?*
C6H12O6 + 60, 600, +6H,0 + energy
6
12
24
36
Answers
Answer:
36
Explanation:
I´m hoping this is correct
If you add 6 + 6 you get 12, multiply the 12 to get 24 and you multipy 6 times 2 to get 12 and add 12 to 24 to get 36.
Calcula el volumen en litros que tendran 2 kg de poliestireno expandidos (densidad = 0,92g/cm3)
Answers
2 kg of expanded polyethylene has a volume of 2.17 liters.
Given that,
Density = 0.9g/cm³
Mass = 2kg = 2000g
Density is the substance's mass per unit of volume. Although the Roman letter D may also be used, the sign most frequently used for density is ρ (the lowercase Greek letter rho). A substance's density changes as a function of pressure and temperature. With solids and liquids, this variance is often slight, but for gases, it is much more pronounced.
Density = Mass ÷ Volume
0.92 = 2000 ÷ Volume
Volume = 2000 ÷ 0.92
Volume = 2.17 liters.
Hence, 2 kg of expanded polyethylene has a volume of 2.17 liters.
To learn more about density, refer to:
https://brainly.com/question/26364788
#SPJ4
Your question is in Spanish. The English translation of the question is:
Calculate the volume of 2 kg of expanded polyethylene in liters. ( Density = 0.92g/cm³ )
The forces between water molecules are stronger
than the forces between ethanol molecules. Which
liquid would probably be most difficult for an insect
to walk on? Explain your answer.
Answers
Answer:
An insect would have an easier time walking on the surface of water than on the surface of ethanol.
Water's stronger intermolecular forces lead to higher surface tension.
Higher surface tension allows water to support the insect.
Answer:
An insect would have an easier time walking on the surface of water than on the surface of ethanol.
Water's stronger intermolecular forces lead to higher surface tension.
Higher surface tension allows water to support the insect.
Explanation:
a sample containing 33.42g of metal pellet is poured into a graduated cylinder initially containing 12.7 ml of water, causing the water level in the cylinder to rise to 21.6ml. calculate the density of the metal in g/cm^3
Answers
Volume of water only=12.7ml
Volume of water and metal pellet=21.6ml
Volume of metal pellet only=21.6ml-12.7ml=8.9ml
Iml=1cm^3
Mass=33.42g
Volume=8.9cm^3
Density=mass/volume=33.42/8.9=3.755g/cm^3
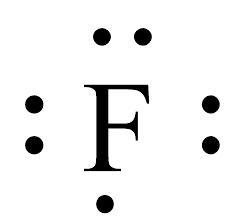
Draw the correct Lewis dot structure from the given shorthand notation below: PLS HELP

Answers
The Lewis structure of the element have been shown in the image attached.
Lewis dot structure of an element:The valence electrons of an atom or molecule are depicted in a simplified manner by the Lewis structure, commonly referred to as the Lewis dot structure or electron dot structure. Gilbert N. Lewis, an American scientist, created it.
The valence electrons of an atom are shown in a Lewis structure as dots surrounding the element's symbol. These dots' placement reveals details about the connectivity and atom-atom bonding in a molecule.
Learn more about Lewis structure:https://brainly.com/question/29756546
#SPJ1
What is the ph of 0.21 M acetic acid to 1.00 L of which 1.43 g of sodium acetate, NaCH3CO2, has been added? (K, for acetic acid is 1.8 x 10^-5)pH =
Answers
To find the pH of the solution, we first need to calculate the concentration of acetate ions in the solution after the addition of sodium acetate.
We can start by calculating the number of moles of sodium acetate added:
1.43 g NaCH3CO2 x (1 mol NaCH3CO2 / 82.03 g NaCH3CO2) = 0.0174 mol NaCH3CO2
Since sodium acetate fully dissociates in water, we know that the number of moles of acetate ions in the solution is also 0.0174 mol.
Next, we need to calculate the new concentration of acetic acid in the solution. We can use the equation for the ionization of acetic acid:
CH3CO2H ⇌ CH3CO2- + H+
Ka = [CH3CO2-][H+] / [CH3CO2H]
At equilibrium, the concentration of acetic acid will be reduced by the amount of acetate ions that were added, so:
[CH3CO2H] = 0.21 M - 0.0174 M = 0.1926 M
We can now use the equilibrium equation and the given Ka value to solve for the concentration of H+ ions, and thus the pH:
1.8 x 10^-5 = (0.0174 M)(x) / (0.1926 M)
x = 1.576 x 10^-4 M
pH = -log[H+] = -log(1.576 x 10^-4) = 3.804
Therefore, the pH of the solution is approximately 3.804.
To learn more about sodium acetate visit;
https://brainly.com/question/12924347
#SPJ11
The process of wearing down by friction-like sand blasting is called
O exfoliiation
O erosion
O Wind Abrasion
O deposition
Answers
Answer:
Erosion
Explanation:
Abrasion is a process of erosion which occurs when material being transported wears away at a surface over time. It is the process of friction caused by scuffing, scratching, wearing down, marring, and rubbing away of materials. ... Objects transported in waves breaking on coastlines cause abrasion.
find the amount of energy transferred to the alpha particle (4he).
Answers
To determine the amount of energy transferred to an alpha particle (4He), we need to know the specific context or process in which the energy transfer occurs.
However, in general, the energy transferred to an alpha particle can be calculated in certain scenarios. For example, in a nuclear reaction such as alpha decay, the energy transferred to the alpha particle can be determined by subtracting the total energy of the parent nucleus from the total energy of the daughter nucleus and any emitted particles.
It is also important to note that energy transfer can occur through various mechanisms, such as collisions, electromagnetic interactions, or chemical reactions. The calculation of energy transfer typically requires specific data and context related to the system and process involved.
If you can provide additional details or clarify the specific scenario in which the energy transfer is occurring, I would be happy to assist you further in calculating the amount of energy transferred to the alpha particle.
Learn more about alpha particle here:
https://brainly.com/question/24276675
#SPJ11
Which of the following describes a nonspontaneous reaction?
A. The Gibbs free energy is more than the activation energy.
B. The Gibbs free energy is less than the activation energy.
C. The Gibbs free energy for the reaction is positive.
D. The reaction does not need added energy to occur.
Answers
The statement that describe nonspontaneous reaction is the The Gibbs free energy for the reaction is positive.
What is non spontaneous reaction?Nonspontaneous reaction is a type of chemical reaction that does not support or give room for products to be formed at given conditions. For a reaction to be nonspontaneous,it must be an endothermic reaction that is heat is absorbed from the surrounding and there must be decrease in entropy, or increase. The Gibbs free energy is positive.
Therefore, The statement that describe nonspontaneous reaction is the The Gibbs free energy for the reaction is positive.
Learn more about nonspontaneous reaction below.
https://brainly.com/question/3754650
#SPJ5
The spontaneous reactions have a positive enthalpy and a decrease in the entropy. In a non-spontaneous reaction, The Gibbs free energy for the reaction is positive. The correct option is C.
What is non-spontaneous reaction?The chemical reactions which require an energy input to proceed or it can or cannot take place without the influence of external factors can be defined as the non-spontaneous reaction. In this case the total energy of the products is higher than that of the reactants.
Gibbs energy change is a better parameter which is used to determine the spontaneity or feasibility of a reaction. If ΔG is negative, the process will be spontaneous and if ΔG is positive, the process is non-spontaneous in the forward direction.
Gibbs free energy is the sum of enthalpy plus the product of temperature in Kelvin and entropy of the system.
Thus the correct option is C.
To know more about Gibbs energy, visit;
https://brainly.com/question/13795204
#SPJ5
Pls help me!!!!!!!!!

Answers
Answer:
Lines in the spectrum were due to transitions in which an electron moved from a higher-energy orbit with a larger radius to a lower-energy orbit with smaller radius. The orbit closest to the nucleus represented the ground state of the atom and was most stable; orbits farther away were higher-energy excited states.
Explanation:
There is an intimate connection between the atomic structure of an atom and its spectral characteristics. Atoms of individual elements emit light at only specific wavelengths, producing a line spectrum rather than the continuous spectrum of all wavelengths produced by a hot object. Niels Bohr explained the line spectrum of the hydrogen atom by assuming that the electron moved in circular orbits and that orbits with only certain radii were allowed. Lines in the spectrum were due to transitions in which an electron moved from a higher-energy orbit with a larger radius to a lower-energy orbit with smaller radius. The orbit closest to the nucleus represented the ground state of the atom and was most stable; orbits farther away were higher-energy excited states. Transitions from an excited state to a lower-energy state resulted in the emission of light with only a limited number of wavelengths Atoms can also absorb light of certain energies, resulting in a transition from the ground state or a lower-energy excited state to a higher energy excited state. This produces an absorption spectrum, which has dark lines in the same position as the bright lines in the emission spectrum of an element. Bohr's model revolutionized the understanding of the atom but could not explain the spectra of atoms heavier than hydrogen.
Identify each of the following compounds as aromatic, nonaro- matic, or antiaromatic. Explain your choice in each
Answers
To determine whether a compound is aromatic, nonaromatic, or antiaromatic, we need to consider the compound's structure and its adherence to the rules of aromaticity. 1. Benzene (C6H6): Benzene is aromatic. It fulfills the criteria for aromaticity, which include a planar ring structure, conjugation of pi electrons, and a Huckel's rule of having 4n+2 π electrons (where n is an integer).
Benzene has a continuous ring of conjugated pi electrons (6 electrons), making it aromatic. 2. Cyclooctatetraene (C8H8): Cyclooctatetraene is nonaromatic. Despite having a planar ring structure and conjugation, it fails to fulfill the aromaticity criteria. It has 8 pi electrons, which is an even number, contradicting Huckel's rule for aromaticity.
3. Cyclobutadiene (C4H4): Cyclobutadiene is antiaromatic. It has a planar ring structure and conjugation; however, it has 4 pi electrons, which is an even number. According to Huckel's rule, for a compound to be aromatic, it must have 4n+2 pi electrons. Since cyclobutadiene has 4 pi electrons, it violates this rule and is classified as antiaromatic.
Learn more about the compound's structure here: brainly.com/question/32635021
#SPJ11
What happens when a base dissolves?OA. It increases the concentration of H* in solution.OB. It is no longer dangerous.OC. It increases the concentration of OH in solution.о D. It adds oxygen to the solution.
Answers
A base, according to the Arrhenius definition, is any species that increases the concentration of OH- in the solution. Some examples of bases are, NaOH, KOH, LiOH, all these substances will increase the concentration of OH when dissolved. Letter C
Write the nuclear equation for the release of a beta particle by 22888Ra
Answers
The nuclear equation :
₈₈Ra²²⁸ ⇒ ₋₁e⁰ + ₈₉Ac²²⁸
Further explanationRadioactivity is the process of unstable isotopes to stable isotopes by decay, by emitting certain particles,
alpha α particles ₂He⁴ beta β ₋₁e⁰ particles gamma particles ₀γ⁰ positron particles ₁e⁰ neutron ₀n¹The sum of the mass number and atomic number before and after decay is the same
Ra = Radium ⇒alkaline earth, group 2, block s, period 7
Atomic mass = 88
If an element releases an β ₋₁e⁰, the atomic number of the new element increases by 1
The new element will have an atomic number: 88+1=89 and mass number 288
The element having the atomic number 89 is Actinium(Ac)
So the nuclear equation :
₈₈Ra²²⁸ ⇒ ₋₁e⁰ + ₈₉Ac²²⁸
what mass (in grams) of NH4Cl is needed to prepare 350 mL of a 0.25 M ammonium chloride solution
Answers
Answer:
4.70 grams of NH4Cl is needed to prepare 350 mL of a 0.25 M ammonium chloride solution.
We need approximately 4.68 grams of NH4Cl to prepare a 0.25 M ammonium chloride solution with a volume of 350 mL.
To determine the mass of NH4Cl needed to prepare the solution, we us use the formula:
m=M x V x MM ... (i)
where,
m= mass in grams
M=molarity of solution
MM= molar mass of compound
V= volume in litres
The number of moles of NH4Cl needed can be calculated using:
Moles = Molarity x Volume ...(ii)
Moles = 0.25 mol/L x 0.350 L
Moles = 0.0875 mol
Hence we can replace M x V with number of moles in equation i.
The molar mass of NH4Cl is :
Molar mass of NH4Cl = (1 x 14.01 g/mol) + (4 x 1.01 g/mol) + (1 x 35.45 g/mol)
Molar mass of NH4Cl = 53.49 g/mol
We have all the variables
Putting them in equation i.
Hence,
Mass (g) = Moles x Molar mass
Mass (g) = 0.0875 mol x 53.49 g/mol
Mass (g) = 4.68 g
Therefore, you would need approximately 4.68 grams of NH4Cl to prepare a 0.25 M ammonium chloride solution with a volume of 350 mL.
To learn more about Stoichiometry,
https://brainly.com/question/16060223
add curved arrows to the reactants to indicate the movement of electrons in this reaction.
Answers
The movement of electrons is always depicted by curved arrows. From the tail to the head, electrons migrate.
What is the point of the curved arrow?The curved arrow's function is to depict the transfer of electrons from one site to another.The majority of the arrows you'll encounter have a double-barb at the head that symbolises the motion of two electrons."Curly arrows" are most frequently used to depict the motion of electron pair.How does stability work?It is more stable for a building to have fewer formal charges than more.The most electronegative atom will hold the negative charge, resulting in the most stable structure.The least electronegative atom will be given a positive charge in the most stable configuration.Learn more about Formal Charge from: https://brainly.com/question/9790241
#SPJ4

using nad and nadh (no structures needed) write out the reaction for the oxidation of methanol (ch3-oh) to formaldehyde, in which nad is the oxidizing agent. we won’t encounter this reaction, but it is a lot like ones we will.
Answers
Using NAD and NADH reaction for the oxidation of methanol CH₃OH to formaldehyde, in which NAD is the oxidizing agent and then alcohol oxidase was crystallized after purification by ammonium sulfate precipitation
Oxidation means a process in which a chemical substance changes because of the addition of oxygen
Methanol was oxidized to formaldehyde by an alcohol oxidase and the reaction are
CH₃OH+O₂ → HCOH + H₂O₂
And the cofactor found in two form of cell and NAD is an oxidizing agent and it accept the electron of other molecule and reduced and this reaction is also with H⁺ form NADH which can be used as the reducing agent to donate electron and this reaction gives alcohol oxidase was crystallized after purification by ammonium sulfate precipitation
Know more about oxidation
https://brainly.com/question/20389059
#SPJ1
What are the factors that affect freezing-point depression
Answers
A solution has a [H*] of 1.0x10-5M.
21. D. As What is its [OH-]?
22.
23.
What is its pH?
What is its pOH?
![A solution has a [H*] of 1.0x10-5M.21. D. As What is its [OH-]?22.23.What is its pH?What is its pOH?](https://i5t5.c14.e2-1.dev/h-images-qa/contents/attachments/cJh7HUAVCHi9mioVu2TwwesNelscAeOr.png)
Answers
As per the given value of \([H^+]\), the pOH of the solution is 9.
To calculate the [OH-] s well as a pH of a solution with a given [H+], we can use the equation:
pH + pOH = 14
Given that:
\([H^+] = 1.0*10^{-5} M\)
First we need to Calculate [OH-]
We know that, water undergoes autoionization to form H+ and OH- ions, we can use the following equation:
\([H^+] * [OH^-] = 1.0*10^{-14} M^2\)
Plugging in the given [H+] value, we have:
\((1.0*10^{-5 }M) * [OH^-] = 1.0*10^{-14} M^2\)
Solving for [OH-]:
[OH-] = \((1.0*10^{-14} M^2) / (1.0*10^{-5} M) = 1.0*10^{-9} M\)
Therefore, the [OH-] of the solution is \(1.0*10^{-9\) M.
Now we need to Calculate pH
Using the relationship between [H+] and pH:
pH = -log[H+]
Plugging in the given [H+] value:
pH = -log(1.0x\(10^{-5\)) = 5
Therefore, the pH of the solution is 5.
Next is to Calculate pOH
Using the equation:
pOH = 14 - pH
Plugging in the calculated pH value:
pOH = 14 - 5 = 9
Therefore, the pOH of the solution is 9.
For more details regarding pH, visit:
https://brainly.com/question/2288405
#SPJ1
10. Liquid changing to gas only at the surface is called
Answers
Answer:
Evaporation
Explanation:
Though it doesn't require high temperatures to do so
Choose the correct alternative for each of the following statements
i. Conjugate bases of strong acids (Give an example of a conjugate base A) do NOT behave as bases in solution B) behave as weak bases in solution C) behave as strong bases in solution.
Answers
The "Conjugate bases of strong acids do NOT behave as bases in solution" A is the correct alternative for the statement.
This is because a strong acid fully dissociates in water, releasing all of its hydrogen ions (H+) and leaving behind its conjugate base. Since the conjugate base is formed from the loss of a proton, it does not have any extra protons to donate and therefore cannot act as a base. An example of a conjugate base would be chloride ion (Cl-) in hydrochloric acid (HCl). When HCl dissociates in water, it releases its hydrogen ion and forms Cl- as its conjugate base. However, Cl- does not have any extra protons to donate and cannot act as a base.On the other hand, the statement "Conjugate bases of weak acids behave as weak bases in solution" is true. Weak acids only partially dissociate in water, meaning that their conjugate bases do have a small tendency to accept protons and act as bases. An example of a conjugate base that behaves as a weak base would be acetate ion (CH3COO-) in acetic acid (CH3COOH). When CH3COOH partially dissociates in water, it releases its hydrogen ion and forms CH3COO- as its conjugate base. CH3COO- has a small tendency to accept protons and can act as a weak base.Overall, the behavior of conjugate bases depends on the strength of their corresponding acids. Strong acids have weak conjugate bases that do not behave as bases in solution, while weak acids have weak conjugate bases that do behave as weak bases in solution.For more such question on Conjugate bases
https://brainly.com/question/14684465
#SPJ11
Explain why the answer is correct and why the others aren’t.
Please and thank you
Answers
Answer:
B. bedrock structure.
Explanation:
A landform refers to a geomorphic or natural feature of the Earth's surface, which typically makes its terrain. Some examples of landforms on planet earth are mountain, plains, valley, hills and plateau.
Basically, the tectonic plates such as the oceanic and continental lithosphere interact in three (3) ways and these are; divergent, transform and convergent boundaries.
A convergent plate boundary can be defined as a boundary where two (2) plates move towards each other, usually, resulting in subduction or collision. This action often causes mountain range such as the Himalayas to form by the collision between the plate carrying Eurasia and that of India; as a result of subduction which causes a plate to be forced underneath the mantle, deep ocean trenches are formed such as the Mariana trench.
The Catskills are commonly called mountains but are actually part of the Allegheny Plateau also referred to as Appalachian Plateau. The Catskills are classified as a plateau because of their bedrock structure which is caused by a valley, continental glaciers, and erosion from various watercourse.
Additionally, the Catskills is a mountain which got its name from early Dutch settlers in the United States of America.
Some tomato ketchup accidentally got onto a piece of white fabric. Your mother suggested that lemon juice, baking soda, or vinegar would be good for removing the tomato stain. Plan and design an experiment that can be used to investigate which of the three substances is most effective in removing the tomato stain.
1. Write a hypothesis for your investigation
2. Identify the dependent, independent, and controlled variables.
3. Outline a proposed method for the experiment you would conduct.
4. What data would be collected? Suggest a suitable way(s) for recording your data.
5. Identify ONE (1) possible source of error and ONE (1) precaution.
Please if you see this help me answer these questions.
Answers
Answer:
3... uhm I'm not sure it could 2
a solution is prepared by dissolving 0.15 g of sodium oxide in water to give 119.5 ml of solution. express the ph to two decimal places.
Answers
To calculate the pH of the solution, we first need to determine the concentration of hydroxide ions (\(OH^{-}\)) in the solution, since sodium oxide is a strong base that dissociates completely in water to give \(Na^{+}\) and \(OH^{-}\) ions.
We can start by calculating the number of moles of sodium oxide dissolved in the solution:
0.15 g \(Na_{2} O\) / (61.98 g/mol \(Na_{2} O\)) = 0.00242 mol \(Na_{2} O\)
Since sodium oxide dissociates completely in water to give two moles of sodium ions and one mole of hydroxide ions per mole of \(Na_{2} O\), we know that the number of moles of \(OH^{-}\) in the solution is:
0.00242 mol \(Na_{2} O\) x (1 mol \(OH^{-}\)/1 mol \(Na_{2} O\)) = 0.00242 mol \(OH^{-}\)
Next, we can calculate the concentration of hydroxide ions in the solution, which is given by:
[\(OH^{-}\)] = moles of \(OH^{-}\) / volume of solution in liters
[\(OH^{-}\)] = 0.00242 mol / (119.5 mL x 1 L/1000 mL) = 0.0203 M
Finally, we can calculate the pH of the solution using the equation:
pH = 14 - log [\(OH^{-}\)]
pH = 14 - log (0.0203) = 11.69
Therefore, the pH of the solution prepared by dissolving 0.15 g of sodium oxide in water is 11.69 to two decimal places.
Learn more about sodium oxide
https://brainly.com/question/29172011
#SPJ4
The element oxygen has a molar mass of 15.999 grams, thus one mole of oxygen is equal to _____ g/mol.
Answers
Answer: 15.999 grams.
Explanation: The molar mass of an element is the mass of one mole of that element. You can also solve this with a little bit of math.
\(15.999gO*\frac{1molO}{15.999gO} =1molO\)
I hope this helps! Pls mark brainliest!! :)
What type of rock is this?

Answers
Answer:
I'm gonna say igneous
Explanation:
The texture looks rough and the rock looks like it's composed of different types of minerals making it an igneous rock
I hope this helps :)
Answer: Igneous
Explanation: Igneous rock, or magmatic rock, is one of the three main rock types, the others being sedimentary and metamorphic. Igneous rock is formed through the cooling and solidification of magma or lava. The magma can be derived from partial melts of existing rocks in either a planet's mantle or crust.