experiment: click play, and this time observe the graph tab as you change the greenhouse gases. what do you notice?
Answers
As you increase the greenhouse gases in the experiment and observe the graph tab, you will notice a correlation between the concentration of greenhouse gases and the temperature change.
Greenhouse gases, such as carbon dioxide, methane, and water vapor, trap heat in the Earth's atmosphere. As the concentration of these gases increases, more heat is trapped, leading to a rise in global temperatures. This is known as the greenhouse effect. In the experiment, the graph tab visually demonstrates this relationship by showing a clear positive correlation between the level of greenhouse gases and the change in temperature.
Understanding the relationship between greenhouse gas concentrations and temperature change is crucial for studying climate change and its potential impacts. By observing the graph tab in the experiment, you can visualize the direct consequences of increasing greenhouse gas emissions on the Earth's climate system. This insight can be used to make informed decisions regarding climate policies and measures aimed at reducing greenhouse gas emissions and mitigating the effects of climate change.
To know more about concentration visit:-
https://brainly.com/question/10725862
#SPJ11
Related Questions
How many moles of NO2 are in a flask with a volume of 28L at a pressure of 121 kPa and a temperature of 45C?
Answers
Answer:
1.2807 moles
Explanation:
From rearranging the equation for the ideal gas equation, you get the equation n=PV/RT, n= moles, P= pressure, V= volume, R= gas constant, T= temperature. Plugging in the numbers and converting kPa to atm and C to K, you get n=1.19418*28/ .0821*318.
Then, you just do the math and get 1.2807 moles.
An aerosol can contains gases under a pressure of4.5atmat16◦C. Ifthecanisleftona hot sandy beach, the pressure of the gases increases to 4.81 atm. What is the Celsius temperature on the beach?
Answer in units of ◦C.
Answers
The Celsius temperature on the beach is approximately 35.8 degrees Celsius.
The pressure-temperature relationship is governed by Charles' Law, which states that as long as pressure is constant, the volume of a gas will be directly proportional to its temperature.The equation used to determine the relationship between temperature and pressure is as follows: PV = nRT, where P is the pressure, V is the volume, n is the number of moles, R is the universal gas constant, and T is the temperature in Kelvin (K).Let's utilize the formula given above to find the temperature of the beach:P1V1/T1 = P2V2/T2We can substitute the values we've been provided to solve for T2 : When the can is at 16 degrees Celsius and 4.5 atm, the pressure is:P1 = 4.5 atmT1 = 16°C + 273 = 289 KV1 and V2, which are the same, can be omitted. P2 = 4.81 atm Substituting these values in the equation : P1V1/T1 = P2V2/T2V1 and V2 are equal and can be eliminated. We can also change the temperature to Kelvin, so:T2 = (P2 * T1 * V1) / (P1 * V2)T2 = (4.81 atm * 289 K) / 4.5 atmT2 = 308.8 KSubtract 273 to convert back to Celsius : T2 = 308.8 K - 273 K = 35.8 degrees Celsius.
for such more questions on temperature
https://brainly.com/question/4735135
#SPJ8
Please answer both
The heat of vaporization for water is 2260 J/g. How much heat in J would be needed to evaporate 8.66g of water?
An unknown salt was dissolved to make a total 1.25g of solution. The temperature of the water decreased from 25.1C to 20.4C when 8mol were dissolved. What is the heat of solution in J/mol?
Answers
(a) We would need 19595.6 J of heat to evaporate 8.66 g of water.
(b) The heat of solution is -3.1 J/mol.
What is the heat needed to evaporate the water?To evaporate 8.66 g of water, we need to use the heat of vaporization for water, which is 2260 J/g.
Therefore, the total amount of heat required to evaporate 8.66 g of water is:
2260 J/g x 8.66 g = 19595.6 J
Therefore,
To find the heat of solution in J/mol, we need to use the formula:
ΔH_solution = -q_solution / n
where;
ΔH_solution is the heat of solution, q_solution is the heat released or absorbed during the solution process, n is the number of moles of solute dissolved.First, we need to calculate the heat released or absorbed during the solution process, which can be found using the formula:
q_solution = m_solution x C_solution x ΔT
We know that 8 mol of the unknown salt were dissolved in 1.25 g of solution, so the mass of the solute is:
m_solute = n x M
We also know that the temperature of the solution decreased from 25.1 ⁰C to 20.4 ⁰C, so ΔT = 4.7 K.
The specific heat capacity of water is 4.184 J/g·K, so we can assume that the specific heat capacity of the solution is also 4.184 J/g·K.
Therefore, the heat released or absorbed during the solution process is:
q_solution = 1.25 g x 4.184 J/g·K x 4.7 K = 24.8 J
Now we can use this value to calculate the heat of solution:
ΔH_solution = -q_solution / n
= -24.8 J / 8 mol
= -3.1 J/mol
Therefore, Note that the negative sign indicates that the solution process is exothermic, i.e., heat is released during the process.
Learn more about heat of solution here: https://brainly.com/question/29055794
#SPJ1
If a mixture of solid nickel(II) oxide and 0.16 M carbon monoxide is allowed to come to equilibrium at 1500 K , what will be the equilibrium concentration of CO2
Answers
To determine the equilibrium concentration of CO2 when a mixture of solid nickel(II) oxide and 0.16 M carbon monoxide is allowed to come to equilibrium at 1500 K, we need to follow these steps:
Step 1: Write the balanced chemical equation
NiO(s) + CO(g) ⇌ Ni(s) + CO2(g)
Step 2: Set up an ICE (Initial, Change, Equilibrium) table
CO CO2
Initial: 0.16 0
Change: -x +x
Equilibrium: (0.16-x) x
Step 3: Write the equilibrium expression using the balanced equation and equilibrium concentrations
Kc = [CO2]/[CO]
Step 4: Find the equilibrium constant (Kc) value for the reaction at 1500 K. For this problem, the value of Kc is not provided. You'll need the Kc value to determine the equilibrium concentration of CO2.
If the Kc value is given, you can proceed with Step 5.
Step 5: Substitute the equilibrium concentrations and Kc value into the equilibrium expression
Kc = x/(0.16-x)
Step 6: Solve for x, which represents the equilibrium concentration of CO2
Once you have found the value of x, the equilibrium concentration of CO2 will be x M.
learn more about equilibrium concentration
https://brainly.com/question/13414142
#SPJ11
Which product is made using bacteria?
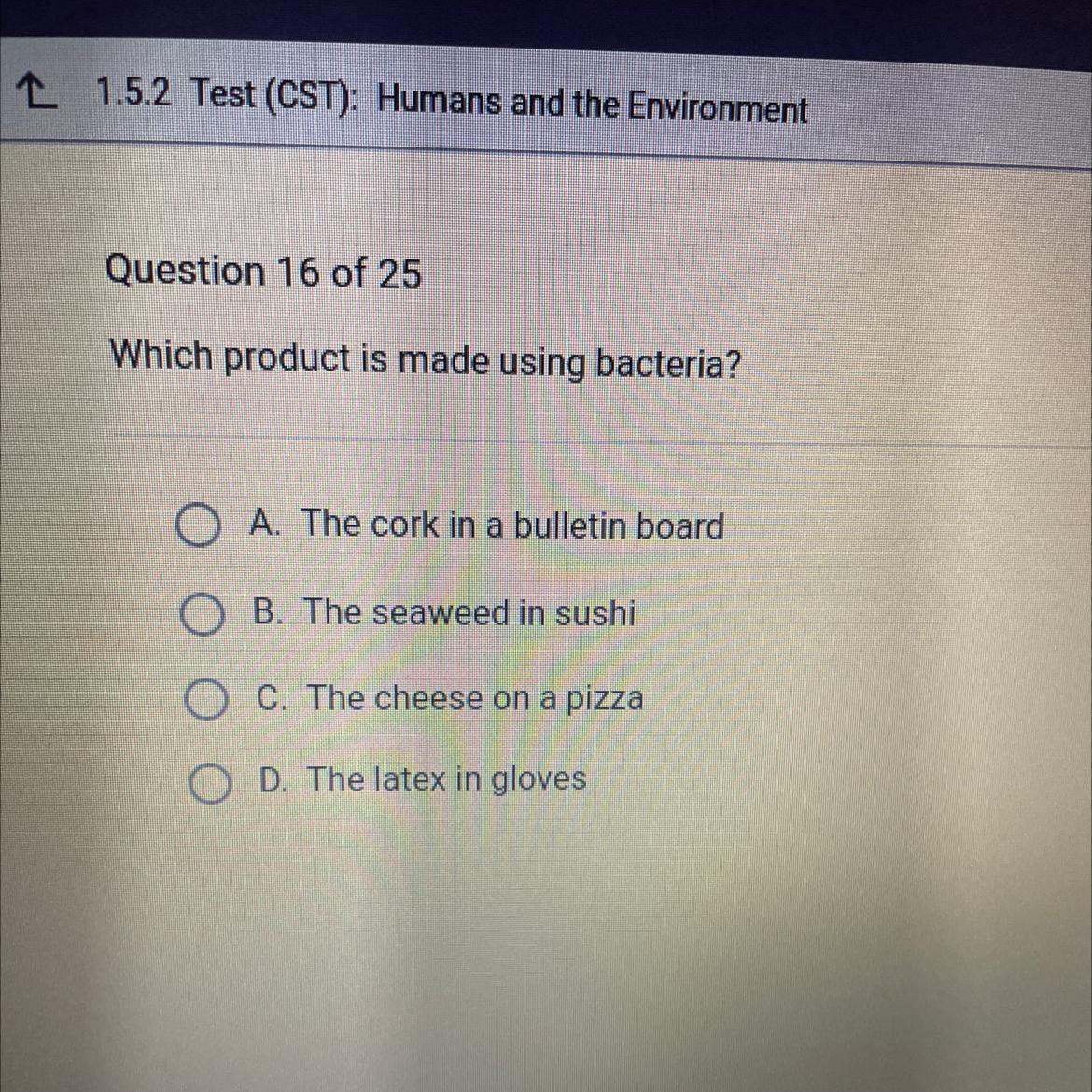
Answers
Answer:
C would be the answer
Explanation:
The solubility of gases in liquids The solubility of gases in liquids increases as temperature increases and increases as pressure increases. increases as temperature increases and decreases as pressure increases. decreases as temperature increases and decreases as pressure increases. decreases as temperature increases and increases as pressure increases. is independent of temperature and increases as pressure increases.
Answers
Answer:
As the kinetic energy of the gaseous solute increases, its molecules have a greater tendency to escape the attraction of the solvent molecules and return to the gas phase. Therefore, the solubility of a gas decreases as the temperature increases.
Explanation:
As the kinetic energy of the gaseous solute increases, its molecules have a greater tendency to escape the attraction of the solvent molecules and return to the gas phase. Therefore, the solubility of a gas decreases as the temperature increases
What is the total pressure of a mixture of he and h2 if the partial pressures are 320 mm hg and 800 mm hg respectively
Answers
Answer:
1120 mm Hg
plus give brainliest
The enthalpy of formation for H2O(l) is –285.8 kJ·mol–1.
Which expression describes the enthalpy change for the reaction:
2 H2O (l) → 2 H2 (g) + O2 (g) ΔH° = ?
A. 1 / (ΔHof)
B. – (ΔHof)
C. – 2 (ΔHof)
D. – ½ (ΔHof)
Answers
The enthalpy change for the given reaction is -2ΔH°f.
option C.
What is the enthalpy change?The enthalpy change for the given reaction is calculated as follows;
ΔH° = ΣnΔH°f(products) - ΣnΔH°f(reactants)
where;
ΔH° is the enthalpy change of the reactionThe balanced chemical equation is given as;
2H₂O (l) → 2H₂ (g) + O₂ (g)
The sum of the standard enthalpies of formation of the products is:
ΣnΔH°f(products) = 2(0 kJ·mol⁻¹) + 0 kJ·mol⁻¹ = 0 kJ·mol⁻¹
The sum of the standard enthalpies of formation of the reactants is:
ΣnΔH°f(reactants) = 2(-285.8 kJ·mol⁻¹) = -571.6 kJ·mol⁻¹
ΔH° = ΣnΔH°f(products) - ΣnΔH°f(reactants)
ΔH° = 0 kJ·mol⁻¹ - (-571.6 kJ·mol⁻¹)
ΔH° = +571.6 kJ·mol⁻¹
+571.6 kJ·mol⁻¹ = -2ΔH°f
Learn more about enthalpy of formation here: https://brainly.com/question/30431725
#SPJ1
What is the purpose of the chemical ammonia (NH3) in hair dyes?
Answers
Answer: Ammonia, an alkaline chemical, is used to raise the pH level (it's levels of acidity) of our hair during the colouring process. This then lifts the cuticles of the hair fibre and allows the colour to be deposited onto the cortex (the inner part of the hair protected by the cuticles). In hair coloring products, ammonium hydroxide is used to support the lightening action of hydrogen peroxide and to prepare hair to accept color pigments. Alkaline properties of ammonia raise the cuticle and allow peroxide and dye molecules to penetrate the hair shaft. (creds to the internet)
Explanation: Hope this helps! :D
Lithium and magnesium bromide react to produce lithium bromide and magnesium metal
Answers
Answer: \(2Li+MgBr_2\rightarrow 2LiBr+Mg\)
Explanation:
Single replacement reaction is a chemical reaction in which more reactive element displaces the less reactive element from its salt solution.
A more reactive element is one which can easily lose or gain electrons as compared to the other element.
The balanced chemical equation is:
\(2Li+MgBr_2\rightarrow 2LiBr+Mg\)
Here Lithium being more reactive than magnesium, it can easily displace magnesium from its salt solution and form lithium bromide and magneium in elemental form.
What element is1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p6
Answers
The electron configuration 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ 5s² 4d¹⁰ 5p⁶ 6s² 4f¹⁴ 5d¹⁰ 6p⁶ corresponds to the element Radon (Rn) with atomic number 86.
In the electron configuration, each number and letter combination represents a specific orbital and the number of electrons occupying that orbital. The numbers represent the principal energy levels (or shells) and the letters represent the sublevels (s, p, d, f).
Breaking down the electron configuration;
1s²; This indicates that the first energy level (n=1) has 2 electrons in the 1s orbital.
2s² 2p⁶; The second energy level (n=2) contains 2 electrons in the 2s orbital and 6 electrons in the 2p orbital.
3s² 3p⁶; The third energy level (n=3) has 2 electrons in the 3s orbital and 6 electrons in the 3p orbital.
4s² 3d¹⁰ 4p⁶; The fourth energy level (n=4) contains 2 electrons in the 4s orbital, 10 electrons in the 3d orbital, and 6 electrons in the 4p orbital.
5s² 4d¹⁰ 5p⁶; The fifth energy level (n=5) has 2 electrons in the 5s orbital, 10 electrons in the 4d orbital, and 6 electrons in the 5p orbital.
6s² 4f¹⁴ 5d¹⁰ 6p⁶; The sixth energy level (n=6) contains 2 electrons in the 6s orbital, 14 electrons in the 4f orbital, 10 electrons in the 5d orbital, and 6 electrons in the 6p orbital.
By referring to the periodic table, we can find that the element with this electron configuration is Radon (Rn) with atomic number 86. Radon is a noble gas and is found in the last group (Group 18) of the periodic table.
To know more about electron configuration here
https://brainly.com/question/14283892
#SPJ4
4th grade. Which of the is a rolling model?
Does anyone know how to help?
Answers
Answer:
we need a picture in order to help you. if you add a picture or text of what you want an answer to I can help!
describe the relationship between pressure and temperature
Answers
It is used in the design of engines, where changes in pressure and temperature are used to convert thermal energy into mechanical work. It is also used in meteorology to predict weather patterns and in the study of the Earth's atmosphere.
Pressure and temperature are two fundamental physical quantities that are closely related in many physical processes. Understanding the relationship between these two quantities is essential in many scientific and engineering fields. This relationship can be described by the ideal gas law, which states that the pressure of an ideal gas is directly proportional to its temperature when the volume and number of particles are constant. In other words, when the temperature of a gas increases, its pressure increases, and vice versa. This can be explained by the kinetic theory of gases, which assumes that gases are made up of a large number of small particles that are in constant motion. The speed of these particles is proportional to the temperature of the gas. As the temperature of the gas increases, the particles move faster and collide more frequently with the walls of the container, resulting in an increase in pressure. Similarly, when the temperature of the gas decreases, the particles move slower and collide less frequently with the walls of the container, resulting in a decrease in pressure. This relationship between pressure and temperature is essential in many scientific and engineering applications.
for more questions on meteorology
https://brainly.com/question/16565664
#SPJ8
Does drinking water undergo a separation process called straining?
Answers
Straining is more likely like exercising or getting better at movements my choice will be yes but 50% of it says maybe
Which describes a velocity?
O A. Flying at 200 miles/hr
O B. Speed changing from 2 km/hr to 5 km/hr
O C. Moving 8 meters in 2 seconds
O D. Moving north at 40 km/hr
Answers
Answer:
D. Moving north at 40 km/hr
Explanation:
velocity is just speed of something in a given direction
18. A student recorded the temperature of a chemical reaction to be 85.0°C. At the end of the reaction the
temperature dropped to 60.0°C. Convert the temperatures into Kelvin.
(You must show all your work to earn the full mark).
HELP ME I DONT UNDERSTAND ANYTHING

Answers
Answer:
and xx xjaakd d sjayas s sjw ddjske dzks sus
szhssbsd
d.ss
s
snas
sjaizhsbszhs z
A piece of chalk (CaCO3) has an initial mass of 43.5 grams. The massof the chalk decreased to 39.6 grams after it was used. Which of these is the closest value to the amount of chalk used?
Answers
Total - left = used
The mass of chalk in this case is 3.90g.
According to the law of conservation of mass, mass can neither be created nor destroyed hence the mass before reaction is equal to the mass after reaction.
Any loss in mass will therefore reflect the mass of chalk used. Therefore, the mass of chalk used in this case is 3.90g.
Learn more about law of conservation of mass: https://brainly.com/question/13383562
Based on the electron configuration of the two atoms, predict the ratio of metal cationic (+) atom to nonmetal anionic (-) atom in the compound. 1522s22p63523p64sl Potassium 1$22s22p63s23p5 Chlorine
Answers
The metal cationic (+) atom to nonmetal anionic (-) atom ratio in the compound formed between Potassium and Chlorine is 1:1.
What is a compound?A compound is made up of two or more atoms that are chemically combined together. In this case, we have the atoms; Potassium and Chlorine.
The electronic configuration of the atoms is not shown here but the metal cationic (+) atom to nonmetal anionic (-) atom ratio in the compound formed between Potassium and Chlorine is 1:1.
Learn more about chemical compounds:
brainly.com/question/12166462
#SPJ1
The _______ is the part of the neuron that carries information away from the cell body.
cell body
axon
dendrite
nucleus
Answers
it is where electrical impulses from the neuron travel away to be received by other neurons!
Answer:
b
Explanation:
A compound contains 19.3% Na, 26.9% S, and 53.8% O. its formula mass is 238g. what
Answers
The empirical formula of the compound, given it contains 19.3% Na, 26.9% S, and 53.8% O is NaSO₄
How to determine empirical formulaThe following paameters were obtained from the question:
Sodium (Na) = 19.3%Sulphur (S) = 26.9%Oxygen (O) = 53.8%Empirical formula =?The empirical formula of the compound can be obtained as follow:
Divide by their molar mass
Na = 19.3 / 23 = 0.839
S = 26.9 / 32 = 0.840
O = 53.8 / 16 = 3.3625
Divide by the smallest
Na = 0.839 / 0.839 = 1
S = 0.840 / 0.839 = 1
O = 3.3625 / 0.839 = 4
Thus, the empirical formula of the compound is NaSO₄
Learn more about empirical formula:
https://brainly.com/question/9459553
#SPJ1
Complete question:
A compound is 19.3% Na, 26.9% S, and 53.8% O. Its formula mass is 238 g/mol. What is the empirical formula? Show your work in a neat and logical manner.
You have prepared a 1.0 M solution of CaCl2 in the laboratory.
What is the concentration of chloride ions in the solution?
a. 0.50 M
b. 1.0 M
c. 1.5 M
d. 2.0 M
Answers
You have prepared a 1.0 M solution of \(CaCl2\) in the laboratory the concentration of chloride ions in the solution is 1.5 M.
What is solution?Solution is defined as huge amount of solute dissolved in solvent. The proportion of solute is limited and after reaching at the point of saturation no more solute will be mixed in the solvent. It is impossible to separate solvent and solute from eye.
Pressure plays a important role in rate of reaction to increase the pressure it increases the rate of reaction by increasing the collision.
Pressure increase the concentration of gases it means that it increases the number of molecules per unit volume due to this collision of gases increases this will increase the temperature. This increase of temperature will increase the rate of reaction.
Therefore, you have prepared a 1.0 M solution of CaCl2 in the laboratory the concentration of chloride ions in the solution is 1.5 M.
Learn more about solution here:
https://brainly.com/question/7932885
#SPJ2
How many feet are in 3.2 miles
Answers
Answer:
16896
Explanation:
5280*3.2=16896
Answer:
The answer is 16896 feet.Explanation:
1 mile = 5280 feetor, 3.2 miles = 3.2 × 5280 feet =16896 feet. Therefore, the answer is 16896 feet. If you like this answer then mark this answer as BRAINLIEST answer.Thank you ☺️☺️
What happens if you mix bleach and rubbing alcohol.
Answers
Mixing bleach and rubbing alcohol produces a toxic substance called chloroform.
When bleach (containing sodium hypochlorite) is mixed with rubbing alcohol (containing isopropyl alcohol), a chemical reaction occurs that produces chloroform.
Chloroform is a dangerous and hazardous chemical, which can cause respiratory issues, dizziness, unconsciousness, and, in severe cases, even death.
It is crucial to avoid mixing these two substances to prevent exposure to harmful fumes or potential health hazards.
Summary: Combining bleach and rubbing alcohol results in the formation of toxic chloroform, posing serious health risks. Always avoid mixing these chemicals.
Learn more about chloroform click here:
https://brainly.com/question/17380113
#SPJ11
NEED ANSWER FAST !!
Using the following equation,
a. Balance the equation by putting the smallest whole number coefficient in each blank
Fe2O3 + H2 à Fe + H2O
b. Use the balanced equation to calculate how many grams of iron can be made from 0.025 mol of Fe2O3
Answer: grams Fe (Round your answer to the nearest hundreths)
Answers
Answer:
.-.
Explanation:
Ethanolic fermentation is used in making beverages such as beer, wine, and pulque. It is carried out by yeast and ...
A) clostridium.
B) zymomonas.
C) leuconostoc.
D) lactobacillus.
E) propionibacterium.
Answers
Ethanolic fermentation, which is the process of converting sugars into ethanol and carbon dioxide by yeast, is primarily carried out by the yeast species known as zymogens. The correct answer is (B).
Zymomonas species are well-known for their ability to ferment sugars and produce ethanol. They are commonly used in the production of various alcoholic beverages, including beer, wine, and pulque. Option (A) clostridium, (C) leuconostoc, (D) lactobacillus, and (E) propionibacterium are not typically associated with ethanolic fermentation. Each of these microorganisms has different metabolic pathways and roles in various other fermentation processes, but they are not the main agents involved in ethanolic fermentation for beverage production. Hence the correct answer is (B).
To know more about Zymomonas, here
brainly.com/question/14869653
#SPJ4
piper rockelle or gavin magnus
Answers
Answer:
Gavin magnus
Explanation:
I don't really care tho
What general trend is seen in the solubility of a solid solute as temperature is increased?
Answers
The general trend in the solubility of a solid solute as temperature is increased is that the solubility typically increases. This is because, as temperature increases, the kinetic energy of the solvent molecules also increases. This increase in kinetic energy allows the solvent molecules to break apart the intermolecular forces holding the solute together and surround the solute molecules, thereby increasing the solubility.
However, there are some exceptions to this general trend. For example, the solubility of gases in liquids generally decreases as temperature increases due to the decrease in solubility resulting from the decrease in gas solubility with increasing temperature. Additionally, there are certain solutes that have solubility curves that are not linear with temperature, such as those that have a solubility maximum at a certain temperature.
To learn more about solubility refer to
brainly.com/question/28170449
#SPJ4
How many moles of carbon are there in a 12.000 g (60.00 carat) diamond?
Answers
this is because: amount of moles=mass/relative formula mass and the relative formula mass of carbon is 12
In a laboratory setting, concentrations for solutions are measured in molarity, which is the number of moles per liter (mol/L). Concentrations are often converted to more common units on the labels of household products. For a particular brand of bleach, the concentration of sodium hypochlorite (NaClO) is reported on the bottle as 7.25% by mass. The following information can thus be used to calculate the molarity of NaClO in the bleach:
• 1L of bleach has a mass of 1,100 grams.
• 7.25% of the mass of bleach is NaClO.
• 1 mol of NaClO has a mass of 74.44 grams.
What is the molarity (mol/L) of NaClO in the bleach?
Answers
The molarity of NaClO in the bleach is 0.101 M (mol/L).
Molarity (M) is the number of moles of solute per liter of solution.
It is calculated by dividing the number of moles of solute by the volume of the solution in liters.
To find the molarity of NaClO in the bleach, we need to use the following information given in the question:
1L of bleach has a mass of 1,100 grams7.25% of the mass of bleach is NaClO1 mol of NaClO has a mass of 74.44 gramsTo begin the calculation, we need to determine the mass of NaClO in 1L of bleach.
To do this, we can use the fact that 7.25% of the mass of bleach is NaClO:Mass of NaClO in 1L of bleach = 0.0725 x 1,100 g = 79.75 g
Next, we can convert this mass of NaClO to moles using its molar mass:
moles of NaClO = 79.75 g / 74.44 g/mol = 1.07 mol.
Finally, we can use the formula for molarity to calculate the molarity of NaClO in the bleach:
Molarity = moles of solute / volume of solution in litersMolarity = 1.07 mol / 10 L = 0.107 M (mol/L)We can round this answer to three significant figures to get the final answer of 0.101 M (mol/L).
For more such questions on molarity
https://brainly.com/question/17138838
#SPJ8
As ocean water salt concentration increases, the _____.
Question 1 options:
water density increases and rises to the top of the sea.
water density increases and sinks to the bottom of the sea.
water density decreases and rises to the top of the sea.
water density decreases and sinks to the bottom of the sea.
Answers
Answer:
B water density increases and rises to the top of the sea
Explanation:
i took a test and got it correct lol