Ferrocene, synthesized in 1951, was the first organic iron compound with Fe-C bonds. An understanding of the structure of ferrocene gave rise to new ideas about chemical bonding and led to the preparation of many useful compounds. In the combustion analysis of ferrocene, which contains only Fe, C, and H, a 0.9437-g sample produced 2.233 g of CO₂ and 0.457 g of H₂O. What is the empirical formula of ferrocene?
Answers
The empirical formula of ferrocene is C10 H10 Fe.
What is empirical formula?
In chemistry, the empirical formula of a chemical compound is the simplest whole number ratio of atoms present in a compound. A simple example of this concept is that the empirical formula of sulfur monoxide, or SO, would simply be SO, as is the empirical formula of disulfur dioxide, S2O2. Thus, sulfur monoxide and disulfur dioxide, both compounds of sulfur and oxygen, have the same empirical formula. However, their molecular formulas, which express the number of atoms in each molecule of a chemical compound, are not the same.
For ferrocene, we see that the empirical formula is identical to the molecular formula, despite have a large number of carbon and hydrogen atoms. This is because the molecular formula has only a single atom of iron. As the empirical formula has to include only whole numbers, we cannot divide the numbers in the formula in any way without ending up with a fraction for the iron molecules. Thus, C10 H10 Fe is the most simplified ration of atoms in ferrocene.
To learn more about molecular formulas click on the link below:
https://brainly.com/question/23948807
#SPJ4
Related Questions
What was the initial volume of a gas that had an initial temperature of 20.65 C if it reached a final temperature of 41.77 C and final volume of 16.14 L.
Answers
If a gas had an initial temperature od 20.65 C and a final temperature of 41.77 C, its initial volume would be 7.979.
How are volume and example used?The volume of any solid in three dimensions is equal to the area it occupy. These solids include shapes like a cube, cuboid, conical, cylinder, and sphere. The form affects how much volume there is.
What does a gas' volume mean?The space occupied by the gaseous particles under typical pressure and temperature circumstances is referred to as the volume of gas. It has the symbol "V." The symbol for volume in the SI is "liters," or "L". When the gas is at normal temperature, a mole has a volume of 24 m3, or 24 000 cm3. The molar volume of a gas is this quantity.
To know more about volume visit:
https://brainly.com/question/24086520
#SPJ13
how do enzymes help with digestion
Answers
Answer: Digestive enzymes play a key role in breaking down the food you eat. These proteins speed up chemical reactions that turn nutrients into substances that your digestive tract can absorb. Your saliva has digestive enzymes in it
Explanation:
Help help help I will give brainliest

Answers
Answer:
BUTANE
Explanation:
H3C--CH2--CH2--CH3
HERE, 4 CARBON AND SINGLE BOND SO, BUTANE
Answer:
D.BUTANE
Explanation:
THIS IS MY ANSWER OK
Flammable liquids are those that have a flashpoint of:.
Answers
Answer:
Flammable liquid is any liquid having a flashpoint at or below 199.4 °F (93 °C).
Explanation:
:)
Flashpoints for flammable liquids are at 100°F. Lower flash points allow for easier ignition of liquids. A liquid's flash point is the lowest temperature at which a concentrated enough layer of vapor accumulates on top of its surface to allow for ignition.
A liquid that is flammable has a flash point* below 37.8 ° C (100 ° F). A flammable liquid has a flash point that ranges from 37.8 to 93.3° C (100 to 200° F), which is above the standard working temperature. Flammable liquids emit a vapor that, at standard working temperatures, is easily ignitable.
The substance ignites more readily the lower the flash point. For instance, petrol is more flammable than ethylene glycol and has a flash point of about -40 degrees C (-40 °F).
To know more about Flammable liquids, visit;
https://brainly.com/question/33716084
#SPJ4
2KCIO3 -> 2KCI+ 302
How many moles of oxygen are produced by
the decomposition of 6.0 moles of potassium
chlorate, KCIO3?
Answers
Answer:
9 moles
Explanation:
The balanced chemical equation for this decomposition reaction is as follows:
2KCIO3 → 2KCI+ 302
Based on this equation, 2 moles of potassium chlorate (KCIO3) decomposes to form 3 moles of oxygen gas (O2).
Hence, 6 moles of pottasium chlorate will decompose to produce;
6 × 3 ÷ 2
= 18 ÷ 2
= 9 moles of O2.
what reaction (oxidation or reduction) occurs at the cathode of a voltaic cell?
Answers
In a voltaic cell, the reaction that occurs at the cathode is the reduction reaction.
A voltaic cell consists of two half-cells, each containing an electrode (cathode or anode) and an electrolyte. In the cell, electrons flow from the anode to the cathode through an external circuit, generating electricity. At the cathode, reduction takes place as the positively charged ions in the electrolyte gain electrons, leading to a decrease in their oxidation state.
A voltaic cell, often called a galvanic cell, is an electrochemical device that produces electricity through uninhibited redox processes, both oxidation and reduction. It is divided into two distinct half-cells. A half-cell is made up of an electrode (a metal strip, M) dissolved in a solution containing Mn+ ions. M can be any metal. A wire from one electrode to the other connects the two half-cells cathode and anode. The half-cells are also connected by a salt bridge.
To learn more about voltaic cell, visit:
https://brainly.com/question/26228780
#SPJ11
Somebody please help me!! What happens when ionic bonds are formed?
Answers
Answer:
The atom that loses the electrons becomes a positively charged ion, while the one that gains them becomes a negatively charged ion
FILL THE BLANK. The difference in energy carried by electrons at different points in a circuit will determine the _____
Answers
The difference in energy carried by electrons at different points in a circuit will determine the voltage or potential difference.
Voltage, often referred to as electric potential difference, is a measure of the electric potential energy per unit charge between two points in an electric circuit. It represents the difference in electric potential energy that an electron possesses when it moves from one point to another in the circuit.
When there is a difference in energy levels between two points in a circuit, it creates an electric field. This electric field exerts a force on the charged particles (such as electrons) within the circuit, causing them to move. The work done by this electric field on the charged particles is what gives rise to the difference in energy and, consequently, the voltage.
The voltage can be visualized as the "push" or driving force that causes electrons to move through a circuit. It is measured in volts (V) and can be either positive or negative, depending on the direction of the energy difference.
To know more about potential difference here
https://brainly.com/question/28566562
#SPJ4
How many grams of He are necessary to fill a balloon having a volume of 4.50 × 10^{3} mL to a pressure of 1.14 × 10^{3} torr at 25.0ºC?
Answers
The mass in grams of He are necessary to fill a balloon having a volume of 4.50×10³ mL to a pressure of 1.14×10³ torr at 25.0ºC is 1.104 grams.
How do we calculate the grams from moles?Mass (W) in grams from moles (n) will be calculated by using the below equation:
n = W/M, where
M = molar mass
Moles of helium gas will be calculated by using the ideal gas equation:
PV = nRT, where
R = universal gas constant = 62.363 L.torr / K.mol
P = pressure of gas = 1.14×10³ torr
V = volume of gas = 4.50×10³ mL = 4.50 L
n = moles of gas = ?
T = temperature = 25.0ºC = 298 K
On putting these values on the ideal gas equation, we get
n = (1140)(4.5) / (62.363)(298) = 5130 / 18584
n = 0.276 moles
Now we convert this moles into mass by using the first equation as:
W = (0.276mol)(4g/mol) = 1.104 g
Hence required mass of helium gas is 1.104 grams.
To know more about ideal gas equation, visit the below link:
https://brainly.com/question/12873752
#SPJ1
Which major branch of chemistry would be most concerned
with studying a series of chemical reactions in order to
measure the amount of heat being released in each one?
Answers
Physical chemistry a major branch of chemistry would be most concerned with studying a series of chemical reactions in order to measure the amount of heat being released in each one.
Understanding the physical characteristics of atoms and molecules, how chemical processes take place, and what these characteristics indicate are the main goals of physical chemists. Their findings are based on an understanding of chemical characteristics and a description of how they behave utilizing physics theories and mathematical calculations.Thermochemistry, which encompasses the study of the heat energy of chemical processes occurring during phase transitions like gas to liquid or vice versa, is one of the main examples of physical chemistry. It provides information on entropy, heat capacity, Gibbs free energy, or formation heat.For more information on chemistry kindly visit to
https://brainly.com/question/13428382
#SPJ1
An oxide of P contains 50% by mass of P. Its Relative molecular mass is 64. The molecular formula of the oxide is (P=32; O=16)
a. PO
b. PO2
c. P2O
d. PO3
Answers
Answer:
B) PO2
Explanation:
first solve empirical formula
50%of P and 50%of O
divide each by molecular massP. O
50/32. 50/16
1.5. 3.0
divide both by the smallest1.5/1.5. 3.0/1.5
1. :. 2
Empirical formula=PO2
Molecular formula=PO2
(PO2)n= 64
(32+(16×2)=64
(32+32)n=64
64n=64
n= 1
what is neutralisation reaction? why is it named so? give one example.
Answers
Answer:
In chemistry, neutralization or neutralisation is a chemical reaction in which acid and a base react quantitatively with each other. In a reaction in water, neutralization results in there being no excess of hydrogen or hydroxide ions present in the solution.Neutralization reactions are the reaction between acid and base. The products formed are water and salt. It is called so because the acid and base neutralize each other to form water and salt.Hint: The neutralization reaction is the one in which an acid reacts with an equimolar amount of base to give salt and water. The example could be a reaction between any strong acid and a base. The sodium chloride formed is a result of neutralization reaction.
Trust me mark me as brainliest trust me
Question 2 of 10
Which of the following describes an important role scientists have in society? A. Scientists provide information to help people make decisions.
B. Scientists tell people which issues are the most important.
C. Scientists know the most about every subject.
D. Scientists use their research to define what is right and wrong.
SUBMIT
Answers
Scientists tell people which issues are the most important describes an important role scientists have in society.
In addition to advising policymakers and other stakeholders about the best and wisest steps to take toward a human-centered society, scientists have a crucial role to play in preventing unwise and dangerous decisions. This promotes scientific knowledge and strengthens cross-cultural relationships and collaborative research. Additionally, they shouldn't overlook the objective, perpetually unfinished nature of science. With the help of a transdisciplinary cross-talks method, we emphasize the significance of information transfer across all scientific disciplines.
Due to the specific ways in which they aid in understanding how the world functions, scientists are crucial to society. It is a mode of thinking that helps us organize our knowledge in order to comprehend how things operate on a deeper level.
Learn more about Scientists here
https://brainly.com/question/22249890
#SPJ9
chemistry between people is the stongest like mine and urs right?
Answers
Answer:
..sure....
Explanation:
Answer:
hmm i dont see it homie sike gimmi that bootiy
94. 2 ml of 3. 8 Molar Rubidium Carbonate is mixed with 38. 2 ml of 5. O Molar Barium Acetate to form a precipitate:
1)Calculate the theoretical mass in grams of the precipitate using only the volume and molartity of the barium acetate
Answers
Given that the volume and molarity of barium acetate are 38.2 ml and 5.0 M, respectively. We need to find the theoretical mass in grams of the precipitate. Let's first write the balanced chemical equation for the reaction taking place: Rubidium Carbonate + Barium Acetate → Barium Carbonate + Rubidium AcetateRb2CO3(aq) + Ba(C2H3O2)2(aq) → BaCO3(s) + 2 RbC2H3O2(aq).
We can see that 1 mole of barium acetate reacts with 1 mole of barium carbonate. Hence, the molar ratio of barium acetate and barium carbonate is 1:1.Using the molarity and volume of barium acetate, we can find the moles of barium acetate as: Moles of barium acetate = Molarity × Volume in litres= 5.0 mol/L × (38.2/1000) L= 0.191 moles. Now, from the balanced chemical equation, we can see that 1 mole of barium carbonate is formed from 1 mole of barium acetate.
Therefore, the number of moles of barium carbonate formed will also be 0.191 moles. Now, let's calculate the mass of barium carbonate using its molar mass. Molar mass of BaCO3= (1 × atomic mass of Ba) + (1 × atomic mass of C) + (3 × atomic mass of O)= (1 × 137.33 g/mol) + (1 × 12.01 g/mol) + (3 × 16.00 g/mol)= 197.33 g/mol. Theoretical mass of BaCO3= Number of moles of BaCO3 × Molar mass of BaCO3= 0.191 mol × 197.33 g/mol= 37.7 g. Therefore, the theoretical mass of the precipitate is 37.7 g (approx) when only the volume and molarity of the barium acetate are taken into account. Note: In order to find the limiting reagent and the actual mass of the precipitate formed, we need to consider the volume and molarity of both the reactants.
To know more about theoretical mass visit
https://brainly.com/question/14527467
#SPJ11
Why do you need to combust benzoic acid before testing the "unknown" sample you are interested in? Why do you need to combust more than one sample of the "unknown" you are testing?
Answers
Various samples of the must be combusted in other to obtain a reliable result.
What is a bomb calorimeter?The bomb calorimeter is used to obtain the energy of a sample. This is called the energy equivalent or the energy value of the sample.
Now, we know that the bomb calorimeter is calibrated by the use of benzoic acid hence it must be combusted first to obtain the basic energy value of the calorimeter.
Various samples of the must be combusted in other to obtain a reliable result.
Learn more about bomb calorimeter:https://brainly.com/question/24245395?
#SPJ1
Why do waves move faster at higher temperatures and in a solid phase?
There is more energy and the particles are farther apart.
There is less energy and the particles are closer together.
There is less energy and the particles are farther apart.
There is more energy and the particles are closer together.
Answers
Answer:
Higher temperatures means more energy, and a solid phase means the particles are close together. This results in highly energized particles that bump into the particles close to them, who in turn bump into more particles.
Explanation:
waves move faster at higher temperatures and in a solid phase there is more energy and the particles are farther apart there is less energy and the particles are closer together there is less energy and the particles are farther apart there is more energy and the particles are closer together.
The waves move faster at higher temperatures and in a solid phase because there is more energy and the particles are closer together.
What is wave?A wave can be described as a medium which transports energy without causing net particle movement. Elastic deformation, pressure change, electric along with magnetic intensity, electric potential, or temperature can all be examples.
What is energy?The quantitative property that's also transferred to a body or a physical system, recognizable in the work performance as well as in the form of heat and light, is called energy.
What is temperature?Temperature is the measurement of a body's degree of hotness or coolness.
The faster the wave is conveyed, the greater the particle interactions are. In general, sound travels quicker through solids than through liquids, and faster through liquids than through gas. Because warmer particles move at a higher pace, the temperature actually speeds up to sound.
Therefore, the correct answer will be option (d).
To more about wave and energy .
https://brainly.com/question/9624826.
#SPJ2
What is the molarity of a solution that contains 4. 25 mol NaCl in 1950 mL of solution? (3 SigFigs)
Answers
The molarity of the solution is 2.18 M
Molarity is a measure of the concentration of a solute in a solution and is defined as the number of moles of solute per liter of solution. Its unit is mol/L or M.
To calculate the molarity of a solution, we need to divide the number of moles of solute (in this case, NaCl) by the volume of the solution in liters.
First, we need to convert volume from milliliters (mL) to liters (L);
1950 mL = 1950/1000 L = 1.95 L
Now we can calculate the molarity;
Molarity = moles of solute/volume of solution in liters
Molarity = 4.25 mol / 1.95 L
Molarity = 2.18 M
To know more about molarity here
https://brainly.com/question/8732513
#SPJ4
what is the mass % of carbon in dimethylsulfoxide (c2h6so) rounded to three significant figures? group of answer choices 7.74 78.1 28.6 25.4 30.7
Answers
Dimethylsulfoxide has the formula C2H6SO.Therefore, the correct answer is option D: 25.4.
Option D.
To determine the mass percent of carbon in this compound, we need to calculate the molar mass of the compound first. Molar mass is the sum of the atomic masses of all the atoms in the molecule. We can use the periodic table to obtain the atomic masses. For this compound, the molar mass will be:2 (atomic mass of carbon) + 6 (atomic mass of hydrogen) + 32 (atomic mass of sulfur + 16 (atomic mass of oxygen) = 78 g/molNext, we need to determine the mass of carbon in one mole of the compound. We can do this by multiplying the number of carbon atoms by the atomic mass of carbon. In this case, there are 2 carbon atoms in one mole of the compound. Therefore, the mass of carbon in one mole of the compound is:2 (number of carbon atoms) x 12.01 (atomic mass of carbon) = 24.02 g/molFinally, we can calculate the mass percent of carbon in dimethylsulfoxide using the formula:mass percent of carbon = (mass of carbon / total molar mass) x 100%Substituting the values we obtained:mass percent of carbon = (24.02 g/mol / 78 g/mol) x 100% = 30.77%Rounding to three significant figures gives us a final answer of 30.7%.
Option D.
For more questions on Dimethylsulfoxide
https://brainly.com/question/10914338
#SPJ8
which statement is true of a rock layer that had another rock layer deposited on top of it?
Answers
Answer:
second rock...I know it
if her particles agglomerated into spherical aggregates with average size of 2 µm, how many aggregates would she have in her scaffold?
Answers
Yennefer would have approximately 4.19 x 10⁻³ mm³ aggregates in her scaffold.
To calculate the number of aggregates Yennefer would have in her scaffold, we need to use the information provided in the question. The hydroxyapatite nanoparticles synthesized by Yennefer have a cylindrical shape with a diameter of 4 nm and length of 42 nm.
She mixed them with PLGA to give a weight ratio of 60/40. Assuming the density of PLGA is 1 g/cm³ and hydroxyapatite is 3 g/cm³, we can calculate the volume fraction of hydroxyapatite in the nanocomposite as follows:
Volume fraction of hydroxyapatite = (Weight fraction of hydroxyapatite / Density of hydroxyapatite) / [(Weight fraction of hydroxyapatite / Density of hydroxyapatite) + (Weight fraction of PLGA / Density of PLGA)]
= (0.4 / 3) / [(0.4 / 3) + (0.6 / 1)]
= 0.126
This means that 12.6% of the volume of the nanocomposite is occupied by hydroxyapatite nanoparticles. To calculate the total volume of hydroxyapatite in the scaffold, we need to multiply the volume fraction by the total volume of the scaffold:
Total volume of hydroxyapatite = 0.126 x (20 x 20 x 1) mm³ = 504 mm³
Since each hydroxyapatite nanoparticle has a volume of πr²l, where r is the radius and l is the length, we can calculate the number of particles in the scaffold as follows:
Number of hydroxyapatite nanoparticles = Total volume of hydroxyapatite / Volume of each nanoparticle
= 504 mm³ / [(4 nm / 2)² x 42 nm x (10⁻³)³ mm³/nm³]
= 2.97 x 10²¹
However, we are asked for the number of aggregates, not individual nanoparticles. If we assume that the particles agglomerated into spherical aggregates with an average size of 2 µm, we can calculate the number of aggregates as follows:
Volume of each aggregate = (4/3) x π x (1 µm)³ = 4.19 x 10⁻³ mm³
Number of aggregates = Total volume of hydroxyapatite / Volume of each aggregate
= 504 mm³ / (4.19 x 10⁻³ mm³)
= 1.20 x 10²⁵
However, we need to remember that the question asked for the number of aggregates with an average size of 2 µm, so we need to adjust our answer accordingly. Assuming that the aggregates are spherical, we can use the formula for the volume of a sphere to calculate the volume of each aggregate:
(4/3) x π x (1 µm)³ = 4.19 x 10⁻³ mm³
So the number of aggregates Yennefer would have in her scaffold is approximately 4.19 x 10⁻³ mm³.
To know more about hydroxyapatite refer here:
https://brainly.com/question/28147631#
#SPJ11
Complete Question:
graduate student (Yennefer) prepared a nanocomposite that contains a polymer matrix (poly-lactide- co-glycolide, abbreviated as PLGA) and hydroxyapatite nanoparticles. She mixed them to give a weight ratio of PLGA/hydroxyapatite =60/40. Yennefer dispersed her hydroxyapatite nanoparticles in the PLGA scaffold, which has a dimension of 20x20x1 mm. The hydroxyapatite nanoparticles she synthesized has a cylindrical shape with a diameter of 4 nm and length of 42 nm. She used PLGA with a lactic acid to glycolic acid ratio of 50:50.
if her particles agglomerated into spherical aggregates with average size of 2 µm, how many aggregates would she have in her scaffold?
HELP PLEASE!! BRAINLIEST!! 5 STARS & THANKS

Answers
step by step explanation
3) Calculate the net force *
3N
5N
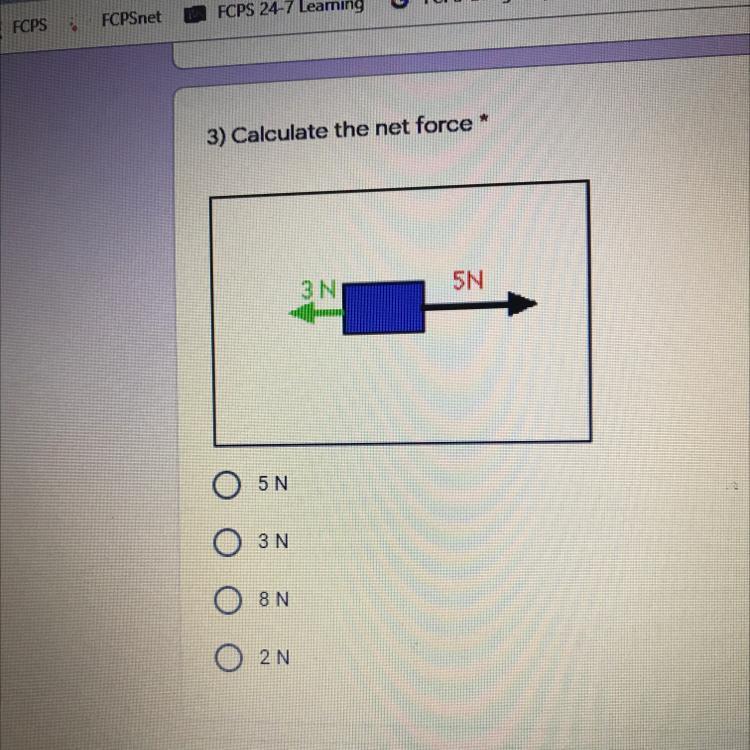
Answers
Answer:
2N
Explanation:
since they are going both sides you subtract
Calculate q, the heat released in each reaction.
Use the equation q = cmAT.
(Use c = 4.18 J/g °C and the total mass, m.)
Record to 2 significant figures.
Reaction 1: blank J
Reaction 2: blank J
Answers
The heat released in each reaction is:
Reaction 1: 3700J
Reaction 2: 3200J
What is heat?
Heat is a transfer of kinetic energy from one medium or object to another or from an energy source to a medium or object. A similar energy transfer can be done in three ways: radiation, conduction, and convection.The standard unit of heat in the International System of Units( SI) is the calorie( cal), which is the quantum of energy transferred needed to raise the temperature of one gram of pure liquid water by one degree Celsius, assuming that the temperature of the water is advanced. advanced than the freezing point and lower than the boiling pointTo know more about heat, click the link given below:
https://brainly.com/question/1429452
#SPJ1
how many moles of h are in 4.56 moles of nh2nh2?
Answers
There are 9.12 moles of H in 4.56 moles of NH2NH2.
To find the number of moles of H in a given number of moles of NH2NH2, you can use the following formula:
moles of H = moles of NH2NH2 x (number of H atoms in one molecule of NH2NH2)
In this case, there are 4 H atoms in one molecule of NH2NH2, so the formula becomes:
moles of H = moles of NH2NH2 x 4
Plugging in the given number of moles of NH2NH2, we get:
moles of H = 4.56 x 4
moles of H = 18.24
Therefore, there are 9.12 moles of H in 4.56 moles of NH2NH2.
You can read more about moles at https://brainly.com/question/29367909#:
#SPJ11
15 grams of water into moles
Answers
0.83 moles present in 15 grams of water .
moles=given mass/molar mass
given mass=15 grams
molar mass, water=18 gram
moles=given mass/molar mass
moles=15/18
moles=0.83
The mole concept is a helpful technique to quantify the amount of a material. When dealing with particles at the atomic (or molecular) level, it is recognized that even one gram of a pure element has a vast number of atoms. The mole concept is widely used in this context. The most used unit of measurement is the "mole," which is a count of a significant number of particles.
The number 6.02214076*1023, also known as the Avogadro constant, is usually denoted by the letter "NA". Among the elementary things that may be represented in moles are atoms, molecules, monoatomic and polyatomic ions, as well as other particles (such as electrons).
To know more about moles visit : brainly.com/question/15209553
#SPJ9
What is Decomposition Reaction
Answers
Answer:
Explanation:
Decomposition reaction, also known as analysis or dissociation, is a type of chemical reaction in which a compound breaks down into simpler substances or elements. In this reaction, a single reactant undergoes a chemical change and produces two or more products.
The decomposition reaction can be represented by the general equation:
AB → A + B
Where AB is the reactant, and A and B are the products. The reactant AB is usually a compound, and it breaks down into its constituent elements or simpler compounds.
There are different types of decomposition reactions, including:
Thermal decomposition: It occurs when a compound is heated, resulting in its decomposition into simpler substances. For example, the thermal decomposition of calcium carbonate (CaCO3) produces calcium oxide (CaO) and carbon dioxide (CO2):
CaCO3 → CaO + CO2
Electrolytic decomposition: It takes place when an electric current is passed through an electrolyte, causing it to break down into its component ions. For instance, the electrolysis of water (H2O) leads to the decomposition into hydrogen gas (H2) and oxygen gas (O2):
2H2O → 2H2 + O2
Photochemical decomposition: It occurs when a compound undergoes decomposition due to exposure to light energy. Chlorine gas (Cl2) can decompose into chlorine atoms (Cl) under the influence of light:
Cl2 → 2Cl
These are just a few examples of decomposition reactions. They are important in various chemical processes and are used in industries, laboratory experiments, and natural phenomena. By understanding and controlling decomposition reactions, scientists can gain insights into the behavior of different compounds and develop practical applications in fields such as chemistry, materials science, and environmental science.
Answer:
Explanation:
reaction in which a compound breaks down into simpler substances or elements
why is it important to not dispose of medicine this way
Answers
Answer:
Because Environmental Risks
Explanation:
Medicines poured down the drain can enter the environment and your community’s drinking water supplies. And it can cause a lot of diseases!
Chlorobenzene, C6H5Cl, is used in the production of chemicals such as aspirin and dyes. One way that chlorobenzene is prepared is by reacting benzene, C6H6, with chlorine gas according to the following BALANCED equation. C6H6 (l) + Cl2 (g) C6H5Cl (s) + HCl (g) a. What is the theoretical yield if 45. 6 g of benzene react? b. If the actual yield is 63. 7 g of chlorobenzene, calculate the percent yield
Answers
The theoretical yield of the reaction is 65.7 grams.
Mass C₆H₆ = 45.6 g
actual yield = 63.7 g
Balanced chemical formula:
C6H6 (l) + Cl2 (g) → C6H5Cl (s) + HCl (g)
Calculate the molar masses of C6H6 and C6H5Cl.
For C₆H₆
Molar mass of C₆H₆ = (12.01 g/mol × 6) + (1.008 g/mol × 6)
Molar mass of C₆H₆ = 78.108 g/mol
For C₆H₅Cl
Molar mass of C₆H₅Cl = (12.01 g/mol × 6) + (1.008 g/mol × 5) + (35.45 g/mol × 1)
Molar mass of C₆H₅Cl = 112.55 g/mol
Since 1 mole of C6H6 = 1 mole of C6H5Cl, the theoretical yield of the reaction is
65.7 grams
Yield calculation = 96.9%
Learn more about Molar mass
brainly.com/question/7585012
#SPJ4
does the substance x have a fixed shape
Answers
Answer:
X is a liquid becoz it a definite volume but gas doesnot have a fixed volume.
Explanation:
Answer:
yes
Explanation: