Answers
Answer:Transamination, a chemical reaction that transfers an amino group to a ketoacid to form new amino acids. This pathway is responsible for the deamination of most amino acids. This is one of the major degradation pathways which convert essential amino acids to non-essential amino acids (amino acids that can be synthesized de novo by the organism).
Explanation:
Related Questions
+
c)
FeCl3 +
NH4OH
Fe(OH)3
NHACI
Answers
The question is incomplete, the complete question is:
Write the net ionic equation for the below chemical reaction:
(c): \(FeCl_3+3NH_4OH\rightarrow Fe(OH)_3+3NH_4CI\)
Answer: The net ionic equation is \(Fe^{3+}(aq)+3OH^{-}(aq)\rightarrow Fe(OH)_3(s)\)
Explanation:
Net ionic equation is defined as the equations in which spectator ions are not included.
Spectator ions are the ones that are present equally on the reactant and product sides. They do not participate in the reaction.
(c):
The balanced molecular equation is:
\(FeCl_3(aq)+3NH_4OH(aq)\rightarrow Fe(OH)_3(s)+3NH_4Cl(aq)\)
The complete ionic equation follows:
\(Fe^{3+}(aq)+3Cl^-(aq)+3NH_4^+(aq)+3OH^{-}(aq)\rightarrow Fe(OH)_3(s)+3NH_4^+(aq)+3Cl^-(aq)\)
As ammonium and chloride ions are present on both sides of the reaction. Thus, they are considered spectator ions.
The net ionic equation follows:
\(Fe^{3+}(aq)+3OH^{-}(aq)\rightarrow Fe(OH)_3(s)\)
objective is equal to to determines the exothermic or endothermic and natural of the reaction between sulfuric acid and sugar apparatus is equal to break and originate water chemical is equal to concerned H2 So4 and sugar producer 1 is equal touch outer surface of beaker and record your observation
Answers
An exothermic reaction is a chemical reaction that releases energy in the form of heat, light, or sound, whereas an endothermic reaction is a chemical reaction that absorbs energy from its surroundings in the form of heat.
What is the he reaction about?In an exothermic reaction, the reactants have more energy than the products, which means that energy is released during the reaction. In contrast, in an endothermic reaction, the products have more energy than the reactants, which means that energy is absorbed during the reaction.
Examples of exothermic reactions include combustion, such as the burning of wood or gasoline, and the reaction between vinegar and baking soda, which produces carbon dioxide gas. An example of an endothermic reaction is the melting of ice, which absorbs heat from its surroundings to convert solid ice into liquid water. An overview was given based on incomplete information.
Learn more about exothermic on
https://brainly.com/question/2924714
#SPJ1
How many kilojoules of heat are required to heat 2550 grams of water from 17.5°C to 100.0°C?
Answers
Answer:
800.209
Explanation:
Can someone help! The diagram shows a cross section of the Earth near its surface.
In the diagram, continental crust is labeled ___, and oceanic crust is labeled ____
A. 1; 2
B. 3; 1
C. 2; 1
D. 1; 3

Answers
The thinner and denser layer labelled "2" in the diagram represents the marine crust, while the thicker and less dense layer labelled "1" represents the continental crust. Therefore, A. 1; 2 is the right response.
Which of the crust layers is thicker and less dense?The land on Earth is made up of the continental crust, which is less dense, thicker (between 35 and 70 km), and primarily composed of the rock granite. The majority of the ocean is made up of oceanic crust, which is denser, thinner (5–7 km), and primarily composed of the rock basalt.
The crust of the continent is dense or less dense.With a density of around 2.7 grammes per cubic cm, continental crust has a largely granitic composition and is slightly lighter than Oceanic crust has a density of roughly 2.9 to 3 grammes per cubic centimetre and is composed of basalt, which is richer in iron and magnesium than granite.
To know more about continental crust visit:-
https://brainly.com/question/30290659
#SPJ1
A buffer system is set up with [A] = 1.5[HA ]. If pKa = 5.4, what is the pH of the buffer?
Answers
5.57 is the pH of the buffer .
What is buffer ?
Buffer, in chemistry, usually a solution containing acids and bases or salts that tends to maintain a constant concentration of hydrogen ions. An ion is an atom or molecule that has lost or gained one or more electrons. A common buffer is a solution of acetic acid (CH3COOH) and sodium acetate. In aqueous solution, sodium acetate completely dissociates into sodium (Na+) and acetate (CH3COO-) ions. Buffer solutions with different hydrogen ion concentrations can be prepared by varying the buffer ratio and choosing acids of appropriate intrinsic strength. Commonly used buffers include phosphate, citrate, or borate and their salts
To learn more about Buffer , click the link below ;
https://brainly.com/question/26416276
#SPJ9
what is the answer to (x+3)=4
Answers
Answer:
1
Explanation:
x + 3 =4
subtract 3 from 4
You are left with one.
Plug in the answer
1+3=4
assume that each tablets mass was 1000 mg, and remember
Answers
The reaction rate to the nearest whole number is 36.1 mg/l/sec.
How to calculate the reaction rateTo calculate the reaction rate we would use the formula already provided which is: mass of tablet/volume of water ÷ Reaction time.
For the tablet with a 3°C Reaction time, we would calculate the rate as follows:
1000 mg * 0.2L/138.5 sec = 36.1 mg/L/sec.
The final result has all three variables and the resulting answer is the reaction rate.
Learn more about reaction rate here:
https://brainly.com/question/12904152
#SPJ1
Complete Question:
Assume that each tablet's mass was 1,000 mg, and remember that you used 0,200 L of water each time,
Compute the reaction rate to the nearest whole number using the formula below,
mass of tablet/volume of water
Reaction Rate = mass reaction time
3°C Reaction time = 138.5 sec
Reaction rate = mg/l/sec
Phosphorus forms two oxides, which have the empirical formulae of P2O3 and P2O5.
a. Which oxide contains the higher percentage of phosphorus? (Show your calculation)
b. What mass of phosphorus will combine with 1mole of oxygen molecules to form P2O3?
c. What is the molecular formula of the oxide that has a formula mass of 284?
d. Suggest a molecular formula for the other oxide.
Answers
The mass percentage is an important method to determine the concentration of a solution. Here among P₂O₃ and P₂O₅, the oxide P₂O₃ contains higher percentage of phosphorous.
What is mass percentage?The mass percentage of a component can be defined as the ratio of the mass of that particular component in the compound to the total mass of the compound. It can be expressed as:
Mass percentage = Mass of the component / Total mass of the component × 100
Molar mass of P₂O₃ = 110 g/mol
Molar mass of P₂O₅ = 142 g/mol
a. % of 'P' in P₂O₃ = 2 × 31 / 110 × 100 = 56.4 %
% of 'P' in P₂O₅ = 2 × 31 / 142 × 100 = 43.66 %
Thus P₂O₃ has higher percentage of 'P'.
b. 1 mol O₂ × 4 P /3 O₂ × 31.0 P / 1 mol P = 41.3 g P
c. P₄O₁₀
d. Aluminium oxide - Al₂O₃
To know more about mass percentage, visit;
https://brainly.com/question/8339943
#SPJ9
5 Boron has two types of atom, shown below.
What is different about these two atoms?
1 What name is given to atoms like these?
Describe each atom in shorthand form, as in 3.
What is the nucleon number of atom A?
is atom B heavier, or lighter than atom A?
1 Give the electronic configuration for A and B.
ii Comment on your answer for i.
foto

Answers
Explanation:
1. atom B has more neutrons than atom A
2. the name for this is Isotopes
3.atom A is lighter
What mass of NaCl is needed to produce a 26.4 mol/L with a 1.7 L volume?
Answers
we would need 2625.13 grams (or 2.62513 kilograms) of NaCl.
To calculate the mass of NaCl required to produce a 26.4 mol/L solution with a 1.7 L volume, we need to use the formula that relates the mass of solute, moles of solute, and molarity:Molarity (M) = moles of solute / liters of solution Rearranging this formula, we get:moles of solute = Molarity (M) x liters of solutionWe can use this formula to find the moles of NaCl needed:moles of NaCl = 26.4 mol/L x 1.7 L = 44.88 molNow, we can use the molar mass of NaCl to convert from moles to grams. The molar mass of NaCl is 58.44 g/mol:mass of NaCl = moles of NaCl x molar mass of NaClmass of NaCl = 44.88 mol x 58.44 g/mol = 2625.13 gTo produce a 26.4 mol/L solution with a 1.7 L volume.
for more question on NaCl
https://brainly.com/question/23269908
#SPJ8
Two samples of carbon come into contact. A heat transfer will occur between sample A and sample B. What must be
true for heat to transfer from sample A to sample B?
O The average kinetic energy of A is greater than that of B.
O The average kinetic energy of B is greater than that of A.
O The average kinetic energy of both samples is equal.
O The average kinetic energy does not determine the direction of heat transfer.
Answers
The direction of heat transfer between two samples of carbon depends on their temperature difference, and not solely on their average kinetic energy. While the average kinetic energy of a substance is related to its temperature, it is not the determining factor for the direction of heat transfer.
When two samples of carbon come into contact, a heat transfer will occur between sample A and sample B. The direction of heat transfer is dependent on the temperature difference between the samples. Heat transfer always flows from a hotter object to a cooler object, so if sample A is hotter than sample B, heat will flow from A to B. If sample B is hotter than sample A, heat will flow from B to A.
The average kinetic energy of the molecules in a substance is related to its temperature. The higher the average kinetic energy, the higher the temperature of the substance. However, the average kinetic energy does not determine the direction of heat transfer.
It is possible for a substance with a lower average kinetic energy (and therefore a lower temperature) to transfer heat to a substance with a higher average kinetic energy (and therefore a higher temperature). This can occur if the substance with the lower temperature has a greater heat capacity, which means it can absorb more heat without a significant increase in temperature.
for more questions on kinetic energy
https://brainly.com/question/25959744
#SPJ8
How many grams of propane contains the same number of carbon atoms as those in 1.0g C2H5OH ?
Answers
Answer:
Exercises Example 6. How Many Moles Of Carbon Atoms And How Many Carbon Atoms Are Contained In 1.0 G Of C,H,OH
Explanation:
The mass of the propane that would have the same number of carbon atoms is 0.63 g.
What is the number of the carbon atoms?We know that we can be able to obtain the number of the carbon atoms by the use of the simple stoichiometry of the reaction as we have it in the question that we have been asked here.
We can see that we have the molar mass of the ethanol as we can see as 46 g/mol. We have to obtain the number of moles of the ethanol that we have in the compound as follows;
Number of moles = 1 g/ 46 g/mol = 0.022 moles
Number of the carbon atoms that we have is; 0.022 moles * 2 * 6.02 * 10^23 = 2.6 * 10^22 atoms
Now the number of the grams of the propane that we have is;
2.6 * 10^22 = x * 3 * 6.02 * 10^23
Where x is the number of moles present
x = 2.6 * 10^22 / 1.806 * 10^24
x = 1.44 * 10^-2 moles
x = 0.0144 moles
Given that the molar mass of the propane is 44 g/mol
mass = 0.0144 moles * 44 g/mol
Mass = 0.63 g
Learn more about number of atoms:https://brainly.com/question/14190064
#SPJ1
The location oon the surface of the Earth directly above the focus of an earthquake
is called the
A.focus point
B.seismic wave
C.epicenter
Answers
Answer:
I think its epicenter
Explanation:
The epicenter is the point on the earth's surface vertically above the hypocenter, point in the crust where a seismic rupture begins.
what is the pH of a 8.27*10^-2 M solution of HClO_4
Answers
Answer: 0.0827
Hope This Will Help You And This Should Be The Correct One.
Which option is the basic unit of water, a compound?
A. A water atom
B. A hydrogen atom
C. A hydrogen molecule
D. A water molecule
Answers
Answer:
D. A water molecule
Explanation:
Using the equation below, how many liters of water can be made from 7.6 L of oxygen gas at STP?
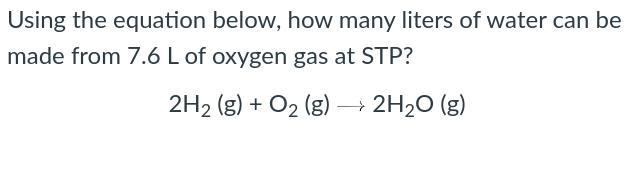
Answers
Answer:
V = 15.2 L
Explanation:
STP means that T = 273 K and P = 1 atm.
We use the PV=nRT equation to convert the given liters of oxygen to moles:
1 atm * 7.6 L = n * 0.082 atm·L·mol⁻¹·K⁻¹ * 273 Kn = 0.340 molNow we convert O₂ moles to H₂O moles, using the stoichiometric coefficients of the equation:
0.340 mol O₂ * \(\frac{2molH_2O}{1molO_2}\) = 0.68 mol H₂OFinally we use the PV=nRT equation once again to convert 0.68 moles of H₂O to liters:
1 atm * V = 0.68 * 0.082 atm·L·mol⁻¹·K⁻¹ * 273 KV = 15.2 Lhow many moles of ions are produced by ionization of 2 moles of MgCl2
Answers
Answer:
number of ions = 12.04 x 10^²³
Explanation:
n = number of ions/Avogadro's constant
2 = number of ions/6.02 x 10^²³
number of ions= 2 x 6.02 x 10^²³
number of ions = 12.04 x 10^²³
Write the symbol for every chemical element that has atomic number less than 5 and atomic mass greater than 8.3 u.
Answers
Atomic m. Element Atomic no. Symbol
1.0079u Hydrogen 1 H
4.0026u Helium 2 He
6.941u Lithium 3 Li
Additional information:-★ Atomic number : The number of protons in one atom of an element is known as atomic number.
Atomic number of an element = No. of protons in one atom of element.The atomic no. of element is denoted by the letter z .The symbol for every chemical element that has an atomic number less than 5 and atomic mass greater than 8.3 u are hydrogen H, helium He, lithium Li and atomic masses 1,4 and 6.94 respectively in the periodic table.
What is a periodic table?A periodic table is an arrangement of the elements in which they are classified on the basis of the number of electrons, neutrons, and protons present in their nucleus and shell of them.
Hydrogen has one electron in it and atomic mass is also one, helium comes in the second number with 4 amu and lithium comes in the third number containing 6.94 mass with it.
Therefore, hydrogen H, helium He, lithium Li and atomic masses 1,4 and 6.94 respectively in the periodic table contain symbols for every chemical element that has an atomic number less than 5 and atomic mass greater than 8.3 u are hydrogen H.
learn more about the periodic table, here:
https://brainly.com/question/16923845
#SPJ2
18.0g of water boils. Hfus of water is 334 J/g. Hvap of water is 2260 J/g.
How much energy is absorbed during the phase change?
Pls help
Answers
406692 j energy is absorbed during the phase change.
Energy is the quantitative property this is transferred to a frame or to a physical gadget, recognizable in the overall performance of work and within the shape of warmth and mild. electricity is a conserved quantity—the law of conservation of power states that electricity can be transformed in form, but no longer created or destroyed
Energy = mΔHfus
= 18×334
=6012 j
Energy = mΔHvap
= 18×2260
=40680 j
Total Energy =46692 J
Learn more about energy here - https://brainly.com/question/13881533
#SPJ1
if there are more products than reactants, does that mean there is an increase in the forward or backward reaction? And if there are more reactants that products, is there an increase in the forward or backward reaction?
Answers
Answer:
If there are more products than reactants, that means the reaction has shifted towards the left, which is the backward direction. If there are more reactants than products, that means the reaction has shifted towards the right, which is the forward direction.
Starting with 0.3500 mol CO(g) and 0.05500 mol COCl2(g) in a 3.050 L flask at 668 K, how many moles of CI2(g) will be present at equilibrium?
CO(g) + Cl2(g)》COCl2(g)
Kc= 1.2 x 10^3 at 668 K
Answers
At equilibrium, the number of moles of \(Cl_2\) (g) will be 0.2025 mol.
1: Write the balanced chemical equation:
\(C_O\)(g) + \(Cl_2\)(g) ⟶ \(C_OCl_2\)(g)
2: Set up an ICE table to track the changes in moles of the substances involved in the reaction.
Initial:
\(C_O\)(g) = 0.3500 mol
\(Cl_2\)(g) = 0.05500 mol
\(C_OCl_2\)(g) = 0 mol
Change:
\(C_O\)(g) = -x
\(Cl_2\)(g) = -x
\(C_OCl_2\)(g) = +x
Equilibrium:
\(C_O\)(g) = 0.3500 - x mol
\(Cl_2\)(g) = 0.05500 - x mol
\(C_OCl_2\)(g) = x mol
3: Write the expression for the equilibrium constant (Kc) using the concentrations of the species involved:
Kc = [\(C_OCl_2\)(g)] / [\(C_O\)(g)] * [\(Cl_2\)(g)]
4: Substitute the given equilibrium constant (Kc) value into the expression:
1.2 x \(10^3\) = x / (0.3500 - x) * (0.05500 - x)
5: Solve the equation for x. Rearrange the equation to obtain a quadratic equation:
1.2 x \(10^3\) * (0.3500 - x) * (0.05500 - x) = x
6: Simplify and solve the quadratic equation. This can be done by multiplying out the terms, rearranging the equation to standard quadratic form, and then using the quadratic formula.
7: After solving the quadratic equation, you will find two possible values for x. However, since the number of moles cannot be negative, we discard the negative solution.
8: The positive value of x represents the number of moles of \(Cl_2\)(g) at equilibrium. Substitute the value of x into the expression for \(Cl_2\)(g):
\(Cl_2\)(g) = 0.05500 - x
9: Calculate the value of \(Cl_2\)(g) at equilibrium:
\(Cl_2\)(g) = 0.05500 - x
\(Cl_2\)(g) = 0.05500 - (positive value of x)
10: Calculate the final value of \(Cl_2\) (g) at equilibrium to get the answer.
Therefore, at equilibrium, the number of moles of \(Cl_2\) (g) will be 0.2025 mol.
For more such questions on equilibrium, click on:
https://brainly.com/question/517289
#SPJ8
-Convert 6.02 x 1020 formula units of MgCl₂ to mol of MgCl₂:
Answers
6.02 x \(10^{20\) formula units of MgCl₂ is equal to 0.1 moles of MgCl₂.
To convert formula units of MgCl₂ to moles of MgCl₂, we need to use Avogadro's number, which relates the number of formula units to the number of moles.
Avogadro's number (NA) is approximately 6.022 x 10^23 formula units per mole.
Given that we have 6.02 x 10^20 formula units of MgCl₂, we can set up a conversion factor to convert to moles:
(6.02 x 10^20 formula units MgCl₂) * (1 mol MgCl₂ / (6.022 x 10^23 formula units MgCl₂))
The formula units of MgCl₂ cancel out, and we are left with moles of MgCl₂:
(6.02 x 10^20) * (1 mol / 6.022 x 10^23) = 0.1 mol
Therefore, 6.02 x 10^20 formula units of MgCl₂ is equal to 0.1 moles of MgCl₂.
It's important to note that this conversion assumes that each formula unit of MgCl₂ represents one mole of MgCl₂. This is based on the stoichiometry of the compound, where there is one mole of MgCl₂ for every one formula unit.
Additionally, this conversion is valid for any substance, not just MgCl₂, as long as you know the value of Avogadro's number and the number of formula units or particles you have.
For more such questions on MgCl₂ visit:
https://brainly.com/question/26311875
#SPJ8
It is an assignment question, so please check it properly to answer it and do use graph to explain it better!

Answers
The instantaneous rate of reaction at 17 minutes is approximately -0.178 mol dm⁻³
To find the instantaneous rate of reaction at 17 minutes, we can use the concept of differential calculus and estimate the slope of the tangent line at t=17 on the graph of rate versus time.
To do this, we can use the formula for the slope of a line
slope = (change in y) / (change in x)
In this case, the "y" values are the rates of reaction and the "x" values are the times. We want to find the slope at t=17, so we can choose two points that are very close to t=17, such as t=15 and t=20. Then, we can use these values to estimate the slope at t=17
slope = (rate at 20 min - rate at 15 min) / (20 min - 15 min)
slope = (0.135 - 0.223) / (20 - 15)
slope = -0.178
This slope represents the instantaneous rate of reaction at t=17. However, since it has a negative value, it means that the rate of reaction is decreasing at t=17.
Therefore, the instantaneous rate of reaction at 17 minutes is approximately -0.178 mol dm⁻³
To know more about instantaneous rate here
https://brainly.com/question/28644129
#SPJ1
A student uses paper towels to clean up a small chemical spill in the lab.
Where should the dirty paper towels be placed?
A in the lab sink
B in the wastebasket
C in the chemical waste container
D in the hazardous waste container
Answers
Answer:
C. in the chemical wastebasket
Some of the water evaporated before its temperature reached 100 degrees. Explain how this affected the student's value for the specific latent heat of vaporisation of water.
Answers
Answer:
The water was flying comfortably but it didnt change the alliance between the wind.
Explanation:
the awnsers can be gotten of your acore key
why do you think they stilk proruxe bright flowers to attract bees?

Answers
Answer:
A
Explanation:
In order for plants to entice pollinators, they must first offer their favorite foods: nectar and protein. Since most pollinators fly, the colors of a flower must attract them; therefore, the brighter the flower, the more likely it will be visited. ... For instance, bees are attracted to bright blue and violet colors.
hope this helps you out...
O Cr³+ because it loses electrons
O Na because it loses electrons
O Cr³+ because it gains electrons
O Na because it gains electrons
Cr³+ + 3Na
3Na+ + Cr
Answers
The symbol is shown as Cr³+ because it loses electrons. Option A
What is oxidation?When we talk about the process of oxidation, what is going on is the loss of electrons. Thus it is possible to say that ocidation is electron loss. The electrons that are lost would lead to the formation of a specie that has a positive ion.
The magnitude of the positive charge that we see in the compound is based on the number of electrons that it has lost in the process of the oxidation of the compound. There are three electrons that have been lost for chromium as shown.
Learn more about oxidation:https://brainly.com/question/9496279
#SPJ1
Metal plating is done by passing a current through a metal solution. For example, an item can become gold plated by attaching the item to a power source and submerging it into an Au³⁺ solution. The item itself serves as the cathode, at which the Au³⁺ ions are reduced to Au(s). A piece of solid gold is used as the anode and is also connected to the power source, thus completing the circuit. What mass of gold is produced when 15.1 A of current are passed through a gold solution for 31.0 min?
Answers
Answer:
172 g
Explanation:
Let's consider the reduction of Au³⁺ to Au.
Au³⁺(aq) + 3 e⁻ → Au(s)
In order to find the mass of gold produced, we will use the following relations.
1 min = 60 s1 A = 1 C/sThe charge of 1 mole of electrons is 96,468 C (Faraday's constant).1 mole of Au is deposited when 3 moles of electrons circulate.The molar mass of Au is 196.97 g/mol.The mass of gold produced when 15.1 A of current are passed through a gold solution for 31.0 min is:
\(31.0min \times \frac{60s}{1min} \times \frac{15.1C}{s} \times \frac{1mole^{-} }{96,468C} \times \frac{3molAu}{1mole^{-} } \times \frac{196.97gAu}{1molAu} = 172 gAu\)
9. It says its wrong? someone help!

Answers
The balanced net ionic equation for the reaction between potassium sulfide and lead(II) nitrate is Pb²⁺(aq) + S²⁻(aq) → PbS(s)
Writing balanced net ionic equation for a reactionFrom the question, we are to write the balanced net ionic equation for the given chemical reaction.
The given chemical reaction is
K₂S(aq) + Pb(NO₃)₂(aq) →
The between potassium sulfide and lead(II) nitrate will produce potassium nitrate and lead sulfide.
That is,
K₂S(aq) + Pb(NO₃)₂(aq) → KNO₃(aq) + PbS(s)
Now, balance the equation
K₂S(aq) + Pb(NO₃)₂(aq) → 2KNO₃(aq) + PbS(s)
Write the complete ionic equation
2K⁺(aq) + S²⁻(aq) + Pb²⁺(aq) + 2NO₃⁻(aq) → 2K⁺(aq) + 2NO₃⁻(aq) + PbS(s)
Cancel out the spectator ions
S²⁻(aq) + Pb²⁺(aq) → + PbS(s)
Hence, the balanced net ionic equation is
Pb²⁺(aq) + S²⁻(aq) → PbS(s)
Learn more on Writing balanced net ionic equation here: https://brainly.com/question/28837770
#SPJ1
is water vapor only found in hot water?