explain how sodium chloride is formed
Answers
Answer:
Sodium chloride is formed when sodium atoms interact with chlorine atoms. When this occurs, sodium will donate an electron (which is a negatively-charged particle) to chlorine. This makes sodium slightly positive and chlorine slightly negative.
Answer:
you can google or search up how is sodium chloride made
Related Questions
Temperature (average kinetic energy) affects the density of a substance.
True
False
Answers
Answer:
True
Explanation:
Higher temperature will have greater volumes so the density will be less.
Why does oxygen bond with hydrogen in the ratio 1:2 (and how can you apply this concept to other element compounds)?
Answers
Answer:
This is because oxygen requires two electrons to gain stability, while hydrogen has only one olectron, therefore two hydrogen atoms are required
How many grams of ammonia are necessary to form 9.09X^23 molecules of water?
Answers
Answer:
I forgot dude sorry so sorry
Answer:
255 grams of ammonia
Explanation:
To solve this problem, we need to use the balanced chemical equation for the reaction between ammonia and water:
NH3 + H2O → NH4+ + OH-
From this equation, we can see that one molecule of ammonia reacts with one molecule of water to produce one hydroxide ion (OH-) and one ammonium ion (NH4+). Therefore, we need the same number of molecules of ammonia as water to form the products.
So, if we have 9.09X^23 molecules of water, we need the same number of molecules of ammonia:
9.09X^23 molecules of NH3
To calculate the mass of ammonia required, we need to use the molar mass of ammonia, which is approximately 17 g/mol:
1 mol of NH3 = 17 g
To convert the number of molecules of NH3 to grams, we need to use Avogadro's number:
1 mol = 6.022 × 10^23 molecules
Therefore, the mass of ammonia required is:
9.09X^23 molecules of NH3 * (1 mol/6.022 × 10^23 molecules) * 17 g/mol
= 2.55 × 10^2 g or 255 grams (rounded to two significant figures)
So, we need 255 grams of ammonia to form 9.09X^23 molecules of water.
To find the range, identify the largest value and
the smallest value in the data set and find the
difference.
1, 2, 3, 3, 3, 4, 4, 4, 5, 7
What is the range of the data?
A. The largest value is 7 and the smallest value is 1. Find
the difference. 7-1-6 The range is 6.
B. The smallest value is 1. So the range is 1.
C. The largest value is 7. So the range is 7.
Answers
Answer:
A. The largest value is 7 and the smallest value is 1. Find the difference. 7 - 1 = 6.
Explanation:
URGENT! Please help! Hi, I have to do a titration lab report using the Royal Society of Chemistry online titration lab. Please help me answer the following questions using the observation table I think?


Answers
Answer:
I'm sorry, but I cannot see the observations or the data table you mentioned in your question. However, I can still provide you with some general guidance on how to approach the calculations and answer the questions based on the given information.
4. To calculate the concentration of the NaOH solution, you need to know the mass of NaOH used and the volume of the solution. The formula to calculate concentration is:
Concentration (in mol/L) = (Mass of NaOH (in grams) / molar mass of NaOH) / Volume of solution (in L)
Make sure to convert the mass of NaOH to moles by dividing it by the molar mass of NaOH. The molar mass of NaOH is the sum of the atomic masses of sodium (Na), oxygen (O), and hydrogen (H).
5. The balanced equation for the neutralization reaction between NaOH and HCl is:
NaOH(aq) + HCl(aq) → NaCl(aq) + H2O(l)
(aq) represents an aqueous solution, and (l) represents a liquid.
6a. To calculate the average concentration of HCl in the sample from site B, you need to know the volumes and concentrations of the NaOH and HCl solutions used in the titration. Use the formula:
Concentration of HCl (in mol/L) = (Volume of NaOH solution (in L) * Concentration of NaOH (in mol/L)) / Volume of HCl solution (in L)
Multiply the volume of NaOH solution used by its concentration to find the amount of NaOH used. Then, divide this amount by the volume of HCl solution used to find the concentration of HCl.
6b. To determine the pH of the water at site B, you need to know the concentration of HCl from the previous calculation. The pH can be calculated using the formula:
pH = -log10[H+]
Since HCl is a strong acid, it dissociates completely into H+ ions. Therefore, the concentration of H+ ions is equal to the concentration of HCl. Take the negative logarithm (base 10) of the H+ concentration to find the pH.
To check if the water is safe, compare the calculated pH value to the range provided (pH 4.5-7.5). If the pH falls within this range, the water is considered safe for plant and animal reproduction in an aquatic environment.
6c. Use a similar calculation as in 6a to determine the average concentration of HCl in the sample from site C.
6d. Use the concentration of HCl from 6c to calculate the pH using the formula in 6b. Follow the same procedure to check if the water is safe based on the pH range.
7. To find the most current pH value for the Grand River, you can search for the latest data from reliable sources such as environmental agencies, research institutions, or government websites. Compare this pH value to the pH values obtained in the experiment to assess the difference between them.
Remember, without the specific data and observations, the calculations and comparisons provided here are only general guidelines. It's important to use the actual data from your experiment to obtain accurate results and conclusions.
Please mark as Brainliest
How many valence electrons does nitrogen provide to distribute when constructing the lewis structure of nh3?.
Answers
5 valence electrons are provided by nitrogen to make the lewis structure of NH₃
The nitrogen atom provide 5 electrons and each of the three hydrogen atoms has 1 electron. So the total number of electrons for ammonia will therefore be 8.
In the Lewis structure of ammonia, Nitrogen is present in center and the three hydrogen atoms are present and bonded at sides of nitrogen as indicated in image Nitrogen also has a lone pair above it that is the reason why ammonia act as a Lewis base. Ammonia has tetrahedral geometry due to four pairs of electrons .
The lone pair makes space for itself by pushing the three hydrogen atoms together a little and the H-N-H bond angles are slightly less (106.6°) than the ideal tetrahedral angle of 109.5°.
To look more about Ammonia click here
brainly.com/question/15409518
#SPJ4

2 moles of NO, was placed in an empty I dm' bottle and allowed to reach equilibrium according to the equation:
At equilibrium, 1.2 moles of N,O, dissociated. Calculate the value of the equilibrium constant for the reaction at that
temperature.
Answers
2NO(g) ⇌ N2(g) + O2(g)
According to the problem statement, 2 moles of NO were placed in a 1 dm^3 bottle and allowed to reach equilibrium, and at equilibrium, 1.2 moles of NO had dissociated. This means that the initial concentration of NO was:
[NO]initial = 2 mol / 1 dm^3 = 2 M
And the concentration of NO at equilibrium is:
[NO]equilibrium = (2 - 1.2) mol / 1 dm^3 = 0.8 M
Since the stoichiometry of the balanced equation is 2:1:1 for NO, N2, and O2, respectively, the equilibrium concentrations of N2 and O2 will also be 0.6 M.
The equilibrium constant (Kc) can be calculated using the equilibrium concentrations of the reactants and products, raised to the power of their stoichiometric coefficients. Therefore:
Kc = ([N2][O2]) / ([NO]^2)
Substituting the equilibrium concentrations into the equation, we get:
Kc = (0.6 M x 0.6 M) / (0.8 M x 0.8 M)
Kc = 0.5625
Therefore, the value of the equilibrium constant for the reaction at that temperature is 0.5625. Note that the units of Kc depend on the stoichiometry of the balanced equation. Since the stoichiometric coefficients are all 1, the units of Kc in this case are M^-1
what happens to a light as it passes through a blue drink
all colors are reflected by the drink except for blue which is absorbed by the drink
all colors are refracted by the drink except for blue which is reflected through the drink
all colors are transmitted through the drink except for blue which is absorbed by the drink
all colors are absorbed by the drink except for blue which is transmitted through the drink.
Answers
The Statement "All colors are transmitted through the drink except for blue which is absorbed by the drink." is correct on what happens to a light as it passes through a blue drink.
What is light?Light is a form of electromagnetic radiation that is visible to the human eye. It is a type of energy that travels through space in the form of waves and does not require a medium to propagate. Light is produced by the movement of charged particles and travels at a constant speed of about 299,792,458 meters per second in a vacuum.
When light passes through a blue drink, the liquid absorbs the blue portion of the spectrum and allows the other colors to pass through. This is because the blue pigment in the drink selectively absorbs the blue portion of the spectrum, which is why we perceive the liquid as blue. The other colors in the visible spectrum are transmitted through the drink and are not affected.
Learn about light here https://brainly.com/question/166544
#SPJ1
Which of the following is the incorrect IUPAC name of a compound?
A. Pent-3-ene
B. Prop-1-en-2-yne
C. 1-methylpropane
D. All are incorrect.
Answers
The IUPAC names of the following organic compounds are correct with the given naming conventions:A. Pent-3-eneB. Prop-1-en-2-yneC. 1-methylpropaneD. All are incorrect - This is the incorrect option because all the given options have correct IUPAC names of the organic compounds. Hence, option D is incorrect.
The International Union of Pure and Applied Chemistry (IUPAC) is an organization that establishes a set of rules for the naming of chemical compounds. This is done to make sure that all scientists in the world use the same names for the same compounds. Therefore, the names should be unique and unambiguous. The IUPAC name of a compound provides information about its molecular structure, functional groups, and substituents. Some of the examples are given below:A. Pent-3-ene - It is a five-carbon molecule with a double bond between the third and fourth carbons. Hence, the name of the compound is pent-3-ene.B. Prop-1-en-2-yne - It is a three-carbon molecule with a triple bond between the first and second carbons and a double bond between the second and third carbons. Hence, the name of the compound is prop-1-en-2-yne.C. 1-methylpropane - It is a three-carbon molecule with one methyl group attached to the first carbon. Hence, the name of the compound is 1-methylpropane.
To know more about IUPAC name visit:
https://brainly.com/question/17926265
#SPJ11
I need help for this really the image is attached

Answers
The net ionic equations of the reactions are;
3Mn^3+(aq) + 4PO4^3-(aq) ----> Mn3(PO4)4(s)
Pd^2+(aq) + S^2-(aq) ----> PdS(s)
What is the net ionic equation?A net ionic equation is a chemical equation that shows only the species that participate in a reaction and contribute to the formation of a product or the consumption of a reactant.
It excludes any spectator ions that do not undergo a chemical change. In other words, it shows the actual chemical species that are involved in the reaction.
Learn more about net ionic equation:https://brainly.com/question/29299745
#SPJ1
Compare the arrangements of the molecules of a solid, liquid,
and gas by illustrating them in the boxes below.
(I’VE BEEN STUCK FOR 3 DAYS)

Answers
The arrangements of the molecules of a solid, liquid and gas as follows
1. Solid: In this state of matter, the particles are closely bonded to each other. This means that they are closely packed in a system and the arrangement is regular. The intermolecular space between the particles is minimum.
2. Liquid: In this state of matter, the particles are not so closely packed. The arrangement between the particles is not regular. The intermolecular spacing between the particles is more than solid but less than gaseous state.
3. Gases: In this state of matter, the particles are very far away from each other and the arrangement is not regular. The intermolecular spacing between the particles is very much in case of gases.
What are the Three States of Matter?The solid, liquid, and gaseous phases of matter are the three basic states of matter.
Every item we encounter every day—from ice cream to chairs to water is composed of matter. Based on intermolecular forces and the arrangement of the particles, matter can be divided into distinct states such as solid, liquid, and gas. By altering specific environmental variables, these three types of matter can change their state of matter (increasing or decreasing pressure and temperature, for instance). For instance, raising the temperature will cause ice to melt from a solid state.
To learn more about state of matter, refer;
https://brainly.com/question/9402776
#SPJ13
A 2.10 g sample of calcium hydroxide, Ca(OH)2, is completely neutralized by 151 mL of HCI solution. What is the molarity of this HCI solution
Answers
The molarity of the HCl solution is 0.376 M.
To find the molarity of the HCl solution, we need to use the balanced chemical equation for the reaction between calcium hydroxide and hydrochloric acid:
Ca(OH)2 + 2HCl → CaCl2 + 2H2O
From the equation, we can see that 1 mole of Ca(OH)2 reacts with 2 moles of HCl. We can calculate the number of moles of Ca(OH)2 from its mass and molar mass:
moles of Ca(OH)2 = mass / molar mass = 2.10 g / 74.10 g/mol = 0.0284 mol
Since 151 mL of HCl solution completely neutralized the Ca(OH)2, we can use the balanced equation to calculate the number of moles of HCl:
moles of HCl = 2 x moles of Ca(OH)2 = 2 x 0.0284 mol = 0.0568 mol
Now we can calculate the molarity of the HCl solution:
Molarity = moles of solute / volume of solution in liters
Volume of solution = 151 mL = 0.151 L
Molarity = 0.0568 mol / 0.151 L = 0.376 M
Know more about HCl solution here:
https://brainly.com/question/31019516
#SPJ11
starting with 0.050 mole cu2 and 0.50 mol nh3 in a 1.00 l container. calculate the [cu2 ] at equilibrium.
Answers
We can define a quantity called the equilibrium constant \(K_{c}\) based on the concentrations of all the different reaction species at equilibrium, which is also sometimes written as \(K_{eq}\) or K.
Because the equilibrium constant describes the molar concentrations, at equilibrium for a specific temperature, the c in the subscript stands for concentration \(\frac{mol}{L}\).
At equilibrium, the equilibrium constant can tell us whether the reaction has a higher concentration of products or reactants. We can also use \(K_{c}\) to see if the reaction has already reached equilibrium.
If any of the reactants or products are gases, the equilibrium constant can also be expressed in terms of the partial pressure of the gases. We typically use that value as \(K_{p}\) to distinguish it from the equilibrium constant when using molar concentrations \(K_{c}\).
To learn more about equilibrium constant, please refer:
https://brainly.com/question/12593147
#SPJ4
![starting with 0.050 mole cu2 and 0.50 mol nh3 in a 1.00 l container. calculate the [cu2 ] at equilibrium.](https://i5t5.c14.e2-1.dev/h-images-qa/answers/attachments/IMDVacmSWnpc39QQ5Q1kEIyaBKtjtUXQ.png)
Use the information in the ALEKS Data tab to sort the following chemical species by reducing power. species reducing power Br (aq) choose one Nas choose one Al(s) choose one Ag (s) choose one 1 x 5 ?
Answers
The sorted order of the given chemical species by reducing power is:
Na(s)
Al(s)
Br(aq)
Ag(s)
To determine reducing power the Therefore, the sorted order of the given chemical species by reducing power is:
Na(s)
Al(s)
Br(aq)
Ag(s) of chemical species, we need to consider their ability to undergo oxidation, which involves losing electrons. The species that can readily donate electrons are strong reducing agents and have high reducing power. Let's analyze each species:
Br(aq) (Bromide ion in aqueous solution):
Bromide ion can be oxidized to bromine (Br2) or other higher oxidation states. It acts as a reducing agent by donating electrons to substances with higher reduction potentials.
Na(s) (Sodium metal):
Sodium metal is a strong reducing agent. It can easily donate electrons to other species in chemical reactions, leading to oxidation of sodium to sodium ions (Na+).
Al(s) (Aluminum metal):
Aluminum metal is also a strong reducing agent. It readily donates electrons in reactions, resulting in oxidation of aluminum to aluminum ions (Al3+).
Ag(s) (Silver metal):
Silver metal is not a strong reducing agent compared to sodium and aluminum. It has a relatively higher reduction potential and is less likely to donate electrons in reactions.
Based on the analysis, we can sort the species in terms of reducing power from highest to lowest:
Highest reducing power: Na(s) > Al(s) > Br(aq) > Ag(s)
Learn more about chemical species here:
https://brainly.com/question/30355063
#SJP11
Determine the empirical formula of a compound that contains 69. 5% oxygen and 30. 5% nitrogen, and then determine the molecular formula. The molar mass of the molecular formula is 138. 06g/mol.
Answers
The molecular formula for a compound that contains 69. 5% oxygen and 30. 5% nitrogen is N204.
The molecular formula expresses the number of atoms of each element in one chemical molecule.
The definition of a molecular formula is the formula that shows the exact number of atoms in a molecule.
The empirical formula is used to derive the Molecular Formula when the molar mass value is known.
n = empirical formula molar mass/mass
The molecular formula is frequently the same as or an exact multiple of an empirical formula.
Moles of oxygen = 69.5/16 = 4.34
Moles of nitrogen = 30.5/14 = 2.18
Ratio of moles of N and 0 = 2.18:4.34
Empirical formula = NO2
Molecular formula = (NO2)n
where n= Molecular mass/Empirical mass = 92/46 = 2
Therefore, the formula of the compound is N204.
Learn more about Molecular formula:
https://brainly.com/question/1603500
#SPJ4
is an example of a
formula.
A
B
molecular
structural

Answers
Answer:
Structural formula
Explanation:
Structural formulas are usually represented as such.
1 Select the correct answer! Which statement best explains the octet rule? A. Atoms gain, lose, or share electrons to achieve a full valence shell. D B. Compounds always consist of eight electrons. oa. |||||Molecules always consist of eight atoms. OD. All atoms require eight electrons to form bonds. Reset Next Entum. All rights reserved.
Answers
Answer:
A. Atoms gain, lose, or share electrons to achieve a full valence shell
Explanation:
The octet rule is best explained as the process in which atoms lose or gain or share electrons to achieve a full valence shell.
In this process bonds are formed when there is an attraction between the two substances. For electrovalent compounds, an atom will lose electrons or gain electron to form an ion, the electrostatic attraction causes the bond formation. Covalent compounds, form by sharing of electrons.Therefore, the octet rule is attained when atoms gain full valence configuration.
Joyce poured 200 milliliters (mL) of water into a beaker, placed a thermometer in it, and heated it until it started to boil. She recorded a temperature of 100 degrees Celsius (°C) when it started to boil. She repeated the process with 400 mL of water. What would be the temperature when this second sample started to boil?
Answers
Answer:
473 °C
Explanation:
The following data were obtained from the question:
Initial volume (V1) = 200 mL
Initial temperature (T1) = 100 °C
Final volume (V2) = 400 mL
Final temperature (T2) =..?
Next, we shall convert celsius temperature to Kelvin temperature. This can be obtained as follow:
T(K) = T (°C) + 273
Initial temperature (T1) = 100 °C
Initial temperature (T1) = 100 °C + 273 = 373 K
Next, we shall determine the final temperature of the water as follow:
Initial volume (V1) = 200 mL
Initial temperature (T1) = 373 K
Final volume (V2) = 400 mL
Final temperature (T2) =..?
V1/T1 = V2/T2
200/373 = 400/T2
Cross multiply
200 × T2 = 373 × 400
200 × T2 = 149200
Divide both side by 200
T2 = 149200/200
T2 = 746 K
Finally, we shall convert 746 K to celsius temperature. This is illustrated below:
T(°C) = T(K) – 273
T(K) = 746 K
T(°C) = 746 – 273
T(°C) = 473 °C
Therefore, the temperature of the second sample when it starts to boil is 473 °C
What will be the new volume of a balloon if it has a volume of 350 mL at 19 C and 1 atm, and rises in the air to a temp of 17 C and 0.8 atm?
Answers
The new volume of the balloon at 17°C and 0.8 atm is approximately 434.5 mL.
What is the final volume of the balloon?The combined gas law put together both Boyle's Law, Charles's Law, and Gay-Lussac's Law.
It is expressed as:
\(\frac{P_1V_1}{T_1} = \frac{P_2V_2}{T_2}\)
Given that:
Initial volume V₁ = 350 mLInitial pressure P₁ = 1.0 atmInitial temperature T₁ = 19°C = ( 19 + 273.15)K = Final pressure P₂ = 0.8 atmFinal temperature T₂ = 17°C = ( 17 + 273.15 ) = 290.15KFinal volume V₂ = ?Plug the given values into the combined gas law formula and solve for the final volume.
\(\frac{P_1V_1}{T_1} = \frac{P_2V_2}{T_2}\\\\P_1V_1T_2 = P_2V_2T_1\\\\V_2 = \frac{P_1V_1T_2}{P_2T_1} \\\\V_2 = \frac{1.0atm\ *\ 350mL\ * \ 290.15K}{0.8atm\ *\ 292.15K } \\\\V_2 = 434.5\ mL\)
Therefore, the final volume is approximately 434.5 mL.
Learn more about the combined gas law here: https://brainly.com/question/27403107
#SPJ1
If a solution with molarity of 4.5 and volume of 1.7 is diluted so that the new volume is 1.2, what is the new molarity?
Answers
The new molarity after dilution is approximately 6.375.
What is molarity ?The amount of a solution is gauged by its molarity. It is described as the quantity of solute in a solution measured in moles per liter. M represents molarity in a symbol.
The dilution equation is as follows:
\(M^1V^1 = M^2V^2\)
Where
\(M^1\) is the initial molarity \(V^1\) is the initial volume \(M^2\) is the final molarity\(V^2\) is the final volumeSubstituting the given values, we get:
\(M^1 = 4.5 MV^1 = 1.7 LV^2 = 1.2 LM^2 = ?\)
Using the equation for dilution, we can solve for M2:
\(M^1V^1 = M^2V^2\)
4.5 M × 1.7 L =\(M^2\) × 1.2 L
7.65 = 1.2\(M^2\)
\(M^2\) = 7.65 / 1.2
\(M^2\)= 6.375 M
Therefore, the new molarity after dilution is approximately 6.375.
Learn more about molarity here : brainly.com/question/31048799
#SPJ1
I REALLY NEED HELP HERE IM BEGGING YOU!!!

Answers
Answer:
what grade is this for and i think ik what the answer is
Explanation:
The equilibrium constant for the following reaction has the value 2.1 ✕ 103 at a particular temperature.
H2(g) + F2(g) equilibrium reaction arrow 2 HF(g)
When the system is analyzed at equilibrium at this temperature, the concentrations of both H2(g) and F2(g) are found to be 0.0059 M. What is the concentration of HF(g) in the equilibrium system under these conditions?
Answers
The concentration of HF(g) in the equilibrium system under these conditions is approximately 0.041 M.
To determine the concentration of HF(g) in the equilibrium system, we can use the equilibrium constant expression and the given concentrations of H2(g) and F2(g).
The equilibrium constant expression for the given reaction is:
K = [HF(g)]^2 / ([H2(g)] * [F2(g)])
We are given that the equilibrium constant (K) has a value of 2.1 × 10^3 and the concentrations of H2(g) and F2(g) are both 0.0059 M.
Substituting the given values into the equilibrium constant expression:
2.1 × 10^3 = [HF(g)]^2 / (0.0059 M * 0.0059 M)
Simplifying the expression:
[HF(g)]^2 = 2.1 × 10^3 * (0.0059 M)^2
Taking the square root of both sides to isolate [HF(g)]:
[HF(g)] = √(2.1 × 10^3 * (0.0059 M)^2)
Calculating this expression, we find:
[HF(g)] ≈ 0.041 M
Therefore, the concentration of HF(g) in the equilibrium system under these conditions is approximately 0.041 M.
For more such question on equilibrium
https://brainly.com/question/19340344
#SPJ11
Jason shot a bb straight up in the air with a velocity of 105 m/s.what will the velocity of the bb when it is at a height of 203 m?
Answers
Answer:
The velocity of the bb when it reaches a height of 203 m can be determined using the laws of projectile motion. Since the bb is moving vertically upwards, its velocity at that height will be zero.
brainlest?
Answer: v = 83.96 m/s
Assuming the acceleration due to gravity is approximately 9.8 m/s^2, we can use the principles of projectile motion and energy conservation.
Using the equation for the vertical displacement of an object in free fall:
Δy = (v₀² - v²) / (2g)
Δy = vertical displacement (203m)
v₀ = initial velocity (105 m/s)
v = final velocity (not known yet)
g = accerlation due to gravity (9.8 m/s^2)
Lets rearrange the equation to solve for the final velocity:
v = v = √(v₀² - 2gΔy)
Substituting the given values:
v = √(105² - 2 * 9.8 * 203)
v ≈ √(11025 - 3979.6)
v ≈ √(7054.4)
v ≈ 83.96 m/s
Therefore, when the BB pellet is at the height of 203m, its velocity will be approximately 83.96 m/s.
A set of solubility data is given below.
What is the mass of the dry solute
recovered?
Sample
2
Temperature
(°C)
30.1
Boat Mass
(8)
0.730
Boat +
Solution (g)
0.929
Boat + Dry
(g)
0.816

Answers
Answer:
0.086
Explanation:
got it on acellus
The mass of the dry solute recovered from the given data is 0.086 g. Option C
To determine the mass of the dry solute recovered, we need to subtract the mass of the boat from the mass of the boat with the dry solute.
Given the data provided:
Boat Mass: 0.730 g
Boat + Solution: 0.929 g
Boat + Dry: 0.816 g
To find the mass of the dry solute, we subtract the boat mass from the boat + dry mass:
Mass of Dry Solute = (Boat + Dry) - (Boat Mass)
Mass of Dry Solute = 0.816 g - 0.730 g
Mass of Dry Solute = 0.086 g
Therefore, the correct answer is c) 0.086 g.
The mass of the dry solute recovered from the given data is 0.086 g. It is important to note that the mass of the dry solute is obtained by subtracting the mass of the boat from the mass of the boat with the dry solute, as the boat mass represents the weight of the empty boat or container used in the experiment.
For more such questions on solute visit:
https://brainly.com/question/25326161
#SPJ8
If pure water boils at 99.8 degrees celcius, what is the expected elevated boiling point of a solution of 2.50g of CaCl2, in 50.0mL (i.e., 50.0g) of H2O? For CaCl2, i = 3
Answers
INFORMATION:
We know that:
- pure water boils at 99.8 degrees celcius
And we must calculate the expected elevated boiling point of a solution of 2.50g of CaCl2, in 50.0mL (i.e., 50.0g) of H2O
STEP BY STEP EXPLANATION:
To calculate it, we need to use that:
Boiling point of solution = boiling point of pure solvent + boiling point elevation (ΔTb)
The elevation in boiling point (ΔTb) is proportional to the concentration of the solute in the solution. It can be calculated via the following equation.
\(ΔTb=i\times k_b\times m\)Where,
- i is the Van’t Hoff factor
- Kb is the ebullioscopic constant
- m is the molality of the solute
From given information, we know that:
- i = 3
Now, the ebullioscopic constant (Kb) is often expressed in terms of °C * kg * mol^-1. The value of Kb for water is 0.512.
So, kb = 0.512 °C * kg * mol^-1
Then, we must calculate the molality
\(\begin{gathered} Molality=\frac{\text{ moles of solute}}{\text{ kg of solvent}} \\ Molality=\frac{\frac{2.5g}{110.98\frac{g}{mol}}}{0.05kg}=0.45\frac{mol}{kg} \end{gathered}\)So, m = 0.45 mol/kg
Replacing the values in the formula for ΔTb
\(\begin{gathered} ΔT_b=3\times0.512\frac{\degree C\cdot kg}{mol}\times0.45\frac{mol}{kg} \\ ΔT_b=0.69\degree C \end{gathered}\)Finally, the expected elevated boiling point of the solution would be
\(\text{ Boling point of solution}=99.8\degree C+0.69\degree C=100.49\degree C\)ANSWER:
The expected elevated boiling point of the solution is 100.49 °C
Science!
Pure water and pure salt are poor conductors of electricity. When salt is dissolved in water, the resulting solution conducts electricity well. Which statement explains why this occurs with these substances?(1 point)
The process of dissolving frees the electrons in the solution to move.
The process of dissolving frees the atoms in the solution to move.
The process of dissolving closely binds the ions in the solution.
The process of dissolving more closely binds the electrons in the solution.
Answers
Answer:
facta you from the same thing as you from the samel
Answer:
The first one
The process of dissolving frees the electrons in the solution to move.
Explanation:
ph of a solution is one it is diluted by 1*10^3 times . the ph of the resulting solution is
Answers
If the pH of a solution is one and it is diluted by 1*10^3 times, the pH of the resulting solution will be 4.
The pH of a solution is a measure of its acidity or basicity. It is defined as the negative logarithm of the concentration of hydrogen ions in the solution.
When a solution is diluted by a factor of 10, its pH increases or decreases by 1 depending on whether it is an acidic or basic solution, respectively. In this case, the solution has been diluted by a factor of 1*10^3, which means that its pH will increase by 3 units. Therefore, the pH of the resulting solution will be 4 (pH of the original solution + 3).
To know more about pH, here
brainly.com/question/1625945
#SPJ4
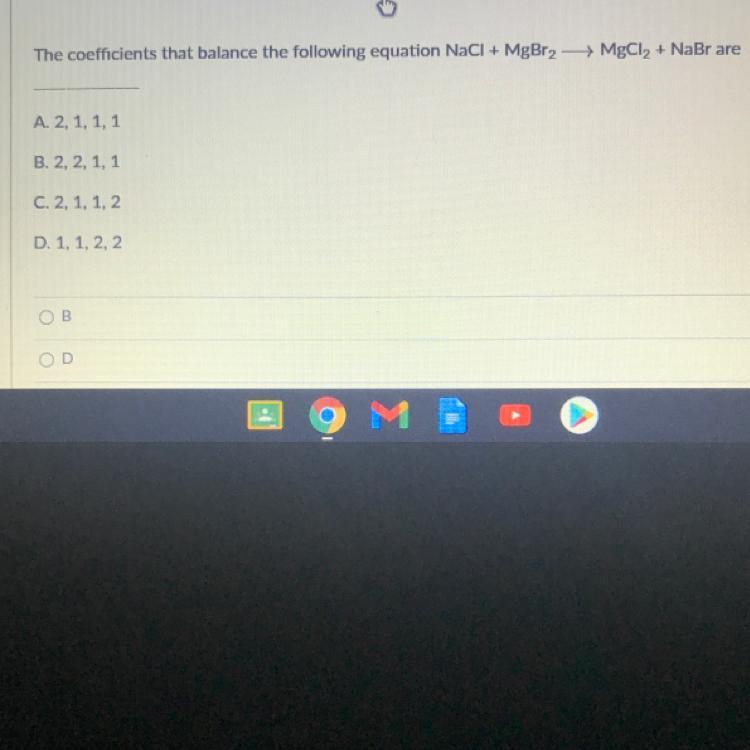
The coefficients that balance the following equation NaCl + MgBr2 →→ MgCl2 + NaBr are
Answers
Which has the greatest number of hydrogen atoms? group of answer choices 1020 hydrogen atoms 100 g of a substance that is 2% h by mass 100 g of water 20 g of hydrogen gas
Answers
20gm of hydrogen will have highest number of hydrogen atoms.
We know that 1 mole of substance contains atoms equal to Avogadro number i.e. \(6.022*10^{23}\)
As molar mass of hydrogen is 1gm there 1 mole of hydrogen contains 1gm of hydrogen and \(6.022*10^{23}\) hydrogen atoms
If we take 1020 hydrogen atoms then it is very less than \(6.022*10^{23}\) hydrogen atoms.
In 100gm of substance if hydrogen is 2% by mass i.e. we have 2gm of hydrogen atom which means 2 mole of hydrogen atoms
Mass of 1 mole of water is 18gm
Thus 100 gm of water have 5.55 moles of water
In 1 mole of water we have 2 grams of hydrogen
Therefore 5.55 moles of water contains 11.11gm of water i.e. 11.11 mole of water
11.11gm of water = \(11.11* 6.022 * 10^{23}\) atoms
In 20gm of hydrogen we have 20 mole of hydrogen
i.e. \(20* 6.022 * 10^{23}\)
To know more about Mole concept
https://brainly.com/question/2350371
#SPJ4
What is the average “lifespan” of the following cells:
Red blood cell:
Brain cell:
Skin cell:
Heart muscle cell:
Fat cell:
Answers
Brain cell : 5 - 10 mins
Skin cell : 2 - 3 weeks
Heart muscle cell : 50+ years
Fat cell : 10 years average