explain why we must balance all chemical equations
Answers
Answer:
The chemical equation needs to be balanced so that it follows the law of conservation of mass.
Explanation:
Explanation:
the chemical equation needs to be balance so that it follows the law of conservation of mass. a balance chemical equation occurs when the number of the different atoms of elements to the reactants side is equal to that of the product side. balancing chemical equation is a process of trial and error .
Related Questions
ten uses of carbon materials in our environment
Answers
Answer:
Carbon material refers to any material that's composed primarily of carbon atoms. Carbon could be a flexible component that can exist in numerous diverse shapes, each with one of a kind physical and chemical properties.
Explanation:
Carbon materials have a wide extend of uses in our environment. Here are ten illustrations:
Fuel: Carbon materials, such as coal, are utilized as fuel to produce power and warm homes and buildings.Transportation: Carbon fiber may be a lightweight and solid fabric that's utilized to fabricate air ship, cars, bikes, and other transportation vehicles.Batteries: Carbon materials are utilized in batteries to move forward their execution, productivity, and life expectancy.Water decontamination: Actuated carbon is utilized in water refinement frameworks to evacuate pollutions, odors, and taste.Horticulture: Carbon materials, such as biochar, are utilized as soil corrections to move forward soil richness, water maintenance, and supplement retention.Therapeutic applications: Carbon materials, such as graphene, are utilized in restorative applications to create unused advances for medicate conveyance, imaging, and diagnostics.Gadgets: Carbon materials are utilized in hardware, such as transistors, sensors, and shows, to make strides their execution and diminish their estimate.Development: Carbon materials, such as carbon nanotubes, are utilized in development materials to improve their quality, toughness, and fire resistance.Sports hardware: Carbon materials, such as graphite, are utilized to make sports hardware, such as tennis rackets, golf clubs, and hockey sticks.Natural remediation: Carbon materials, such as activated carbon, are utilized in natural remediation to clean up sullied soils and groundwater.To learn more about carbon material,
https://brainly.com/question/17185984
https://brainly.com/question/30712449
A 10.0 g sample of water was heated from 20.0°c to 50.0°c. how much energy did the water gain? the specific heat of water is 4.18 j/g·°c.
Answers
The energy gained by water is 1254 J.
Given,
Mass, m = 10.0g
Initial Temperature = 20°C
Final Temperature = 50°C
Specific heat capacity, c = 4.18 J/g·°C
H = mcΔT
where,
ΔT = Final Temperature - Initial Temperature
ΔT = 50 - 20
ΔT = 30 °C
Substituting the given values,
H = 10 * 4.18 * 30
H = 1254 J
Therefore, the energy gained by water is 1254 J.
For more information, regarding Energy Gain refer to the link given below:
brainly.com/question/18980657
Imagine two pure samples of gas in identical closed containers: sample A and sample B.
Sample A has a higher temperature than sample B.
Each of the statements below is true about the samples. Which statements describe a
property that contributes to the difference in temperature? Select all that apply.
The total mass of sample A is higher than the
total mass of sample B.
The particles in sample A have a higher average
speed than the particles in sample B.
Each particle in sample A has more mass than
each particle in sample B.

Answers
The statements that contributes to the difference in temperature are the particles in Sample A have a higher average speed than the particles in sample B and Each particle in sample A has more mass than each particle in sample B and the correct options are option 2 and 3.
In Kinetic Theory of Gas, we assume speed of a gas molecule to be constant at constant temperature.
If it collides with walls of the container or with other molecules, then its speed remain constant and direction reverses as collision is assumed to be elastic.
The average velocity of gas molecules is the speed at which they move around in a space. This can be affected by various factors such as temperature, pressure, and the type of gas.
The average velocity is important because it determines how fast a gas will expand or contract in response to changes in these conditions.
Thus, the ideal selections are option 2 and 3.
Learn more about Average speed, here:
https://brainly.com/question/12322912
#SPJ1
Use the word stable to explain why the alkali metals tend to lose 1 valence electron.
Answers
Answer:
becoz vvv is always with you
Answer:
Alkali metals have one valence electron on their outer shell. They are more stable when they have eight valence electrons so they tend to loose that one electron and obtain a full octet, therefore become stable
A sample of neon gas occupies a volume of 752 mL at
298K. What volume will the gas occupy at 320.K?
please helppppp
Answers
Answer:
815mL am 10000000% sure
Which of the following is True about nitrogen -14 and nitrogen -16
A, They are completely different Atoms
B, they are the exact same atom
C, they are the same except that carbon -14 has 2 extra electrons
D, they are isotopes of the same atom
Answers
Cations are
because?
Answers
Answer:
They are positively charged ions.
Explanation:
Though, I am not exactly sure about what do you actually mean by your question, but cations are positively charged ions.
Describe and explain the significance of research published by
F.S. Rowland in 1991 titled Stratospheric ozone in the
21st century: the chlorofluorocarbon problem?
Answers
The research titled "Stratospheric Ozone in the 21st Century: The Chlorofluorocarbon Problem" by F.S. Rowland was published in the journal Science in 1991. The study's significance is evident in the way it paved the way for global action on the depletion of the ozone layer.
The study outlined the link between chlorofluorocarbons (CFCs) and the depletion of the ozone layer in the stratosphere. These chemicals have long been utilized in refrigerants, air conditioning systems, foam insulation, and various industrial applications. They have been shown to destroy ozone molecules when they rise to the stratosphere, allowing ultraviolet radiation to penetrate the Earth's atmosphere. Rowland's research proved beyond a doubt that human activity is significantly affecting the ozone layer, resulting in an increased risk of skin cancer, blindness, and other problems associated with exposure to UV radiation.
The research is vital in the sense that it helped to initiate international agreements, such as the Montreal Protocol, aimed at phasing out the use of CFCs. These agreements have been instrumental in lowering the production and use of CFCs, resulting in a reduction in the depletion of the ozone layer. As a result, the world has benefited from a decrease in the risks associated with exposure to UV radiation. In conclusion, Rowland's research was groundbreaking in the sense that it confirmed the link between CFCs and ozone depletion, providing a basis for a global reaction to this critical problem.
To know more about Stratospheric Ozone visit:
https://brainly.com/question/32816779
#SPJ11
A cook had a jar containing a sweet food and a jar containing a sour food. The image above shows the sweet and sour foods. At room temperature, both foods are liquids. The same amount of energy was transferred into both substances. Later, one of the foods had changed phase while the other had not. Which food changed phase, and how did it change?
pls help rn asap
Answers
Answer:
the sour food changed
Explanation:
because the molecules were able to move fast enough to overcome the attraction between them, it's molecules now move in place
The solubility of a gas in a liquid varies in proportion to the partial pressure of that gas in the overlying space. This relationship is known as:
a) Henry's Law
b) Guy-Lussac's Law
c) The Henderson-Hasselbach equation
d) Dalton's Law
Answers
The correct answer is a) Henry's Law. This law states that the solubility of a gas in a liquid is directly proportional to the partial pressure of the gas in the overlying space.
This means that as the partial pressure of the gas increases, more gas molecules will dissolve in the liquid. Henry's Law is important in many areas of science, including chemistry, environmental science, and biology.
For example, it is used to understand the behavior of gases in the atmosphere and their impact on climate change, as well as the ability of aquatic organisms to obtain oxygen from water.
Henry's Law can also be applied to industrial processes such as gas purification and carbonation of beverages.
To know more about Henry's Law. please visit.....
brainly.com/question/24136715
#SPJ11
please help quickly
Rubia was given a type of inclined plane called a ramp in her science class. Her teacher told her that the ramp should have a mechanical advantage of 3.
Rubia pulled a block up the ramp, but afterward she calculated that the mechanical advantage of the ramp was 2.8 instead of 3. Her teacher said she did not make a mistake. What did Rubia calculate?
A.
the actual operating power of the ramp
B.
the actual mechanical advantage of the ramp
C.
the ideal mechanical advantage of the ramp
D.
the ideal operating power of the ramp
Answers
Answer: B. The actual mechanical advantage of the ramp.
Explanation:
The professor saying that the ramp SHOULD have a mechanical advantage of 3 indicates that that would be the ideal mechanical advantage. Since Rubia calculated it to be 2.8, that means that it is the acutal mechanical advantage. The actual advantage will be reduced from the ieal due to things like friction.
What elements in a reaction is likely to react just like Zinc?
Answers
Answer:
Explanation:
Single replacement
Double replacement
Combustion (and synthesis)
synthesis
Decomposition
Calculate the mass of Sb produced when 0.122 moles of is produced. Show work.
Answers
Answer:
14.9 g Sb
Explanation:
The mass of Sb produced can be calculated using the conversion:
Molar Mass (g/mol) = mass (g) / moles
Antimony (Sb) has the molar mass 121.76 g/mol. This value is found on the periodic table. You have been given the moles produced. Therefore, you can find the mass of Sb by plugging these values into the formula and simplifying.
Molar Mass = mass / moles
121.76 g/mol = mass / 0.122 moles
14.9 = mass
ASAP! PLEASE!
For the reaction below calculate how many moles of HCI are produced along with 2.9 moles of H3P04.
PCI5 + 4 H20 ---> H3P04 + 5 HCI

Answers
The number of moles of HCl produced along with the 2.9 moles of H₃PO₄ is 14.5 moles
Calculating number of moles of HCl produced in a reactionFrom the question, we are to determine the number of moles of HCl that are produced along with 2.9 moles of H₃PO₄
The given balanced chemical equation of the reaction is
PCI₅ + 4H₂O ---> H₃PO₄ + 5HCI
From the balanced chemical equation,
1 mole of PCI₅ reacts with 4 moles of H₂O to produce 1 mole of H₃PO₄ and 5 moles of HCI
Now,
If 2.9 moles of H₃PO₄ are produced from the reaction,
Then,
5 × 2.9 moles of HCl will be produced along with the H₃PO₄
5 × 2.9 = 14.5 moles
Hence, the number of moles of HCl produced is 14.5 moles
Learn more on Calculating the number of moles produced here: https://brainly.com/question/8049130
#SPJ1
what is the classification for this unbalanced reaction? fe + hcl → fecl3 + h2
A) single replacement B) combination C) double replacement D) combustion
E) decomposition
Answers
The given unbalanced reaction, Fe + HCl → FeCl\(_{3}\) + H\(^{2}\), can be classified as a single replacement reaction. Option A.
This is because the iron (Fe) is replacing the hydrogen (H) in hydrochloric acid (HCl) to form iron (III) chloride (FeCl\(_{3}\)) and hydrogen gas (H\(^{2}\)). In a single replacement reaction, one element replaces another in a compound. In this case, Fe replaces H in HCl to form FeCl\(_{3}\) and H\(^{2}\). The other reaction types are not applicable to this reaction.
A combination reaction involves two or more reactants combining to form a single product. A double replacement reaction involves two compounds exchanging ions to form two new compounds. A combustion reaction involves the reaction of a fuel with oxygen to produce carbon dioxide and water. A decomposition reaction involves a single reactant breaking down into two or more simpler products. Therefore, the classification for this unbalanced reaction is single replacement. Option A.
More on single replacement reaction: https://brainly.com/question/31311955
#SPJ11
Determine the pH of a 0. 580 M KCH3CO2 solution at 25°C. The Ka of CH3CO2H is 1. 80 × 10^-5. A) 9. 25
B) 4. 75
C) 7. 00
D) 12. 5
E) 1. 47
Answers
The given problem involves calculating the pH of a 0.580 M KCH3CO2 solution at 25°C using the given Ka value of CH3CO2H.
The chemical equation for the dissociation of CH3CO2H in water is as follows:
CH3CO2H + H2O ⇌ CH3CO2- + H3O+
The expression for the equilibrium constant (Ka) is given as:
Ka = [CH3CO2-][H3O+] / [CH3CO2H]
Since the acid is weak, we can assume that [H3O+] is approximately equal to [CH3CO2-]. Therefore, the above equation can be simplified as:
Ka = [CH3CO2-]^2 / [CH3CO2H]
We can now use the given Ka value to calculate the concentration of [H3O+] using the ICE table.
Initial: CH3CO2H + H2O ⇌ CH3CO2- + H3O+
0.580 M + 0 M ⇌ 0 M + 0 M
Change: -x + x + x + x
Equilibrium: 0.580 - x + x ⇌ x + x
Using the Ka expression and the equilibrium concentrations, we get:
1.80 x 10^-5 = x^2 / (0.580 - x)
Assuming x is negligible compared to 0.580, we can simplify the equation as:
1.80 x 10^-5 = x^2 / 0.580
Solving for x, we get:
x = 0.0021 M
The pH of the solution can now be calculated using the formula:
pH = -log[H3O+]
pH = -log(0.0021)
pH = 2.68
Therefore, the pH of the 0.580 M KCH3CO2 solution at 25°C is 2.68.
Learn more about pH here:brainly.com/question/2288405
#SPJ11
substances, such as menthol, that open the lungs' airways are called ______.
Answers
The substances that open the lungs' airways are called bronchodilators.
Bronchodilators are a class of medication used to treat respiratory diseases such as asthma, chronic obstructive pulmonary disease (COPD), and bronchitis.
They function by relaxing and widening the air passages of the lungs, making it easier to breathe.
A variety of bronchodilators are available. Inhalers, nebulizers, tablets, and syrups are examples of them.
Bronchodilators come in a variety of forms, and their effects vary depending on the form.
For example, nebulized bronchodilators, which are inhaled in the form of a mist, work quickly and are used to treat severe respiratory distress.
To know more about bronchodilators visit:
https://brainly.com/question/31569609
#SPJ11
If you wanted to perform a controlled experiment to test the effect of temperature on the pressure of a bicycle tire, which of the following would be necessary?
Answers
Determine the kinds of intermolecular forces that are present in each of the following elements or compounds. CH3COOH, Br2, He
Answers
CH3COOH, also known as acetic acid, is a polar molecule due to the presence of electronegative atoms such as oxygen and the polar covalent bonds between them. The intermolecular forces present in CH3COOH are hydrogen bonding and dipole-dipole interactions.
Br2, also known as molecular bromine, is a nonpolar molecule due to the presence of two identical bromine atoms. The only intermolecular force present in Br2 is London dispersion forces.
He, also known as helium, is a nonpolar molecule due to its symmetrical electron distribution. The only intermolecular force present in He is also London dispersion forces.
In summary, CH3COOH exhibits both hydrogen bonding and dipole-dipole interactions, Br2 exhibits London dispersion forces, and He exhibits only London dispersion forces. It is important to note that the type and strength of intermolecular forces present in a molecule or compound can greatly affect its physical properties such as melting and boiling points, solubility, and viscosity.
To know more about Elements visit:
https://brainly.com/question/8460633
#SPJ11
which four areas does hurricanes happen in the ocean
Answers
Answer:
It’s just North Atlantic Ocean, Atlantic Ocean, Pacific Ocean and the North Pacific Ocean. NOT THE PACIFIC OCEAN ON THE RIGHT SIDE NOR THE INDIAN OCEAN.
which micropipette should you use to most accurately dispense 125 microliters of solution?
Answers
An adjustable-volume micropipette with a range of 0.5-10 μL would be the most accurate for dispensing 125 μL of solution.
What is the micropipette ?
A micropipette is a precision instrument used to accurately measure and transfer very small volumes of liquid, typically between 0.5 µL and 10 mL. It is commonly used in laboratories to prepare samples for chemical analysis and in medical applications to dispense precise amounts of medication. The micropipette is composed of a plunger, a tip, and a cylinder. The plunger is used to draw liquid into the tip, and the cylinder is used to release the liquid. The micropipette is usually operated using a thumbwheel or a push button that controls the plunger. The tip of the micropipette is designed to fit a range of different sized microtubes, allowing for accurate and repeatable transfer of liquids. The micropipette can be calibrated for accuracy, making it an invaluable tool for laboratories that need precise measurements of liquids.
To learn more about micropipette
https://brainly.com/question/28425080
#SPJ4
a balloon contains 0.76 mol n2, 0.18 mol o2, 0.031 mol he and 0.026 mol at 739 mm hg. what is the partial pressure of o2?
Answers
Assume that every gas in the list will act perfectly. Using the mole fraction of O2 and the specified pressure, the partial pressure of O2 may be computed (P).
What is specified?
The following diagram illustrates the mathematical link between partial pressure of oxygen (P1) and oxygen mole fraction (X1):
P₁=X₁P
By dividing the total number of moles of gases (0.76 + 0.18 + 0.031 + 0.026) by the number of moles of O2 (0.18 mol), it is possible to determine the mole fraction of O2 as follows:
X₁= 0.18 / (0.76+0.18+0.031+0.026)= 0.1805
In this manner, the partial pressure of O2 (P1) is determined:
P1=0.1805x739mmHg, or 133.4mmHg
According to the estimate above, the partial pressure of oxygen is almost equal to 130 mm Hg. As a result, option C is the best one.
To learn more about specified visit
https://brainly.com/question/14137273
#SPJ4
when solid has a mass of 15.0 g and volume of 12.4 mL, what is the density?
Answers
The actual decimal was 1.0967742
HELP PLZ ILL GIVE BRAINLIEST
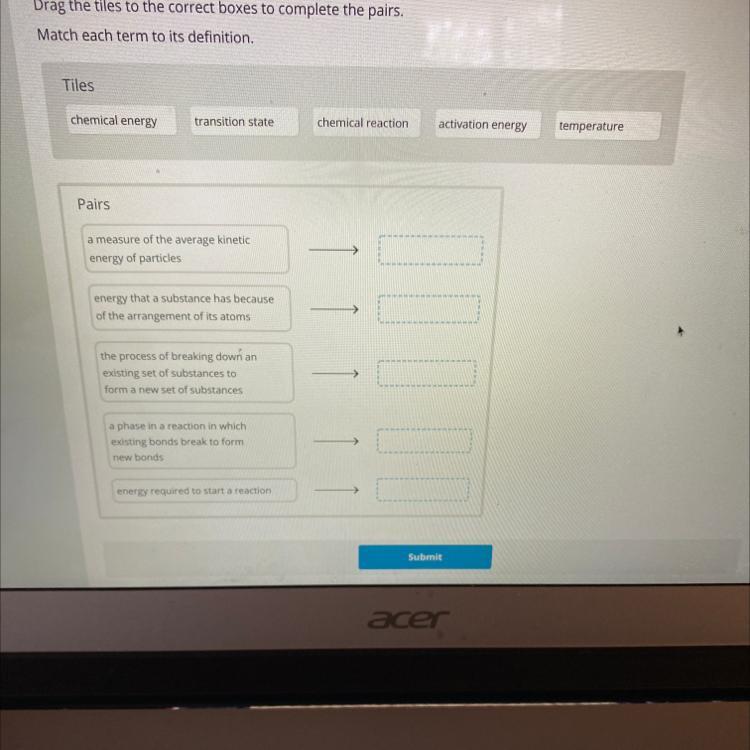
Answers
ons below.
Which diagram shows the correct electron
configuration for fluorine (F)?

Answers
Option A is correct way of representation of electronic configuration.
100.0 g of saltwater is weighed out and all of the water evaporated. the remaining salt is found to have a mass of 23.8 g. what was the molality of the original solution?
Answers
The molality of the original saltwater solution is approximately 5.34 mol/kg.
To determine the molality of the original saltwater solution, we need to calculate the amount of salt (solute) in moles and the mass of water (solvent) in kilograms.
Given:
Mass of salt = 23.8 g
Mass of saltwater solution = 100.0 g
First, we convert the mass of salt to moles using the molar mass of salt (NaCl). The molar mass of NaCl is approximately 58.44 g/mol.
Number of moles of salt = Mass of salt / Molar mass of salt
= 23.8 g / 58.44 g/mol
≈ 0.407 mol
Next, we need to calculate the mass of water in kilograms. Since we have 100.0 g of saltwater solution, and the mass of the salt is 23.8 g, the mass of water can be calculated as:
Mass of water = Mass of saltwater solution - Mass of salt
= 100.0 g - 23.8 g
= 76.2 g
To convert this mass to kilograms:
Mass of water = 76.2 g * (1 kg / 1000 g)
= 0.0762 kg
Now we can calculate the molality (m) using the formula:
Molality (m) = Moles of solute / Mass of solvent (in kg)
= 0.407 mol / 0.0762 kg
≈ 5.34 mol/kg
Learn more about saltwater here:
https://brainly.com/question/30363741
#SPJ11
Typically, when two atoms form a chemical bond, the expected result is that the electrons.
Answers
When atoms form chemical bonding then the atoms attain Noble gas configuration.
Noble gas configuration of an atom includes the fundamental image of the ultimate noble fueloline previous to that atom, observed via way of means of the configuration of the ultimate electrons.so for sodium, we make the substitution of [Ne] for the 1s22s22p6 a part of the configuration. Sodium's noble fueloline configuration becomes [Ne]3s1.
Covalent bonds, atoms percentage pairs of electrons, at the same time as in ionic bonds, electrons are absolutely transferred among atoms in order that ions are formed.During the formation of a chemical bond, the predicted end result is that the electrons will whole a noble fueloline configuration in each atoms. Typically, while atoms shape a chemical bond, the predicted end result is that the electrons.
Read more about atom:
https://brainly.com/question/621740
#SPJ4
Question
545 J of work is done on a gas and changes the volume by-2.50 L. What is the external pressure? Assume that the
external pressure is constant over the change in volume. Give the answer to three significant figures.
Answers
W = -P_ext ΔV
545 J = -P_ext × (-2.50 L)
545 J = 2.50 P_ext L
P_ext = -218 Pa
IG: whis.sama_ent
Where are both earthquakes and volcanoes common?
a) in the earth’s interior
b) at transform fault boundaries
c) at focal points
d) at subduction zones
Answers
When you leave a wine exposed to the air, the ethanol in it reacts to form what?
Answers
Answer:
(ethanol) into acetic acid or vinegar. When wine is exposed to the air, Oxygen in the air interacts with the wine. The wine changes colour and turns both white and red wines brown.
Explanation: