Answers
In 1963, the united states passed the clean air act which sets a maximum amount for emissions of pollutants or the presence of pollutants in ambient air.
The Clean Air Act of 1963 was the first piece of federal law to address "regulating" air pollution. This was done by the 1963 legislation, which authorized research into methods for monitoring and regulating air pollution and established a government program inside the U.S. Public Health Service. To reduce air pollution in the United States, significant legislation known as the Clean Air Act was passed. less air pollution overall. The overall amount of pollutants generated may be decreased in a number of methods, including smaller automobiles, the removal of sulfur from coal, and lower combustion temperatures.
learn more about air pollution here:
https://brainly.com/question/23196476
#SPJ4
Related Questions
convert 7.54 x 10^-8 m to nanometers
Answers
7.54 *\(10^8\) meters is 75.4 nanometers.
To convert 7.54 * \(10^8\) meters to nanometers, you can multiply the value by \(10^9\)
as, \(10^9\)nanometers = 1 meter.
7.54 * \(10^8\) m * \(10^9\) = 7.54 x \(10^1\) nm
Therefore, 7.54 *\(10^8\) meters is equal to 75.4 nanometers.
learn more about conversion:
https://brainly.com/question/13076223
To convert 7.54 x 10^-8 meters to nanometers, you multiply 7.54 x 10^-8 by 1 x 10^9 to get 75.4 nanometers.
Explanation:To convert meters to nanometers, you need to know that 1 meter is equivalent to 1 x 109 nanometers. Therefore, if you were to convert 7.54 x 10-8 m to nanometers, you would multiply 7.54 x 10-8 by 1 x 109.
Here's how you'd do it: 7.54 x 10-8 m * 1 x 109 nm/m = 75.4 nm. So, 7.54 x 10-8 meters is equivalent to 75.4 nanometers.
Learn more about Unit Conversion here:https://brainly.com/question/32030244
#SPJ2
An unknown alcohol (C4H10O) reacted slowly with metallic sodium and gave positive results with NaOI. Have you sufficient information to be reasonably certain which of the butyl alcohols the unknown may be? Explain
Answers
The unknown butyl alcohol which reacted slowly with metallic sodium and gave positive results with NaOI is: 1-iodo, butan-2-ol.
Discussion:
When metallic sodium reacts with an unknown alcohol, and NaOI is produced. In essence, the alcohol compound has an iodo-group on one of it's carbon atoms and therefore has it's alcoholic, -OH group on carbon position 2.
Read more on organic alcohols;
https://brainly.com/question/16770113
What mass of NaCl is needed to produce a 26.4 mol/L with a 1.7 L volume?
Answers
we would need 2625.13 grams (or 2.62513 kilograms) of NaCl.
To calculate the mass of NaCl required to produce a 26.4 mol/L solution with a 1.7 L volume, we need to use the formula that relates the mass of solute, moles of solute, and molarity:Molarity (M) = moles of solute / liters of solution Rearranging this formula, we get:moles of solute = Molarity (M) x liters of solutionWe can use this formula to find the moles of NaCl needed:moles of NaCl = 26.4 mol/L x 1.7 L = 44.88 molNow, we can use the molar mass of NaCl to convert from moles to grams. The molar mass of NaCl is 58.44 g/mol:mass of NaCl = moles of NaCl x molar mass of NaClmass of NaCl = 44.88 mol x 58.44 g/mol = 2625.13 gTo produce a 26.4 mol/L solution with a 1.7 L volume.
for more question on NaCl
https://brainly.com/question/23269908
#SPJ8
What is the atomic number of an atom?
Answers
The atomic number or proton number (symbol Z) of a chemical element is the number of protons found in the nucleus of every atom of that element.
A molecule of which compound has a multiple covalent bond?
1. CH4
2. C2H4
3. C3H8
4. C4H10
Answers
Answer:
The second one
Explanation:
Because not only did I take a castle learning in this today but c2h4 has 6 covalent bonds
There are several types of molecules that have multiple bond. A molecule of which compound has a multiple covalent bond is C2H4.
Covalent bonding is the sharing of one or more electron pairs. In many covalent bonding situations,
Multiple chemical bonds exist when there is more than one electron pair that is being shared.
A nitrogen atom often fill its octet by sharing three electrons with another nitrogen atom thereby creating three covalent bonds.
Multiple bonds are commonly found in organic compound and they have carbon molecules.
Learn more from
https://brainly.com/question/4985972
5. If you have 5.0 L of a 3 molar saline solution of sodium chloride in water, how many
grams of NaCl would you find if you boiled off all the water?
Answers
You would have 175.32 grams of NaCl after boiling out all the water from 5.0 L of a 3 M NaCl solution.
When sodium chloride is boiled in water, what happens?Salt dissolves in water, forming sodium and chloride ions. If all the water were to be evaporated by boiling, the ions would join once more to produce solid salt. NaCl is safe to boil without harm, nevertheless. 2575 F, or 1413 C, is the boiling point of sodium chloride.
Three moles of NaCl would remain after boiling off all the water from a 5.0 L solution of 3 M NaCl. You can use the molar mass of NaCl, which is 58.44 g/mol, to get the mass of NaCl.
Mass of NaCl = moles of NaCl x molar mass of NaCl
Mass of NaCl = 3 mol x 58.44 g/mol
Mass of NaCl = 175.32 g
To know more about solution visit:-
https://brainly.com/question/30886177
#SPJ1
1. A sample of commercial concentrated hydrochloric acid is 11.8 M HCl and has a density of 1.190 g/mL. Calculate (a). the mass % of HCI (b). the molality of HCI (c). the mole fraction of HCI
Answers
(a) The mass percent of HCl in the solution is approximately 36.1%.
(b) The molality of HCl in the solution is approximately 15.5 mol/kg.
(c) The mole fraction of HCl in the solution is approximately 0.218.
(a) To calculate the mass percent of HCl, we need to determine the mass of HCl in a given volume of the solution.
Given: Concentration of HCl = 11.8 M
Density of the solution = 1.190 g/mL
First, we need to calculate the mass of the solution. Since density is mass per unit volume, the mass of 1 mL of the solution is 1.190 g.
Next, we need to calculate the mass of HCl in 1 mL of the solution. Since the concentration is given in moles per liter (M), and the molar mass of HCl is 36.46 g/mol, we can calculate the mass of HCl in 1 mL as follows:
Mass of HCl = concentration × volume × molar mass
= 11.8 mol/L × 0.001 L × 36.46 g/mol
= 0.430 g
Now, we can calculate the mass percent of HCl using the following formula:
Mass percent = (mass of solute ÷ mass of solution) × 100
= (0.430 g ÷ 1.190 g) × 100
≈ 36.1%
(b) The molality of HCl is calculated by dividing the moles of solute (HCl) by the mass of the solvent (water) in kilograms.
Since the density of the solution is given as 1.190 g/mL, the mass of 1 mL of the solution is 1.190 g. However, we need to consider the density of the solvent (water) to calculate the mass of water in the solution.
Assuming the density of water is 1 g/mL, the mass of water in 1 mL of the solution is (1.190 g - 0.430 g) = 0.760 g.
To calculate the molality of HCl, we need to convert the mass of water to kilograms:
Mass of water (kg) = 0.760 g ÷ 1000 = 0.000760 kg
The molality (m) is calculated using the formula:
Molality = (moles of solute ÷ mass of solvent in kg)
= (11.8 mol/L × 0.001 L) ÷ 0.000760 kg
≈ 15.5 mol/kg
(c) The mole fraction (X) of HCl is calculated by dividing the moles of HCl by the total moles of all components in the solution.
To calculate the mole fraction, we need to consider the volume of the solution and convert it to liters.
Given: Concentration of HCl = 11.8 M
Volume of the solution = 1 mL
Volume of the solution (L) = 1 mL ÷ 1000 = 0.001 L
To calculate the mole fraction of HCl, we need to calculate the moles of HCl and the moles of water (solvent) in the solution.
Moles of HCl = concentration × volume
= 11.8 mol/L × 0.001 L
= 0.0118 mol
Moles of water = mass of water ÷ molar mass of water
= 0.760 g ÷ 18.015 g/mol (molar mass of water)
= 0.0422 mol
Total moles in the solution = moles of HCl + moles of water
= 0.0118 mol + 0.0422 mol
= 0.054 mol
Mole fraction of HCl = moles of HCl ÷ total moles
= 0.0118 mol ÷ 0.054 mol
≈ 0.218
For such more questions on molality
https://brainly.com/question/14366957
#SPJ8
FeCI2 + Na2CO3 = FeCO3 + NaCI Balance this equation please
No links
Answers
FeCl2 + Na2CO3 = FeCO3 + 2NaCl
The Sun has been shining on this swimming pool all day. The water is much warmer than it was in the morning. Describe what is happening to the water in terms of temperature, particle speed, and kinetic energy.
Answers
Answer:
The waters' temp increased
Explanation:
The temperature of the water in the swimming pool has increased due to the heat from the Sun. As a result, the particles in the water are moving faster and have a higher kinetic energy than in the morning.
Which of the following functions are acceptable wave functions over the indicated interval?
Match the words in the left column to the appropriate blanks in the sentences on the right.

Answers
The function \(e^(-x^2)\)/2,\(e^{-ix}\), x\(e^{-x}\) are acceptable wavefunctions and \(x^2e^{-2}\)piex is a non-acceptable wavefunction.
An acceptable wavefunction is defined at every point in the specified interval.
The function \(e^{-x^2}/2\) is an acceptable wavefunction as it is defined at every point in the interval.It becomes finite at the pointx tends to infinity and x tends to -infinityThe function e^-ix=cosx-isinxIt is an acceptable wave function because of is defined at every point in the interval. It becomes finite.f(x)-[-1,1]f(x)=\(x^2\)\(e^{-2}\)pieix=\(x^2\)(cos2piex-isin2piex)It is not an acceptable wavefunction as it tends to infinite.The function is x\(e^{-x}\) is acceptable wavefunction as it is defined at every point in the interval.Learn more about wavefunction at:
https://brainly.com/question/15082946
#SPJ9
A sample of lithium has a mass of 0.0624 grams. How many atoms are present in this sample?
Answers
Answer:
\(5.412*10^{23}\)
Explanation:
We can find the moles of lithium by doing 0.0624 divided by the molar mass of lithium (which is 6.941). Then, we can multiply that number by 6.02*10^23 which is the avogadro number. Then, we get our answer.
How many liters of carbon dioxide can be produced if 37.8 grams of carbon disulfide react with excess oxygen gas at 28.85 degrees Celsius and 1.02 atmospheres?
CS2(l) + 3O2(g) yields CO2(g) + 2SO2(g)
2.78 liters
5.95 liters
12.1 liters
11.9 liters
Answers
The volume of carbon dioxide produced is approximately (d) 11.9 liters.
To determine the amount of carbon dioxide (C\(O_2\)) produced when 37.8 grams of carbon disulfide (C\(S_2\)) reacts with excess oxygen gas (\(O_2\)), we need to use stoichiometry and the given balanced chemical equation:
C\(S_2\)(l) + 3\(O_2\)(g) → C\(O_2\)(g) + 2S\(O_2\)(g)
First, we calculate the number of moles of C\(S_2\) using its molar mass:
Molar mass of (C\(S_2\)) = 12.01 g/mol (C) + 32.07 g/mol (S) × 2 = 76.14 g/mol
Number of moles of (C\(S_2\)) = mass / molar mass = 37.8 g / 76.14 g/mol ≈ 0.496 mol
From the balanced equation, we can see that the stoichiometric ratio between (C\(S_2\)) and C\(O_2\) is 1:1. Therefore, the number of moles of C\(O_2\) produced will also be 0.496 mol.
Now we can use the ideal gas law to calculate the volume of C\(O_2\) at the given temperature and pressure. The ideal gas law equation is:
PV = nRT
where P is the pressure, V is the volume, n is the number of moles, R is the ideal gas constant (0.0821 L·atm/mol·K), and T is the temperature in Kelvin.
Converting the temperature from Celsius to Kelvin:
T(K) = 28.85°C + 273.15 = 302 K
Using the ideal gas law:
V = nRT / P = (0.496 mol) × (0.0821 L·atm/mol·K) × (302 K) / (1.02 atm) ≈ 11.9 L
The correct answer is 11.9 liters.
for more questions on carbon dioxide
https://brainly.com/question/26150306
#SPJ8
What is the correct formula that would result from the combination of the two ionic species? Cu2+ and SO42-
Answers
CuSO4.
brainliest???
what volume litters of oxygen would be ptoduced in the electrolysis which forms 548 litters of hydrogen both gases measured at stp?
Answers
The ideal gas law may be used to determine the volume of oxygen created in the electrolysis that produces 548 litres of hydrogen at STP (Standard Temperature and Pressure). PV = nRT, where P is the pressure, V is the volume, n is the number of moles, R is the gas constant, and T is the temperature, according to the ideal gas equation.
The pressure is 1 atm, the temperature is 273 K, and the number of moles of hydrogen is 548/22.4 = 24.5 in this example. We may compute the volume of oxygen created by rearranging the ideal gas law: V = nRT/P = 24.5*0.082*273/1 = 483.3 litres.
As a result, the volume of oxygen created in the electrolysis at STP that produces 548 litres of hydrogen is 483.3 litres.
Learn more about oxygen at:
https://brainly.com/question/2272415
#SPJ1
What is the change in enthalpy when 90.6 g
of steam at 100◦C is converted to liquid water
at the same temperature and pressure? The
heat of vaporization of water is 40.7 kJ/mole.
Answers
The change in enthalpy when 90.6 g of steam at 100◦C is converted to liquid water at the same temperature and pressure is 204.7 KJ
How do i determine the change in enthalpy?First, we shall obtain the number of mole water converted to steam. details below:
Mass of water = 90.6 grams Molar mass of water = 18 g/mol Mole of water =?Mole = mass / molar mass
Mole of water = 90.6 / 18
Mole of water = 5.03 moles
Finally, we shall determine the change in enthalpy. Details below:
Mole of water (n) = 5.03 molesHeat of vaporization (ΔHv) = 40.7 KJ/molChange in enthalpy (ΔH) =?ΔH = n × ΔHv
ΔH = 5.03 × 40.7
ΔH = 204.7 KJ
Thus, we can conclude that the change in enthalpy is 204.7 KJ
Learn more about enthalpy change:
https://brainly.com/question/24170335
#SPJ1
Answer the following questions about specific elements. a. Click on the symbol for calcium (Ca). Where does calciumcome from? Is calcium a natural element? b. Scroll down to the first synthetic element that is listedafter calcium. What is its name? How was this elementdiscovered? c.Click back to the periodic table. Click on the element inGroup 7 and Period 5. Is this a natural element or asynthetic element? Explain your answer
Answers
Calcium is a natural element, synthetic element after calcium is technetium, element in group 7 period 5 is also technetium.
a. Calcium (Ca) is a natural element that comes from the Earth's crust. It is the fifth most abundant element in the Earth's crust and can be found in minerals such as calcite, gypsum, and limestone.
b. The first synthetic element that is listed after calcium is technetium (Tc). It was discovered in 1937 by Carlo Perrier and Emilio Segrè, who were able to produce it artificially by bombarding molybdenum with deuterons.
c. The element in Group 7 and Period 5 is Technetium (Tc). It is a synthetic element.
Technetium can only be produced artificially because most forms or isotopes of it (atoms of the same chemical element with different numbers of neutrons) have an excess of neutrons, making it very unstable.
To know more about synthetic elements, refer here:
https://brainly.com/question/13520853#
#SPJ11
Please help some one I’ve been trying this all day and it’s due in 5 min

Answers
Answer:
1st box = compound
2nd box = pure compound
Describe the model rigid rotor, both mathematically and its meaning.
Answers
Answer: An arbitrary rigid rotor is a 3-dimensional rigid object, such as a top
Explanation: The rigid rotor is a mechanical model of rotating systems. ... A special rigid rotor is a linear rotor requiring only two angles to describe, for example, a diatomic molecule. More general molecules are 3-dimensional, such as water (asymmetric rotor), ammonia (symmetric rotor), or methane (spherical rotor).
What do I write for 3

Answers
The above model is a good representation of a molecule because it clearly displays the atoms (white and black circles) and the chemical bond sticks that are present in the atom.
A drawback of the above model is that it does not show clearly the various freedom of movement that may be found in molecules nor does it show the movement of electrons.
The model can be improved by designing it to have relative freedom between the bond to indicate the freedom of movement of bonds.
What are scientific models?Scientific models are structures that are used to represent or explain scientific concepts.
Scientific models may be pictures, three-dimensional objects, illustrations., etc.
Scientists use different models to represent concepts or ideas. For example, the planetary model of the atom, and the ball and stick model of molecules.
The ball and stick model shows the atoms and the bonds that are present in the compound.
Learn more about models at: https://brainly.com/question/18603376
#SPJ1
What is the mass of oxygen, in grams, required to completely react a 24 gram sample of methane gas?
Answers
Answer: 96 g O2
Explanation:
this is combustion
CH4 + 2 O2 --> CO2 + 2 H2O
24 g CH4 X 1 mole Ch4 / 16.043 g CH4) X ( 2 moles O2 / 1 moles CH4) X
( 31.999 G O2 / 1 mole O2 ) = 95.74 g O2 = 96 g O2 in correct sig figs
Calculate strain energy for the conformer pictured below, using strain energy increments from the table.

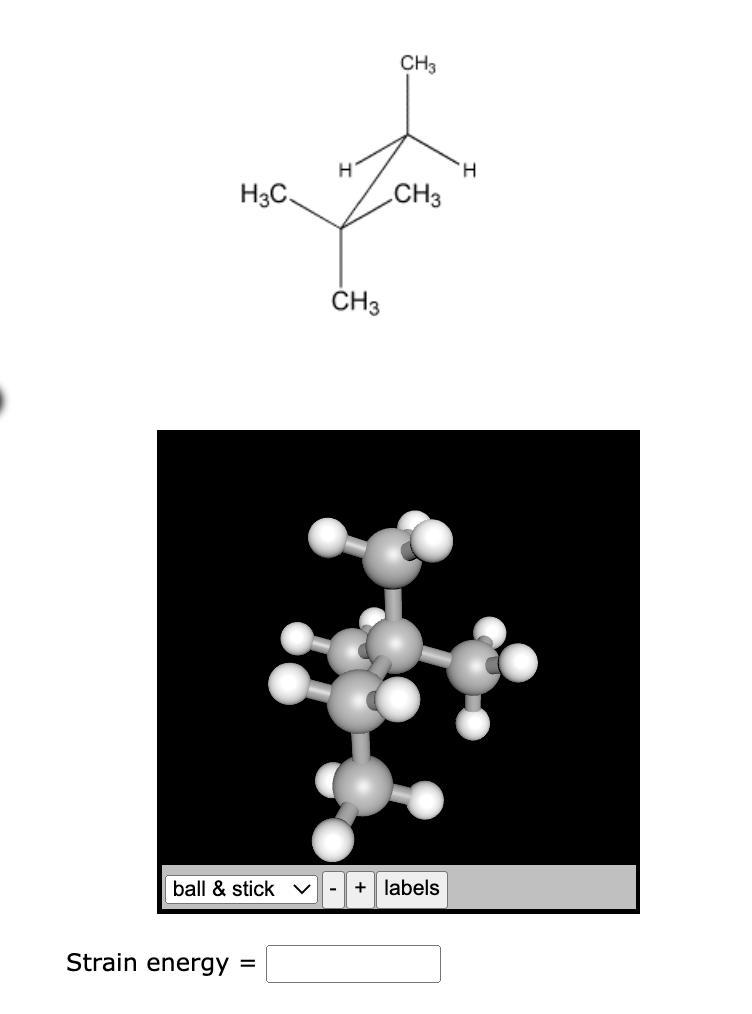
Answers
Strain energy for the conformer is 13.8 kj/mol
Energy of eclipsed H-H bond is 4.0 kJ mol
Energy of eclipsed Н-СH3 bond is 5.8 kJ mol
Total strain energy of conformer (S,) E=2×ECLIPSED(H-H)+eclipse(H-CH3)
Substitute, 4.0 kJ mol for eclipsed (H-H) and 5.8 kJ mol for eclipsed (H-CH thus,
E =2x4.0 kJ mol +5.8 kJ mol
E=13.8 kJ mol
When rotated about the C-C single bond and the dihedral angle is 60°, conformational isomers give various spatial configurations of a molecule. Eclipsed conformation and Staggered conformation are the two different forms of conformational isomers.Because of the larger torsional strain brought on by the hydrogen atoms linked closest to one another, the eclipsed conformation is least stable.To know more about conformation visit : https://brainly.com/question/15215912
#SPJ1
Identify two ways to measure mass
Answers
Answer:
Putting it in water, and see the displacement of the water, or just measuring its weight and devide it by the gravitational pull
1. Liver Balance
2. Physical balance
3. Electronic Balance
Which element is in the same group as Lithium (Li)?
Carbon (C)
Potassium (K)
Chlorine (Cl)
Hydrogen (H)
Answers
Answer: Potassium (K)
Explanation:
Module #5: Countin
REVIEW QUESTIONS FO
1. Can an element undergo a decomposition
Answers
Answer:
Elements cannot undergo decomposition reactions.
Explanation:
Elements cannot undergo decomposition reactions. 2. Classify the following reactions as decomposition, formation, complete combustion, or none of these: a. C₆H₁₂O₆ (s) + 6O₂ (g) → 6CO₂ (g) + 6H₂O (g)
80. When water forms ice, hydrogen bonds around a water molecules are at ... apart.
1.
30 °C
2. 45 °C
3.
90 °C
4.
180 °C
5. 35 °C
6. I do not know.
N
Answers
Answer:
6
Explanation:i dont know
Two objects of equal mass have a force of gravity of 6 N between them. Imagine the mass of one is cut in half and the other stays the same, what is the force due to gravity?
Answers
Answer:
F' = 3 N
Explanation:
Given that,
Two objects of equal mass have a force of gravity of 6 N between them.
If the mass of one is cut in half and the other stays the same such that,
m₁' = m₁ and m₂' = (m₂/2)
We need to find the new force. The gravitational force between two objects is given by :
\(F=G\dfrac{m_1m_2}{r^2}\)
We have, F = 6 N
New force,
\(F'=G\dfrac{m_1'm_2'}{r^2}\\\\F'=\dfrac{Gm_1\times \dfrac{m_1}{2}}{r^2}\\\\=\dfrac{1}{2}\times \dfrac{Gm_1m_2}{r^2}\\\\F'=\dfrac{F}{2}\\\\F'=\dfrac{6}{2}\\\\F'=3\ N\)
So, the new force becomes 3 N.
A doorbell uses an electromagnet for its operation.
Question 7 options:
True
False
Answers
Explanation:
The heart of the doorbell is an electromagnetic so yeah it does.
Electromagnets are coils of wire wrapped around a small piece of magnetic metal. When electricity passes through the wire, it creates a magnetic field around the wire. When you press a doorbell button, you complete an electrical circuit that allows household electricity to flow through the doorbell's internal electromagnet.
An amateur entomologist captures a particularly excellent ladybug specimen in a plastic jar. The internal volume of the jar is 0.5L, and the air within the jar is initially at 1 atın. The bug-lover is so excited by the catch that he squeezes the jar fervently in his sweaty palm, compressing it such that the final pressure within the jar is 1.25 atm. What is the final volume of the ladybug's prison?
Answers
The final volume of the ladybug's prison is approximately 0.4 liters.
To determine the final volume of the ladybug's prison, we can use Boyle's Law, which states that the pressure and volume of a gas are inversely proportional at constant temperature. The equation for Boyle's Law is:
P1 * V1 = P2 * V2
Where P1 and V1 are the initial pressure and volume, and P2 and V2 are the final pressure and volume, respectively.
In this scenario, the initial volume (V1) is given as 0.5 L, and the initial pressure (P1) is 1 atm. The final pressure (P2) is 1.25 atm. We need to find the final volume (V2).
Plugging the given values into the equation, we have:
1 atm * 0.5 L = 1.25 atm * V2
Simplifying the equation, we find:
0.5 L = 1.25 atm * V2
Dividing both sides of the equation by 1.25 atm, we get:
0.5 L / 1.25 atm = V2
V2 ≈ 0.4 L
For such more questions on volume
https://brainly.com/question/31454001
#SPJ8
Club soda is an aqueous solution of carbon dioxide. A sample of club soda is titrated with 0.04202M NaOH(aq) according to the reaction equation below:
CO2(aq)+2NaOH(aq)→Na2CO3(aq)
If it takes 32.14 mL of 0.04202M NaOH(aq) to react with a 25.00 mL sample of club soda, what is the concentration of CO2 in club soda (in g/L )?

Answers
The concentration of CO2 in club soda is approximately 1.1964 g/L.
To find the concentration of CO2 in club soda, we need to use the stoichiometry of the reaction and the volume and concentration of the NaOH solution used.
The balanced equation for the reaction is:
CO2(aq) + 2NaOH(aq) → Na2CO3(aq)
From the stoichiometry of the equation, we can see that 1 mole of CO2 reacts with 2 moles of NaOH. Therefore, the moles of CO2 can be calculated using the volume and concentration of NaOH solution used.
Given that 32.14 mL of 0.04202 M NaOH solution was used, we can calculate the moles of NaOH:
moles of NaOH = volume (L) × concentration (M)
moles of NaOH = 32.14 mL × 0.04202 mol/L
moles of NaOH = 0.001351 mol
According to the stoichiometry of the equation, 1 mole of CO2 reacts with 2 moles of NaOH. Therefore, the moles of CO2 can be calculated as:
moles of CO2 = (moles of NaOH) / 2
moles of CO2 = 0.001351 mol / 2
moles of CO2 = 0.0006755 mol
Now, we need to convert the moles of CO2 to grams. The molar mass of CO2 is approximately 44.01 g/mol.
mass of CO2 = moles of CO2 × molar mass of CO2
mass of CO2 = 0.0006755 mol × 44.01 g/mol
mass of CO2 = 0.02979 g
Finally, we need to express the concentration of CO2 in club soda in g/L. We are given that the sample of club soda used is 25.00 mL.
concentration of CO2 = (mass of CO2) / (volume of club soda in L)
concentration of CO2 = 0.02979 g / (25.00 mL × 0.001 L/mL)
concentration of CO2 = 1.1964 g/L
For more such questions on concentration visit:
https://brainly.com/question/28564792
#SPJ8
the reaction of -COOH and -NH₂:
a) forms urea
b) creates a peptide bond
c) forms glycogen
d) forms a ketone body
Answers
The reaction of -COOH and -NH₂ creates a peptide bond.
What is peptide bond?Peptide bond is defined as a type of amide type covalent chemical bond which is formed between the two simultaneous molecules of amino acid in such a way that carboxyl group of one molecule of amino acid get attached to the NH₂ group of another molecules of Amino acid.
Formation of Peptide bondPeptide bond is formed by the elimination of water molecules.
The hydroxyl group from the carboxyl and proton hydrogen cation from NH₂ group.
Thus, we concluded that the reaction of -COOH and -NH₂ creates a peptide bond by losing—OH from the -COOH and H+ from the -NH₂.
learn more about Peptide bond:
https://brainly.com/question/11559138
#SPJ9