Explain why warm air temperature has lower air pressure than cold air?
Answers
Answer:
Differences in temperature cause differences in air density. Warmer air is less dense than cold, which is why warm air tends to rise and cold air sinks.
temparature affects particles. warm particles are more spread out.
Explanation:
. Being acted on by gravity, colder, denser air weighs more and exerts greater pressure per unit area.
Related Questions
In addition to not causing damage to the sample, what is another advantage of using a microspectrophotometer to analyze fibers?
The fiber’s spectra can also be polarized.
The sample can be quite small.
The sample can originate from a known source.
The fiber sample’s dye can be extracted.
Answers
Another advantage of advantage of using a microspectrophotometer to analyze fibers asides not causing damage to the sample is that the sample can be quite small.
What is a microspectrophotometer?Microspectrophotometry is a biological technique used to measure the absorption or transmission spectrum of a solid or liquid material in either transmitted or reflected light.
Microspectrophotometry can also measure the emission of light by a sample, which is usually small as the micro implies.
One advantage of microspectrophotometry is that the sample does not get damaged. However,
However, another advantage of advantage of using a microspectrophotometer to analyze fibers asides not causing damage to the sample is that the sample can be quite small.
Learn more about microspectrophotometry at: https://brainly.com/question/5832827
I NEED HELP ASAP FOR ALL OF #13

Answers
When a solid is heated, its surface area expands due to a type of thermal expansion known as superficial expansion.
What do you meant by matter ?zero percent lowest possible temperature, around - 273 Celsiusthe melting point temperature at which a substance transforms from solid to liquid or from a liquid to a gas Direct transition of a substance from a solid to a gasthe Fahrenheit temperature scale. Water boils at 212 degrees Fahrenheit and freezes at 32 degreesMolecules a collection of two or more atoms held together by chemical bondsElements are pure compounds that cannot be transformed into simpler substances by conventional chemical processes.homogeneous mixtureCelsius liquid CatenationDepositionmatterAn element is a pure substance that cannot be divided into smaller elements using physical or chemical methods.Atomic compound mixturescombination heterogeneousA reliablesuperficial expansionBoiling point intermolecular forcesTo learn more about matter refer to :
https://brainly.com/question/1172629
#SPJ1
Which event most likely occurs at point v? cooling erosion heating melting
Answers
The event which most likely occurs at point V during a rock cycle is cooling.
What is point V?Point V is present in the rock cycle, where changes in the state or nature of rock takes place.
When mangma comes towards the surface of the earth then due to change in temperature and pressure it will concerts into the igneous rock by cooling process and coverts into the harder form.
Hence at point V, colling is occur.
To know more about rock cycle, visit the below link:
https://brainly.com/question/6073070
Answer:
A. cooling
Explanation:
edge 22'
calculate the mass defect in fe-56 if the mass of an fe-56 nucleus is 55.921 amu. the mass of a proton is 1.00728 amu and the mass of a neutron is 1.008665 amu, and the mass of an electron in 0.00055 amu
Answers
The mass defect in Fe-56 is equal to 0.52823 amu.
What is Mass Defect?The mass defect can be defined as the difference between the actual atomic mass and the theoretical mass calculated by addition of the mass of protons and neutrons in the nucleus.
The actual atomic mass is always less than the predicted mass determined by adding the masses of nucleons. This additional mass is due to the binding energy that is released when a nucleus is formed.
\(\triangle M = (Zm_p + Nm_n) - M_A\)
Given the mass of the proton = 1.00728 amu
The given mass of a neutron = 1.008665 amu
The given mass of an electron = \(0.00055 amu\)
The mass of an Fe-56 nucleus = 55.921 amu
The mass defect for Fe-56 can be calculated as:
\(\triangle M = (26\times 1.00728 + 30 \times 1.008665) - 55.921\)
ΔM = 0.52823 amu
Learn more about mass defect, here:
https://brainly.com/question/11624098
#SPJ1
A ion of the element oxygen (o) has overall charge -2. Therefore,the number of elections in this electrons in this oxygen ion is
Answers
Answer:
18 electrons
Explanation:
Each electron carry a -1 charge.
Since Oxygen atom originally has 16 electrons (same no. as no. of protons, where net charge as 0), it must add 2 electrons to bring the net charge to -2. Hence, the number of electrons in this ion is 16 + 2 = 18.
which compound will form the most intensely colored 0.01 m aqueous solution?
Answers
The compound that will form the most intensely colored 0.01 M aqueous solution will depend on the specific compound and its ability to exhibit color in solution.
The compound that will form the most intensely colored 0.01 M aqueous solution will depend on the specific compound and its ability to exhibit color in solution. Without knowing the specific compounds being considered, it is difficult to provide a definitive answer. Different compounds can exhibit a wide range of colors based on their molecular structure and electronic transitions.
In general, compounds with highly conjugated systems or transition metal complexes tend to exhibit intense colors in solution. These compounds often have extended delocalized π-electron systems that allow for absorption of light in the visible range, resulting in the observed color.
To determine which compound would form the most intensely colored solution, it would be necessary to consider the specific compounds and their molecular structures, including the presence of conjugated systems or transition metal ions. Conducting experiments or consulting references specific to the compounds in question would provide more accurate information regarding their coloration in aqueous solution.
To learn more about ions, click here: brainly.com/question/10942834
#SPJ11
6.
Increasing light
This type of response is a main function of the -
A.
excretory system.
B.
circulatory system.
C.
endocrine system.
D
nervous system.

Answers
Answer: d: nervous system
Explanation:
When focused light is projected onto the retina, it stimulates the rods and cones. The retina then sends nerve signals are sent through the back of the eye to the optic nerve. The optic nerve carries these signals to the brain, which interprets them as visual images.
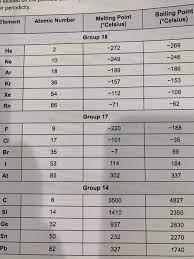
Do the boiling points and melting points of these elements support the claim that elements of the same group have similar physical characteristics?
Answers
Answer:
Elements in the same group has different physical properties such as boiling and melting points. However there are trends of their physical properties down the group.
Explanation:
You should look at the perdoic table to for extra help
The boiling points and melting points of the given elements does not support the claim that elements of the same group have similar physical properties.
What are boiling point and melting point?The boiling point of a substance is the temperature at which it changes from liquid state to gaseous state. Melting point is the temperature at which the substance changes from solid to liquid state.
Elements in the same group shows similar physical and chemical properties. But from the boiling and melting point we cant say that they are in the same group.
Elements in a group shows a trend in boiling point and melting point but cannot be distinguished with this from other groups.
Hence, boiling point and melting point of the elements do no support for the similar properties in a group.
To learn more about groups of elements, refer the link below:
https://brainly.com/question/4418354
#SPJ2
Your question is incomplete, but most probably your full question was,
Do the boiling points and melting points of these elements support the claim that elements of the same group have similar physical characteristics? (image uploaded)
What are 3 possible reasons why a percentage recovery might be low? What are 3 possible reasons why a percentage recovery might be well over 100%?
Answers
Low percentage recovery can be attributed to three possible reasons: experimental errors, incomplete reactions, and losses during purification.
What are three potential factors that can result in a low percentage recovery?Experimental errors, incomplete reactions, and losses during purification are three factors that can lead to a low percentage recovery.
When the percentage recovery is low, it indicates that the actual amount of a desired substance obtained is significantly less than the expected amount based on theoretical calculations.
Firstly, experimental errors such as incorrect measurements, equipment malfunctions, or procedural mistakes can contribute to a low recovery. Secondly, incomplete reactions occur when the reaction does not proceed to completion, resulting in unreacted starting materials remaining in the mixture.
Lastly, losses during purification techniques, such as filtration or evaporation, can lead to the loss of the desired substance.
To improve the percentage recovery, it is essential to minimize experimental errors, optimize reaction conditions to maximize conversion, and develop efficient purification techniques. Regular calibration of equipment, careful procedural execution, and thorough understanding of the reaction mechanism can help in obtaining higher recovery rates.
Learn more about Percentage recovery
brainly.com/question/28625279
#SPJ11
A farmer wants to build a pond for her cows. What step must she take in order to build the pond?
A. Dig into the zone of aeration.
B. Place a permeable material on the ground.
C. Keep the soil moist.
D. Place an impermeable material on the ground.
Answers
Answer:
Option A:
Dig into the zone of aeration
Explanation:
Within the lithosphere of the earth's surface, the zone of aeration is the zone directly above the water table, with a lot of pore spaces within the rocks. These pore spaces contain air and water, which can be used to water the cows.
Once the zone of aeration has been dug into, the farmer can tap into the underground reserve of water, which is stored within the pores of rocks. This water can now sip into the hole that was dug up by the farmer, forming a pond for the cows.
How many g of CO2 can be produced from 256 g Fe2O3?
Answers
Answer:
if i consider this reaction
Fe2O3+ 3CO---》2Fe+ 3CO2
so let's calculate first moles of Fe2O3 i.e. = 256/159.69= 1.6 moles
So the one moles of Fe2O3 is forming three moles of CO2
hence 1.6 moles will form 4.8 moles of CO2
one mole of CO2 is 44 g so 4.8 moles of Co2 is 44×4.8= 211.2 g
so the conclusion is 211.2 g of CO2 can be produced from 256 g Fe2O3!!
i d k it's right or wrong but i tried my best :)
what might be a formula for calculating distance
Answers
Answer:
Distance = speed * Time
hope it helps
Answer:
Distance formula (x2 - a1) ° + (y2 - Yı)?
d - d = distance
(x1, Y1) coordinates of the first point
(x2, Y2) = coordinates of the second point
What is the mass of the oxygen in a 125 g of copper (II) sulfate?
Answers
125 g CuSO4 • 1 mol CuSO4 / 159.609 g CuSO4 • 4 mol O / 1 mol CuSO4 • 16.0 g O / 1 mol O = 50.1 g oxygen
how many sigma and pi bonds, respectively, are in this aldehyde? ch3ch2cho.
Answers
There are six sigma (σ) bonds: three C-H sigma bonds and three C-C and C-O sigma bonds. There is one pi (π) bond: the double bond between the carbon and oxygen atoms (C=O).
To determine the number of sigma (σ) and pi (π) bonds in an aldehyde, such as CH₃CH₂CHO (ethanal or acetaldehyde), let's examine the structure:
H H
| |
H - C - C - O
| |
H H
In this structure, the carbon atom (C) in the aldehyde group (CHO) is bonded to three other atoms: two hydrogen atoms (H) and one oxygen atom (O).
Sigma (σ) bonds occur when two atomic orbitals overlap end-to-end. Each single bond, whether it's a carbon-hydrogen (C-H) or carbon-oxygen (C-O) bond, is a sigma bond. Therefore, the molecule CH₃CH₂CHO has a total of six sigma (σ) bonds: three C-H sigma bonds and three C-C and C-O sigma bonds.
Pi (π) bonds occur when two parallel p-orbitals overlap sideways. Pi bonds are formed in multiple bond situations, such as double or triple bonds. In the given aldehyde, there is only one double bond between the carbon and oxygen atoms (C=O). Therefore, there is one pi (π) bond present in CH₃CH₂CHO.
To summarize:
There are six sigma (σ) bonds: three C-H sigma bonds and three C-C and C-O sigma bonds.
There is one pi (π) bond: the double bond between the carbon and oxygen atoms (C=O).
It's worth noting that sigma and pi bonds are types of covalent bonds, and they play a crucial role in determining the structure and reactivity of organic compounds.
To know more about covalent bonds, refer to the link below:
https://brainly.com/question/19382448#
#SPJ11
What kind of intermolecular forces act between a chloromethane (CH3CI) molecule and a sodium cation?
Note: If there is more than one type of intermolecular force that acts, be sure to list them all, with a comma between the name of each force
Answers
Chloromethane (CH₃Cl) and a sodium cation (Na⁺) interact through two main types of intermolecular forces: ion-dipole forces and van der Waals forces.
Ion-dipole forces occur when an ion, such as the sodium cation, interacts with a polar molecule, like chloromethane. Chloromethane has a polar covalent bond between the carbon and chlorine atoms, resulting in a dipole moment. The positively charged sodium ion is attracted to the negatively charged chlorine atom in the CH₃Cl molecule, forming an ion-dipole interaction.
Van der Waals forces, specifically London dispersion forces, also play a role in the interaction between chloromethane and a sodium cation. These forces arise due to temporary fluctuations in the electron distribution around the atoms in both species, leading to transient dipoles. The induced dipoles result in weak, temporary attractions between the CH₃Cl molecules and the sodium ions.
In summary, the intermolecular forces acting between a chloromethane molecule and a sodium cation are ion-dipole forces and van der Waals forces (London dispersion forces). These forces facilitate the interactions and attractions between the two species.
Learn more about intermolecular forces here: https://brainly.com/question/13588164
#SPJ11
Which of the following ions could exist in either the high-spin or low-spin state in an octahedral complex?
A. Sc3+
B. Ni2+
C. Mn2+
D. Ti4+
E. Zn2+
Answers
Ni²⁺ is the only ion on the list that can exist as both a high-spin and a low-spin octahedral complex. The correct option is B.
An electrostatic model called the crystal field theory (CFT) assumes that the metal-ligand connection is ionic and results only from electrostatic interactions between the metal ion and the ligand. When dealing with anions, ligands are viewed as point charges, and when dealing with neutral molecules, as dipoles.
The crystal field splitting theory predicts that some transition metal ions can exist as either high-spin or low-spin octahedral complexes, depending on the magnitude of the crystal field splitting parameter (Δ) relative to the pairing energy (P).
Of the ions listed, the only one that could exist as either a high-spin or a low-spin octahedral complex is Ni²⁺ (B).
Mn²⁺ (A) is a d⁵ ion and will always form a high-spin octahedral complex due to its large number of unpaired electrons.
Sc³⁺ (C) is a d⁰ ion and does not form octahedral complexes with ligands.
Cu²⁺ (D) is a d⁹ ion and typically forms a low-spin octahedral complex due to the stability of the half-filled d⁹ configuration.
Zn²⁺ (E) is a d¹⁰ ion and does not have any unpaired electrons to undergo spin pairing, so it will always form a low-spin octahedral complex.
Therefore, the correct answer is B) Ni²⁺.
To know more about crystal field theory here
brainly.com/question/23840749
#SPJ4
An atom has a mass number of 22 and an atomic number of 12. How many neutrons
does it have?
Answers
Answer:
this isn't correct answer but the equation is correct by using this methid you can got your answer .
Explanation:
Given - Mass number of atom = 23
Atomic number of atom = 11
As, no. of electrons in an atom is equal to an atomic number of the element.
So, number of electrons = 11
Secondly , number of protons are equal to number of electrons.
so, number of protons = 11
And number of neutrons = mass number - atomic number = 23−11 = 12
so, number of neutrons = 12
So , the atom contains 11 protons, 11 electrons, 12 neutrons.
What is one thing that is the same about a mole of sodiums and a mole of carbons?
A) The weight
B) All of these
C) The total number of atoms
D) The mass
Answers
what information can be gained from the quantum mechanical treatment of the optical properties of metals which cannot be obtained by the classical treatment?
Answers
The quantum mechanical treatment of the optical properties of metals provides a more accurate and detailed understanding of the electronic behavior in metals. This approach allows us to gain information on the following aspects that are not accessible through classical treatment:
1. Quantization of energy levels: Quantum mechanics describes the discrete energy levels of electrons in metals, whereas the classical treatment assumes a continuous range of energy levels. This quantization is crucial for understanding the specific optical properties of metals.
2. Fermi surface: Quantum mechanics allows for the calculation of the Fermi surface, which is the boundary between occupied and unoccupied electron states in a metal. This is essential for understanding how electrons in metals interact with light and contribute to their optical properties.
3. Electron-electron interactions: Quantum mechanics takes into account electron-electron interactions, which are neglected in the classical treatment. These interactions play a significant role in determining the optical response of metals, especially when dealing with phenomena like plasmonics and surface plasmon resonances.
4. Transition probabilities: Quantum mechanics calculates the probabilities of electron transitions between different energy levels, providing insights into the absorption and emission spectra of metals. This is crucial for understanding the interaction of metals with light and their response to different wavelengths.
To know more about Quantum mechanics :
https://brainly.com/question/23780112
#SPJ11
Which amino acid is achiral?
A. AlanineB. ValineC. ProlineD. Glycine
Answers
The achiral amino acid is glycine. Glycine contains a hydrogen atom as its side chain, which implies it lacks stereocenters and is thus the only amino acid that is not chiral.
Proteins are made up of amino acids, which all have the same structure: an amino group (-NH2), a carboxyl group (-COOH), and a side chain (-R) connected to a core alpha carbon atom.
The side chain, commonly known as the R-group, differs amongst amino acids and dictates the chemical and physical characteristics of each. In some situations, the side chain can have a chiral centre, which means it can be linked to four distinct groups and exist in two mirror-image forms known as enantiomers.
Chiral amino acids can occur in two forms known as L and D enantiomers. The L form is more typically seen in proteins, but the D form is uncommon in nature. Conversely, achiral amino acids, such as glycine, lack a chiral centre on their side chain and so do not occur in enantiomeric forms.
Glycine's side chain is merely a single hydrogen atom, indicating that it is not chiral. As a result, glycine has no L or D forms and just one distinct structure.
For more such questions on amino acid, click on:
https://brainly.com/question/30265108
#SPJ4
The molecular formula of an antibacterial drug is C, H, FN,O,. How many fluorine atoms are in a 150-mg tablet of this drug?
Answers
Answer:
To find the number of fluorine atoms in a 150-mg tablet of the antibacterial drug, you need to know the molecular weight of the drug and the number of moles in 150 mg.
Since the molecular formula is given as C, H, F, N, O, you can determine the molecular weight by adding the atomic weights of each element.
For example, if the atomic weights of C, H, F, N, and O are 12, 1, 19, 14, and 16, respectively, the molecular weight of the drug is (12 x C) + (1 x H) + (19 x F) + (14 x N) + (16 x O).
Knowing the molecular weight and the number of moles, you can use Avogadro's number to find the number of fluorine atoms. Avogadro's number is 6.022 x 10^23.
Since the information about the molecular weight, number of moles, and molecular formula is not given, it's not possible to determine the number of fluorine atoms in a 150-mg tablet of this drug.
Explanation:
According to Avogadro's number, there are 0.0145×10²³ atoms present in a 150-mg tablet of this drug.
What is Avogadro's number?Avogadro's number is defined as a proportionality factor which relates number of constituent particles with the amount of substance which is present in the sample.
It has a SI unit of reciprocal mole whose numeric value is expressed in reciprocal mole which is a dimensionless number and is called as Avogadro's constant.It relates the volume of a substance with it's average volume occupied by one of it's particles .
According to the definitions, Avogadro's number depend on determined value of mass of one atom of those elements.It bridges the gap between macroscopic and microscopic world by relating amount of substance with number of particles.
Number of atoms can be calculated using Avogadro's number as follows: mass/molar mass×Avogadro's number, substitution of values in formula gives number of atoms= 0.15/62×6.022×10²³= 0.0145×10²³.
Learn more about Avogadro's number,here:
https://brainly.com/question/11907018
#SPJ2
how long would it take to electroplate a flute with 28.3 g of silver (107.87 g/mol) at a constant current of 2.0 amps using AgNO3
Answers
It would take 211 hours to plate 28.3 g of silver on a flute.
The equation of the reaction;
Ag^+(aq) + e -----> Ag(s)
We know that 1 F of electricity is required to deposit 107.87 g of Ag. Also 1F = 96500 C
Now;
Since 107.87 g is deposited by 96500 C of electricity
28.3 g is deposited by 28.3 g × 96500 C/ 107.87 g
= 25317 C
Also;
Q = It
I = current
t = time
25317 C = 2.0 amps × t
t = 25317 C /2.0 amps
t = 12658.5 seconds or 211 hours
Learn more about electrolysis: https://brainly.com/question/12054569
Answer:
It would take \(3.5hrs\) to electroplate the fluteExplanation:
Electrolysis equation is:
\(Ag1+ + 1e- ------> Ag\)
1 mol of Ag requires 1 mol of electron
1 mol of electron = 96485 C
So,
1 mol of Ag requires 96485 C
let us calculate mol of element deposited:
molar mass of Ag = \(107.87 g/mol\)
number of mol of Ag, \(n = \frac{mass of Ag}{molar mass of Ag}\)
\(n = \frac{28.3}{107.87}\\\\n = 0.2623 mol\)
\(total charge = mol of element deposited * charge required for 1 mol\\\\total charge = 0.2623*9.649*10^4\\\\total charge = 2.531*10^4 C\)
Therefore,
\(time = \frac{Q}{i}\\\\= \frac{2.531*10^4}{2}\\\\= 1.265*10^4 seconds\\\\= 3.515 hr\)
For more information, visit
https://www.homeworklib.com/qaa/1142722/5-how-long-would-it-take-to-electroplate-a-flute
A certain element exists as three different isotopes. 24.1% of all the isotopes have a mass of 75.23 amu, 48.7% have a mass of 74.61 amu, and 27.2% have a mass of 75.20 amu. What is the average atomic mass of this element?
Answers
Answer: A certain element exists as three different isotopes. 24.1% of all the isotopes have a mass of 75.23 amu, 48.7% have a mass of 74.61 amu, and 27.2% have a mass of 75.20 amu. What is the average atomic mass of this element?
Explanation: A certain element exists as three different isotopes. 24.1% of all the isotopes have a mass of 75.23 amu, 48.7% have a mass of 74.61 amu, and 27.2% have a mass of 75.20 amu. What is the average atomic mass of this element? 74.92 amu. Use your periodic table to determine which element this is. As. An element exists
The average atomic mass of the element is 74.93 amu
Let the 1st isotope be A
Let the 2nd isotope be B
Let the 3rd isotope be C
From the question given above, the following data were obtained:
For A (i.e 1st isotope)Abundance of A (A%) = 24.1%
Mass of A = 75.23 amu
For B (i.e 2nd isotope)Abundance of B (B%) = 48.7%
Mass of B = 74.61 amu
For C (i.e 3rd isotope)Abundance of C (C%) = 27.2%
Mass of C = 75.23 amu
Average atomic mass =?The average atomic mass of the element can be obtained as follow:
Atomic mass = [(mass of A × A%)/100] + [(mass of B × B%)/100] + [(mass of C × C%)/100]= [(75.23 × 24.1)/100] + [(74.61 × 48.7)/100] + [(75.23 × 27.2)/100]
= 18.13043 + 36.33507 + 20.46256
Average atomic mass = 74.93 amuThus, the average atomic mass of the element is 74.93 amu
Learn more: https://brainly.com/question/12315958
why is sulfuric acid considered as a positive catalysts in esterification
Answers
Answer:
Because it is a strong acid
Explanation:
All strong acids are consideredpositive
Which of the following is made of cells?
A. a rock
B. a snowflake
C. a raindrop
D. a leaf
Answers
Answer:
a snowflake
Explanation:
Snowflake Symmetry. All ice crystals (of ordinary 'Ice 1h' that is) have the same basic hexagonal symmetry. The fundamental building block is a unit cell containing four water molecules. Ice crystals can be thought of as made of untold stacks of these unit cells.
What is the frequency of this?: 3.3×10−19J
Answers
For the following reaction, 9.82 grams of phosphorus (P4) are allowed to react with 37.7 grams of chlorine gas.Phosphorus (P4)(s) + Chlorine(g) → Phosphorus trichloride(l).a) What is the maximum mass of phosphorus trichloride that can be formed? (Answer in grams)b) What is the formula for the limiting reagent?c) What mass of the excess reagent remains after the reaction is complete?
Answers
a) The maximum mass of phosphorus trichloride that can be formed is 12.1 g.
b) The formula for the limiting reagent is Cl₂.
c) 7.08 g of P₄ is the mass of the excess reagent that remains after the reaction is complete.
a) To find the maximum mass of phosphorus trichloride that can be formed, we need to determine which reactant is the limiting reagent. We can do this by calculating the amount of product that each reactant can produce and comparing them.
First, we need to write a balanced chemical equation for the reaction:
P₄(s) + 6Cl₂(g) → 4PCl₃(l)
The balanced equation shows that one mole of P₄ reacts with six moles of Cl₂ to produce four moles of PCl₃.
Next, we need to calculate the amount of product that can be produced from each reactant:
From P₄:
- Moles of P₄ = mass / molar mass = 9.82 g / 123.9 g/mol = 0.0792 mol
- Moles of PCl₃ produced = 4 x moles of P₄ = 4 x 0.0792 mol = 0.3168 mol
- Mass of PCl₃ produced = moles x molar mass = 0.3168 mol x 137.3 g/mol = 43.5 g
From Cl₂:
- Moles of Cl₂ = mass / molar mass = 37.7 g / 70.9 g/mol = 0.531 mol
- Moles of PCl₃ produced = (1/6) x moles of Cl₂ = (1/6) x 0.531 mol = 0.0885 mol
- Mass of PCl₃ produced = moles x molar mass = 0.0885 mol x 137.3 g/mol = 12.1 g
Since the amount of PCl₃ that can be produced from P₄ (43.5 g) is greater than the amount that can be produced from Cl₂ (12.1 g), we can conclude that Cl₂ is the limiting reagent. Therefore, the maximum mass of PCl₃ that can be formed is 12.1 g.
b) The limiting reagent is the reactant that is completely used up in the reaction and limits the amount of product that can be formed. In this case, we have determined that Cl₂ is the limiting reagent.
c) The excess reagent is the reactant that is not completely used up in the reaction. In this case, we can calculate the amount of excess P₄ as follows:
- Moles of P₄ used = (1/4) x moles of PCl₃ produced = (1/4) x 0.0885 mol = 0.0221 mol
- Moles of P₄ remaining = total moles of P₄ - moles of P₄ used = 0.0792 mol - 0.0221 mol = 0.0571 mol
- Mass of P₄ remaining = moles x molar mass = 0.0571 mol x 123.9 g/mol = 7.08 g
Therefore, 7.08 g of P₄ is the mass of the excess reagent remaining after the reaction is complete.
Learn more about limiting reagents at https://brainly.com/question/26905271
#SPJ11
Which one of the following salts produces neutral solutions when it is dissolved in water?
a. NaCN
b. NaOCl
c. NaF
d. NaBr
e. NaCH3COO
Answers
Out of the given salts, NaCH3COO (sodium acetate) produces a neutral solution when dissolved in water. This is because it is the conjugate base of a weak acid, acetic acid (CH3COOH). When sodium acetate is dissolved in water, it hydrolyzes to form acetate ions and sodium ions.
The acetate ions react with water to produce hydroxide ions (OH-) and acetic acid (CH3COOH). However, since acetic acid is a weak acid, it does not dissociate completely in water and the solution remains neutral. Therefore, the net effect of dissolving NaCH3COO (sodium acetate) in water is the production of equal amounts of OH- and H+ ions, resulting in a neutral solution.
On the other hand, NaCN (sodium cyanide), NaOCl (sodium hypochlorite), NaF (sodium fluoride), and NaBr (sodium bromide) all produce basic or acidic solutions when dissolved in water. NaCN and NaOCl are strong bases and strong oxidizing agents, respectively, while NaF and NaBr are weak bases. Their dissolution in water leads to the formation of OH- ions, H+ ions, or both, resulting in either basic or acidic solutions. Therefore, out of the given salts, only NaCH3COO produces a neutral solution when dissolved in water.
learn more about sodium acetate here
https://brainly.com/question/12924347
#SPJ11
complete by describing what the change of the state of the matter will be for the process to occur
1. Solidification or freezing:
2. evaporation:
3.condensation:
4.sublimation:
5. Deposition or reverse sublimation:
Answers
Answer:
Freezing: Liquid turns into solid. Evaporation: Liquid turns into gas. Condensation: Gas turns into liquid. Sublimation: solid into gas. Deposition: Gas into solid.
Explanation:
Describe the proper handling of explosive materials to prevent
initial combustion that leads to explosion?
Answers
Explosive materials need to be handled carefully and stored appropriately to avoid the possibility of initial combustion. The following are some of the precautions to take when handling explosive materials: Avoid any type of friction, impact, or shock, whether small or large, when handling explosive materials.
Keep the containers of explosive materials tightly sealed to prevent the infiltration of moisture or contaminants. Store the explosives in a cool, dry, and well-ventilated environment, keeping them away from any heat sources or flammable materials. Keep the explosives away from direct sunlight to prevent the heat from building up and causing an explosion.
In conclusion, explosive materials should be handled and stored with care to prevent initial combustion, which may lead to an explosion. Explosives should be stored in a dry, cool, and well-ventilated area, and containers should be kept tightly sealed to prevent moisture or contaminants from entering. Explosives should also be kept away from any heat sources or flammable materials.
You can learn more about Explosive materials at: brainly.com/question/30756602
#SPJ11