Elements in columns with the same number of valence electrons are in the same___
Answers
Answer:
group
Explanation:
The periodic table also has a special name for its vertical columns. Each column is called a group. The elements in each group have the same number of electrons in the outer orbital.
Related Questions
The greater in ecosystems blank the more likely it is that some organisms could survive caster traffic environmental changes
Answers
Answer:
The answer is species diversity
Explanation:
Hope this helps:)......if not then sorry for wasting your time and may God bless you:)
When the Florida summer brings hot temperatures, what seasonal changes can be seen in plants?
Answer choices:
Florida plants are full of leaves and make fruit.
Florida plants begin to sprout seeds.
Florida plants go dormant from the heat.
Florida plants start to change color.
Answers
Have a nice day and hope this helps : )
Answer:I think it’s the first option because of high temperatures there would be more evaporation and precipitation which would give plants water and exposure to the sun makes plants make food because of the photosynthesis process.
Explanation: Have a nice day and hope this helps : )
The mole ratio is a comparison of how many moles of one substance are required to participate in a chemical reaction with another substance, based on the balanced chemical equation.
a. True
b. False
Answers
True. The mole ratio is a comparison of how many moles of one substance are required to participate in a chemical reaction with another substance, based on the balanced chemical equation.
The mole ratio is a fundamental concept in stoichiometry, which describes the quantitative relationship between reactants and products in a chemical reaction.
It is determined from the coefficients of the balanced chemical equation and represents the ratio of moles of one substance to moles of another substance in the reaction. The mole ratio is crucial for calculating the amount of reactants consumed and products formed in a chemical reaction.
Learn more about mole ratio, here:
https://brainly.com/question/14425689
#SPJ4
How many grams of aluminum oxide (Al2O3) will be formed from 10.0 grams of aluminum (Al)? 4 AL + 3 02 --> 2 AL203
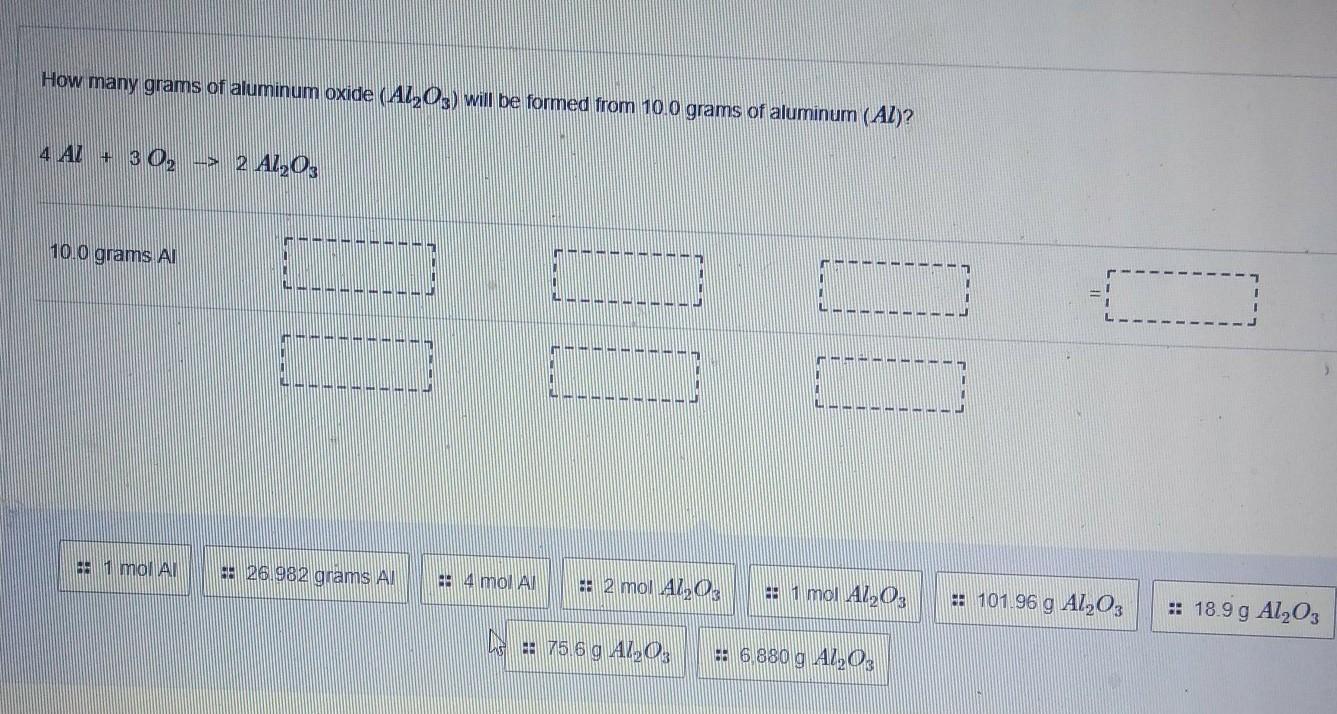
Answers
Answer:
Mass = 18.9 g
Explanation:
Given data:
Mass of Al₂O₃ formed = ?
Mass of Al = 10.0 g
Solution:
Chemical equation:
4Al + 3O₂ → 2Al₂O₃
Number of moles of Al:
Number of moles = mass/molar mass
Number of moles = 10.0 g/ 27 g/mol
Number of moles = 0.37 mol
Now we will compare the moles of Al and Al₂O₃.
Al : Al₂O₃
4 : 2
0.37 : 2/4×0.37 = 0.185 mol
Mass of Al₂O₃:
Mass = number of moles × molar mass
Mass = 0.185 mol × 101.9 g/mol
Mass = 18.9 g
is sodium oxide an acidic oxide or a basic oxide
Answers
Answer:It a Basic oxide
Plz trust Me
Explanation:
Answer:
Sodium oxide is a simple strongly basic oxide. It is basic because it contains the oxide ion, O2-, which is a very strong base with a high tendency to combine with hydrogen ions. Reaction with water: Sodium oxide reacts exothermically with cold water to produce sodium hydroxide solution.
Explanation:
what is the third quantum number of a 3 s 2 electron in phosphorus, 1 s 2 2 s 2 2 p 6 3 s 2 3 p 3 ?
Answers
The third quantum number (m_l) of a 3s² electron in phosphorus is 0.
The third quantum number, denoted as m_l, represents the magnetic quantum number and describes the orientation of an orbital within a subshell. It can have integer values ranging from -l to +l, where l is the azimuthal quantum number.
In the electron configuration of phosphorus, we see that the 3s subshell is being filled. The azimuthal quantum number (l) for the 3s subshell is 0. Since the electron is in the 3s² subshell, there are two electrons present in the 3s orbital.
For the two electrons in the 3s orbital, they will have opposite spins due to the Pauli exclusion principle. However, the magnetic quantum number (m_l) for both electrons in the 3s orbital will be the same, which is 0.
Therefore, the third quantum number (m_l) of a 3s² electron in phosphorus is 0. This means that both electrons in the 3s orbital have the same orientation within the subshell.
To learn more about quantum number, here
https://brainly.com/question/31955577
#SPJ4
Silicon carbide, SiC (m.w. = 40.10 g/mol), is used as an abrasive for many industrial processes. If you want to produce 1.00 kg of silicon carbide from the reaction of SiO2 (m.w. = 60.08 g/mol) and carbon (m.w. = 12.011 g/mol), what is the minimum amount of SiO2 that is needed?SiO2(s) + 3C(s) → SiC(s) + 2CO(g)
Answers
It is needed 1498.25 g of SiO2.
- From the chemical equation, we know that to produce 40.10 g of SiC, it is needed 60.08 g of SiO2 and 36.033 g of C (because in the reaction there are 3 moles of C).
- Calculating we obtain that:
\(\frac{1000\text{ . 60.08 }}{40.10}=1498.25\text{ g}\)So, it is needed 1498.25 g of SiO2. This can be expressed as 1.50x10^3 g.
describe the temperature, moisture and air pressure associated with a continental polar air mass.
Answers
The variance in the US continental region is brought on by the shift in daytime and nighttime weather patterns.
Continental polar air mass -Cold, dry, and stable air masses are found in the continental polar (cP) or continental arctic (cA) regions. Radiative cooling causes these air masses to form over northern Canada and Alaska. They travel south, then east via the Plains and the Rockies.
During the winter, a continental polar air mass can develop over the land. It comes from northern Canada or Alaska in the Northern Hemisphere. It transports dry weather to the United States as it goes south. Low humidity and temperature are both present.
These factors contributed to the polar air mass:
Breezeextreme humiditythe evening's low temperatureDuring the colder months of the year, continental polar air typically forms over vast land masses.
A cool breeze blows across the upper section of the area, while a warm breeze blows through the lower part.
To learn more about continental polar air mass from given link
https://brainly.com/question/22790966
#SPJ4
The way Earth's rotation makes winds curve.
Answers
Answer:
it is called the prevailing westerlies
Explanation:
why does water boil at less than 100 drgrees celsius in boulder colorado
Answers
Explanation:
Because boiling point of water is not 100 degrees Celsius but it depends on atmospheric pressure. Liquid boils at temperature when partial pressure of liquid becomes equal to atmospheric pressure.
Molarity of Kool Aid solutions can be calculated by comparing the concentrations of Kool Aid powder and sugar added to a given volume of water. The molar mass of Kool Aid will be the same as that of sugar for our purpose. The molecular formula for sugar is C12H22O11- Your objective for this lab will be to calculate the molarity of Kool Aid desired based on package directions. You will then be provided two concentrated Kool Aid solutions. You will use dilution calculations to determine the amount of water and concentrated solution you will need in order to prepare 65 mL of the desired molarity.
Calculate the molarity of Kool Aid desired based on the following information from the package directions.
1 package Kool Aid powder = 4. 25 grams 1 cup sugar = 192. 00 grams
2. 00 quarts of water (1. 06 quarts = 1 liter)
Answers
The amount of concentrated solution needed is (0.286 M)(65 mL) / C M, and the amount of water needed is 65 mL minus the volume of the concentrated solution.
To calculate the molarity of Kool Aid desired, we need to determine the number of moles of Kool Aid powder and sugar in the package. Since the molecular formula for sugar is C12H22O11, we can calculate its molar mass as follows:
Molar mass of C12H22O11 = (12 * 12.01) + (22 * 1.01) + (11 * 16.00)
= 144.12 + 22.22 + 176.00
= 342.34 g/mol
Given that the package contains 4.25 grams of Kool Aid powder, we can calculate the number of moles of Kool Aid powder using its molar mass:
Number of moles of Kool Aid powder = Mass / Molar mass
= 4.25 g / 342.34 g/mol
≈ 0.0124 mol
Similarly, for the sugar, which has a molar mass of 342.34 g/mol, we can calculate the number of moles of sugar using its mass:
Number of moles of sugar = Mass / Molar mass
= 192.00 g / 342.34 g/mol
≈ 0.5612 mol
Now, to calculate the molarity of the desired Kool Aid solution, we need to determine the volume of water. Given that 1.06 quarts is equal to 1 liter, and we have 2.00 quarts of water, we can convert it to liters as follows:
Volume of water = 2.00 quarts * (1.06 liters / 1 quart)
= 2.12 liters
To find the molarity, we use the formula:
Molarity (M) = Number of moles / Volume (in liters)
Molarity of Kool Aid desired = (0.0124 mol + 0.5612 mol) / 2.12 L
≈ 0.286 M
To prepare 65 mL of the desired molarity, we can use dilution calculations. We need to determine the volume of concentrated solution and the volume of water needed.
Let's assume the concentration of the concentrated Kool Aid solution is C M. Using the dilution formula:
(C1)(V1) = (C2)(V2)where C1 is the initial concentration, V1 is the initial volume, C2 is the final concentration, and V2 is the final volume.
Given that C1 = C M and V1 = V mL, and we want to prepare a final volume of 65 mL (V2 = 65 mL) with a final concentration of 0.286 M (C2 = 0.286 M), we can rearrange the formula to solve for the volume of the concentrated solution:
(C M)(V mL) = (0.286 M)(65 mL)
V mL = (0.286 M)(65 mL) / C M
So, the amount of concentrated solution needed is (0.286 M)(65 mL) / C M, and the amount of water needed is 65 mL minus the volume of the concentrated solution.
For more such questions on concentrated visit:
https://brainly.com/question/28564792
#SPJ8
what is the purpose of washing the crude product with aqueous nahco3? give equations.
Answers
The purpose of washing the crude product with aqueous NaHCO₃ is to remove unwanted acidic impurities that may be present in the crude product. This process is known as acid-base extraction or alkaline extraction.
The aqueous NaHCO₃ solution acts as a base that reacts with the acidic impurities to form a salt. The salt thus formed is more polar and can be easily removed from the crude product by washing with water. The equation for this reaction is as follows:
Acidic impurity + NaHCO₃ → Salt + CO₂↑ + H₂O
The CO₂ gas produced is responsible for the effervescence observed during the reaction. The equation for the reaction of acetic acid, an acidic impurity commonly found in organic synthesis, with NaHCO₃ is as follows:
CH₃COOH + NaHCO₃ → CH₃COONa + CO₂↑ + H₂O
The acetic acid reacts with the NaHCO₃ to form sodium acetate, carbon dioxide gas, and water.
Learn more about crude at https://brainly.com/question/14281081
#SPJ11
According to the bohr model of atomic quantization, what energy do emitted photons have?
Answers
The difference between any two stationary state energy is the right response.
In atoms, the orbits of electrons are quantized. Electrons can travel to a higher orbit by absorbing some energy and can move to a lower orbit by releasing energy. Each orbit has distinct energy. Discrete spectra result from the quantization of the orbits, which also quantizes the energy received or emitted. One of the most common ways to introduce and remove energy from atoms is by photon absorption or emission, and the energy involved is equivalent to the change in electron energy as it moves from one orbit to another.
What is atomic quantization?
Quantized energy refers to the fact that only specific discrete energy values are authorized for electron possession; values outside of these quantized values are forbidden. Although strictly speaking, the Bohr model only functions for one-electron atoms or ions, both contain a very massive nucleus with electrons circulating about it.So the more about atomic quantization visit.
https://brainly.com/question/7980294
#SPJ4
Both covalent-network solids and ionic solids can have melting points well in excess of room temperature, and both can be poor conductors of electricity in their pure form. However, in other ways their properties are quite different. a. Which type of solid is more likely to dissolve in water
Answers
Answer:
IonicEvidence: It can be proved by a simple experiment, sand doesn't dissolve in water but NaCl (table salt) does. (excluding cases like BaSO4 and sugar and amino acids)Cause: The electrostatic bonds among ions in a lattice only hold them together like two magnets with unlike poles facing each other. The water molecules can split them through the similar process with several molecules ganging up on them, the H+ parts of the molecules attracts the anion and O2- part; the cations. If this electrostatic attraction is big enough, the bonds between the solid's ions break (into separate ions). And so the solid dissolves. This is usually the case with most.
Why not covalent structures?: Sure, covalent solids have a low m.p and b.p for low intermolecular forces, the covalent bonds here are strong and cannot easily be 'pulled apart' by water molecules. They usually have stronger intermolecular forces than that of water.
But... if their intermolecular forces are weaker or similar, sure they can dissolve easily. Like any often-used alcohol (glacial).
50 POINTS!! What is the relationship between the molecular structure of polypropylene and its macroscopic properties such as strength, flexibility, electrical conductivity, etc?
Answers
Explanation:
The structure (e.g., extent of branching) determines how the individual polymer molecules can orient (or "pack") in the solid state. This, in turn, influences physical properties such as density, crystallinity, melting point, and strength.Thus this is the relationship between the molecular structure of polypropylene and its macroscopic properties such as strength, flexibility, electrical conductivity, etc
Is atomic spectrum of hydrogen due to the ionization of hydrogen molecules?
Answers
Answer:
Emission Spectrum of Hydrogen. When an electric current is passed through a glass tube that contains hydrogen gas at low pressure the tube gives off blue light. When this light is passed through a prism (as shown in the figure below), four narrow bands of bright light are observed against a black background.
Explanation:
that hydrone molecules Diprotic acids
Complete the table by filling in the missing information.
Options for A
- pressure, volume
- temperature, pressure
- volume, temperature
Options for B
- pressure, moles of gas
- temperature, moles of gas
- volume, moles of gas
Options for C
- Gay-Lussac’s law
- Charles’s law
- Dalton’s law
Options for D
- V = kT
- V = kP
- V = KP
Options for E
- pressure, volume
- temperature, pressure
- volume, temperature
Options for F
- PT = k
- P = kT
- T = kV
Options for G
- pressure only
- temperature, volume
- number of moles only
- pressure, temperature

Answers
Answer:
A. Pressure,Volume
B. Temperature, moles of gas
C. Charles Law
D. V=kT
E. Temperature, Pressure
F. P= kT
G.Number of Moles only
Explanation:
can someone please help me:(

Answers
Answer:
I think (B) is right answer because here oxygen is a combustible material and combustible is always take place when oxygen is burn
how to distinguish between aqueous potassium bromide and aqueous potassium iodide TEST AND RESULT
Answers
It is possible to conduct a test to distinguish between potassium chloride and potassium iodide using a silver nitrate solution. Silver nitrate solution and ammonia solution are used in the testing for halide ions.
What happens when potassium iodide and aqueous bromine interact?When bromine-water is introduced to a potassium iodide solution, hydrobromic acid is produced as a byproduct of the oxidation to iodate, which is indicated by a sharp rise in conductivity and a fall in pH.
What is the iodide and bromide ion confirmatory test?The Layer's test is conducted using "carbon disulphide" and "dilute hydrochloric acid." This produces an orange layer when bromide ions are present, and a violet layer when iodide ions are present.
To know more about potassium chloride visit:-
https://brainly.com/question/22528097
#SPJ1
how many grams of zinc will be formed if 32 G of copper reacts with zinc nitrate copper 1 nitrate is the other product
Answers
Answer:
Mass = 32.69 g
Explanation:
Given data:
Mass of copper = 32 g
Mass of zinc formed = ?
Solution:
Chemical equation:
Cu + Zn(NO₃)₂ → Cu(NO₃)₂ + Zn
Number of moles of copper:
Number of moles = mass/molar mass
Number of moles = 32 g/ 63.55 g/mol
Number of moles = 0.5 mol
now we will compare the moles of zinc with copper.
Cu : Zn
1 : 1
0.5 : 0.5
Mass of Zn:
Mass = number of moles × molar mass
Mass = 0.5 mol × 65.38 g/mol
Mass = 32.69 g
what two major economic or global problems could be alleviated if we based our energy on hydrogen
Answers
The hydrogen can be produced from water using renewable energy sources, which makes it more sustainable.
If we based our energy on hydrogen, two major economic or global problems that could be alleviated are:
1. Climate change: This is a global issue that requires an immediate response. The world needs to move away from carbon-emitting fossil fuels. Burning hydrogen fuel emits only water and does not release greenhouse gases. If the world shifts to hydrogen fuel, it will reduce carbon emissions and help to slow down climate change.
2. Dependence on Oil: Most countries are dependent on oil. The price of oil is volatile, and the demand and supply fluctuate due to political, economic, and weather events. This dependence on oil is a major economic challenge for many countries. If we based our energy on hydrogen, we could reduce our dependence on oil and decrease oil imports, which could significantly improve the economy of countries that do not produce oil.
To know more about the hydrogen, visit:
https://brainly.com/question/31018544
#SPJ11
there are several isomers with the formula c3h9n. one of them cannot form an h-bonding interaction with another identical molecule, but can do so with a water molecule. draw that isomer.
Answers
The isomer that cannot form an H-bonding interaction with another identical molecule, but can do so with a water molecule, is the primary amine isomer (C3H9N).
The primary amine isomer (C3H9N) has the following structure: H3C-CH2-CH2-NH2.This isomer cannot form H-bonding interactions with another identical molecule because it lacks a hydrogen atom bonded to the nitrogen atom. In order for H-bonding to occur between two molecules, there must be a hydrogen atom bonded to an electronegative atom (such as nitrogen, oxygen, or fluorine) and a lone pair of electrons on the electronegative atom.
In the case of the primary amine isomer, there is only one hydrogen atom bonded to the nitrogen atom, and it is already involved in forming an H-bond with a water molecule.However, the primary amine isomer can form an H-bonding interaction with a water molecule. The lone pair of electrons on the nitrogen atom can act as a hydrogen bond acceptor, while the hydrogen atom bonded to the nitrogen can act as a hydrogen bond donor. This interaction can occur between the lone pair of electrons on the oxygen atom of water and the hydrogen atom bonded to the nitrogen of the primary amine isomer.
In summary, the primary amine isomer (C3H9N) cannot form an H-bonding interaction with another identical molecule due to the lack of a hydrogen atom bonded to the nitrogen. However, it can form an H-bonding interaction with a water molecule.
Learn more about: H-bonding
brainly.com/question/2575711
#SPJ11
Question 1
Which of the following is a false

Answers
Answer:
1.A 2.C 3.B
4. d
Explanation:
Enter your answer in the provided box. Calculate the wavelength of a
photon of electromagnetic radiation with a frequency of 61.7 MHz. m
Be sure to answer all parts. Calculate the energy of a photon of
electromagnetic radiation with a wavelength of 582.8 nm. * 10 Report
your answer in scientific notation using the provided boxes.
Answers
we find the energy to be approximately \(3.41 * 10^-19\) Joules is the answer.
To calculate the wavelength of a photon with a frequency of 61.7 MHz, we can use the formula: wavelength = speed of light / frequency. The speed of light is approximately\(3 * 10^8\) meters per second.
Converting the frequency to Hz (\(1 MHz = 10^6 Hz\)), we have \(61.7 * 10^6\)Hz.
Plugging these values into the formula, we get: wavelength =\((3 * 10^8 m/s) / (61.7 * 10^6 Hz).\)
Simplifying, we find the wavelength to be approximately 4.862 meters.
Now, to calculate the energy of a photon with a wavelength of 582.8 nm, we can use the equation: energy = Planck's constant × speed of light / wavelength.
Planck's constant is approximately \(6.63 * 10^-34\) Joule-seconds.
Converting the wavelength to meters (\(1 nm = 10^-9 m\)), we have \(582.8 * 10^-9 m.\)
Plugging these values into the equation, we get: energy =\((6.63 * 10^-34J·s) * (3 * 10^8 m/s) / (582.8 * 10^-9 m).\)
Simplifying, we find the energy to be approximately \(3.41 * 10^-19\) Joules.
know more about wavelength
https://brainly.com/question/31143857
#SPJ11
ffg full form
gti
ccc
Answers
Answer:
Grand Tourer Injection
the radioactive isotope 14 c has a half-life of approximately 5715 years. a piece of ancient charcoal contains only 88 % as much of the radioactive carbon as a piece of modern charcoal. how long ago was the tree burned to make the ancient charcoal? (round your answer to the nearest integer.)
Answers
The tree was burned to make the ancient charcoal approximately 17,130 years ago.
We can use the formula for radioactive decay to solve this problem. The formula is:
N = N0 * (1/2)^(t/T)
Where:
N = the amount of radioactive material at a given time
N0 = the initial amount of radioactive material
t = the time that has elapsed since the material was created
T = the half-life of the material
Let's use this formula for both the modern and ancient charcoal:
For modern charcoal:
N = N0
t = 0
T = 5715 years
For ancient charcoal:
N = 0.88*N0
t = ?
T = 5715 years
Now we can set up an equation using the two formulas:
0.88*N0 = N0 * (1/2)^(t/5715)
Simplifying this equation:
0.88 = (1/2)^(t/5715)
Taking the natural logarithm of both sides:
ln(0.88) = (t/5715)*ln(1/2)
Solving for t:
t = (ln(0.88)/ln(1/2))*5715
t ≈ 17,130 years
Therefore, the tree was burned to make the ancient charcoal approximately 17,130 years ago.
Learn more about radioactive material
https://brainly.com/question/3542572
#SPJ11
A student performing this lab measured the initial ph of one of his buffer solutions to be 7.68. after adding 0.25 ml of 0.50 m naoh, the ph of the same solution changed to 8.05. calculate the buffer capacity (δ mmol oh-/δ ph) of this solution to two decimal places.
Answers
The buffer capacity of this solution is 0.34.
Buffer solution is defined as a solution whose pH is not altered to any great extent by the addition of small quantities of either an acid or base is called buffer solution. Buffer is also known as the solution of reserve acidity or alkalinity which resists change of pH upon the addition of a small amount of acid or alkali.
Buffer capacity is number of moles of acid or base added to 1 lit buffer to bring 1 unit change in pH.
buffer capacity = (0.25x0.5) / (8.05-7.68) = 0.34
Hence, the buffer capacity of this solution is 0.34.
Learn more about buffer solution from the link given below.
https://brainly.com/question/24262133
#SPJ4
Which of the following is true about the behavior of an organism?
A.
The behavior of an organism is influenced only by the traits it inherits from its parents.
B.
The behavior of an organism is influenced only by the environment in which it lives.
C.
The behavior of an organism is not influenced by either its heredity or its environment.
D.
The behavior of an organism is influenced by both its heredity and its environment.
Answers
Answer:
C. The behavior of an organism is not influenced by either its heredity or its environment.
First period in the periodic table has ____ elements and they are called____
Answers
We need ti find how many elements there are in the first period and what are these.
To know what is the first period we must use that
Seeing the periodic table, the first row has two elements.
We can see that these elements are H ( hydrogen ) and He ( helium ).
ANSWER:
Firs period in the periodic table has two elements and they are called hydrogen and helium.

What might the student have done that caused this
error? List all possible causes.
Answers
Answer:
The capillary tube was too close to the bottom of the beaker.
The ruler may have moved.
Water got into the capillary tube.
The temperature was not allowed to equilibrate in the 2-4 minutes.
Explanation: