Earth Science, 25 points.
Review the properties and uses of the unknown mineral. It is...
(a) talc
(b) gypsum
(c) hematite
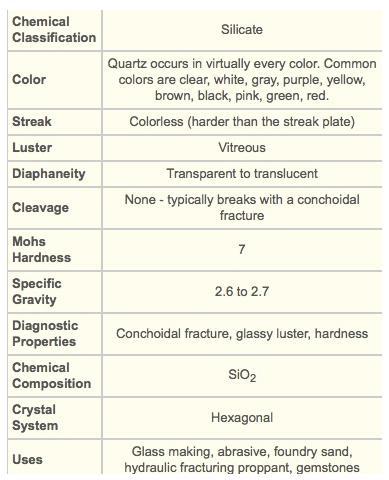
(d) quartz
Answers
The mineral that has been described in the question is quartz. Option D
What is a mineral?We know that the inner part of the earth's crust is filled with a lot of substances that can be able to extracted. The extraction of the substances that is a major field of business as they are used for the production of several materials on earth.
The substances that we can find either on the surface of the earth or in the interior of the earth that we can can be able to use for the production of many different substances or that are useful for further production are called minerals.
The structure of a mineral shows us how it works and that the mineral can be applied in the production certain materials. The mineral that we have contains silicon and oxygen hence the mineral is quartz.
Learn more about quartz:https://brainly.com/question/12822959
#SPJ1
Related Questions
Collect information regarding different laboratory apparatus volume measurement, mass measurement and fire producers
Answers
Volume Measurement Laboratory Apparatus:
Graduated Cylinder: A cylindrical container with volume markings along its vertical axis, used for measuring liquid volumes with good accuracy.
Burette: A long, graduated tube with a stopcock at the bottom, used for precise measurement of volumes of liquids during titrations.
Pipette: A slender tube with a tapered tip used to transfer specific volumes of liquids accurately. Common types include volumetric pipettes and micropipettes.
Volumetric Flask: A flask with a precise volume marking, typically used for preparing solutions of known concentrations.
Mass Measurement Laboratory Apparatus:
Analytical Balance: A highly accurate balance used to measure the mass of solid samples or substances with high precision.
Top-loading Balance: A balance that measures the mass of larger samples or substances with slightly lower accuracy compared to an analytical balance.
Weighing Boat/Dish: A small container used to hold substances being weighed on a balance.
Weights: Standard weights of known mass used to calibrate and verify the accuracy of balances.
Fire Producers Laboratory Apparatus:
Bunsen Burner: A gas burner commonly used for heating, sterilization, and flame-related experiments.
Alcohol Burner/Spirit Burner: A small burner that uses alcohol as fuel to produce a controlled flame for heating applications.
Meker Burner: A type of gas burner that produces a hot, intense flame with multiple openings, commonly used in laboratory settings.
Safety Matches/Laboratory Matches: Matches designed to be more stable and resistant to accidental ignition, often used to light Bunsen burners and other flames safely.
It's important to note that when working with fire-producing laboratory apparatus, proper safety protocols, such as wearing protective equipment and handling flammable substances with caution, should always be followed to prevent accidents and ensure laboratory safety.
learn more about Laboratory here
https://brainly.com/question/30753305
#SPJ11
How do secretions of apocrine glands differ from those of the eccrine sweat glands
Answers
An object with a mass of 15.3kg accelerates 3.5.0 m/s2
Answers
The force that acts on the body is 53.55 N
What is the force?According to the Newton law, the force is the product of the mass and the acceleration of a body. This is in accordance the Newton second law of motion. We now have to obtain the force that acts on the body.
Mass of the object = 15.3kg
Acceleration of the object = 3.5.0 m/s2
Force = 15.3kg * 3.50 m/s2
Force = 53.55 N
Learn more about force:https://brainly.com/question/13191643
#SPJ1
How is the number of shells in an atom related to the position of the element on the periodic table?
Answers
Answer:
The number of shells are called period ,so in the periodic table there are places under a column written periods in bib charts
A 1. 00 m long beam of stainless steel with a square 2. 00 cm x 2. 00 cm cross section has a mass of 3. 02 kg. What is it’s density in grams per cubic centimeter?
Answers
The density of the given stainless steel is 7.55 g/cm³.
Density is defined as the measure of how much “stuff” is in a given amount of space.
Determine the volume first to get the density , and the volume formula is,
Volume = Area × Thickness
Given,
Area = 2 cm × 2 cm = 4 cm²
Thickness = 100 cm
Substitute the values to get the volume,
Volume = 4 × 100 = 400 cm³
Where,
Density = mass / volume
Let's convert mass from kg to g = 3020 g
Substitute the values to get density,
Density = 3020 g / 400 cm^3
= 7.55 g/cm³
Learn more about volume from the link given below.
https://brainly.com/question/13586826
#SPJ4
How many kilograms is 0.22 lb?
O A. 0.22 kg
O B. 0.454 kg
C. 0.0998 kg
O D. 0.49 kg
Answers
Answer:
C)0.0998kg
explanation:
1 POUND=0.453592...kg
What is the mRNA sequence that will be formed from the DNA sequence below?
TAC CGG ATG CCA GAT CAA ATC
Answers
It will form the following: mRNA sequence will be 3' AUG CCA GGG UCG AAU UUC GAA UAG GCC CA 5'.
What is mRNA sequence?
High-throughput sequencing methods are used in RNA sequencing (RNA-Seq) to reveal the transcriptome of a cell. Compared to prior Sanger sequencing- and microarray-based techniques, RNA-Seq provides far greater coverage and better resolution of the dynamic dynamics of the transcriptome. In addition to allowing for the quantification of gene expression, RNA-Seq data also allows for the identification of novel transcripts, the recognition of alternatively spliced genes, and the detection of allele-specific expression.
Recent advancements in the RNA-Seq approach, which entails sample preparation, library building, and data analysis, have allowed researchers to better comprehend the functional complexity of the transcription. In addition to polyadenylated messenger RNA (mRNA) transcripts, total RNA, pre-mRNA, and noncoding RNA, including microRNA and long ncRNA, can all be explored using RNA-Seq.
Learn more about RNA here:
brainly.com/question/25979866
#SPJ1
Which statements describe Newton and the law of universal gravitation? Check all that apply.
Answers
Answer:
Newton believed that gravity was a force.
The law of universal gravitation offers a mathematical explanation for the attraction between the moon and Earth.
Newton was the first to have the idea that gravity is everywhere.
Answer:every object attract every other object
Explanation:
1)Predict whether the following solutions are acidic, basic or nearly neutral:
(a)
N
a
B
r
(b)
K
2
S
O
3
(c)
N
H
4
N
O
2
(d)
K
2
H
P
O
4
Answers
NaBr: Nearly neutral, K2SO3: Basic, NH4NO2: Acidic, K2HPO4: Basic
NaBr: Sodium bromide is a salt formed from a strong base (NaOH) and a strong acid (HBr). Salts of strong acids and strong bases are neutral, so NaBr is nearly neutral.
K2SO3: Potassium sulfite is a salt formed from a strong base (KOH) and a weak acid (H2SO3). Salts of strong bases and weak acids are basic, so K2SO3 is basic.
NH4NO2: Ammonium nitrite is a salt formed from a weak base (NH3) and a weak acid (HNO2). Salts of weak acids and weak bases can exhibit acidic or basic properties depending on their relative strengths. In this case, ammonium ions (NH4+) act as a weak acid, making NH4NO2 acidic.
K2HPO4: Dipotassium hydrogen phosphate is a salt formed from a strong base (KOH) and a weak acid (H3PO4). Similar to (b), salts of strong bases and weak acids are basic. Therefore, K2HPO4 is basic.
The predicted acidity or basicity of the given solutions is as follows: (a) nearly neutral, (b) basic, (c) acidic, and (d) basic. The nature of the solutions depends on the specific salts formed and the relative strengths of the acids and bases involved.
To learn more about Salts , visit
brainly.com/question/13818836
#SPJ11
If a handsaw does the same amount of work on a log is a chainsaw does, which has more power? Why?
Answers
Since both does the same amount of work, don’t worry about the variable W
Let’s talk about t, time
A chainsaw can cut into a log at a faster time than a handsaw can
Let’s assume chainsaw = 2 minutes
A handsaw = 6 minutes
Let’s pretend work = 12
12/2 = 6 power (chainsaw)
12/6 = 2 power (handsaw)
Based on this example, you can see that chainsaw has more power.
Which factor most often affects wind and weather patterns on Earth?
Answers
Answer:
i think its solar radiation
You have 10 Ci of tritium on july 1, 1998:
(i) How much 3He will you have on Jan, 1, 2000 in atoms and grams?
(ii) How much (in grams) 3H will you have?
Answers
(i) Tritium (3H) undergoes radioactive decay to produce helium-3 (3He) with a half-life of 12.32 years.
Using the radioactive decay law, we can calculate the amount of 3H that will decay into 3He over the given period:
t1/2 = 12.32 years
t = 1.5 years (from July 1, 1998, to Jan 1, 2000)
λ = ln(2) / t1/2 = 0.0562 year^-1
N(t) = N0 * e^(-λt)
where N(t) is the number of atoms at time t, N0 is the initial number of atoms, and e is the mathematical constant approximately equal to 2.718.
Plugging in the given values, we get:
N(1.5 years) = 10 Ci * (3.7 * 10^10 disintegrations/s/Ci) * (1 - e^(-λt)) = 2.76 * 10^19 atoms
To convert this into grams, we need to know the molar mass of 3He, which is 3.016 g/mol. Therefore, the mass of 3He produced will be:
m = N * M = 2.76 * 10^19 atoms * (3.016 g/mol / 6.022 * 10^23 atoms/mol) = 0.138 g
Therefore, on Jan 1, 2000, there will be 2.76 * 10^19 atoms of 3He and 0.138 grams of 3He.
(ii) To calculate the remaining amount of 3H, we can use the fact that the initial amount of 3H was 10 Ci, and we have already calculated the amount of 3H that has decayed into 3He. Therefore, the remaining amount of 3H is:
10 Ci - 2.76 Ci = 7.24 Ci
To convert this into grams, we can use the conversion factor:
1 Ci = 3.7 * 10^10 disintegrations/s
Therefore,
7.24 Ci * (3.7 * 10^10 disintegrations/s/Ci) * (1 mol/6.022 * 10^23 atoms) * (3.016 g/mol) = 0.015 g
Therefore, on Jan 1, 2000, there will be 0.015 grams of 3H remaining.
For mre such questions on helium
https://brainly.com/question/29392730
#SPJ11
A sample of gas at 23c is placed in an expandable container with a volume of 23 ml. the gas is heated and expands to a volume of 30 ml. what conversion(s) must take place to determine the final temperature of the gas?
Answers
To determine the final temperature of the gas, you need to apply Charles's Law and perform a temperature conversion from Celsius to Kelvin.
Charles's Law states that the volume of a gas is directly proportional to its temperature when pressure and the amount of gas are held constant. Mathematically, it can be represented as V1/T1 = V2/T2, where V1 and T1 are the initial volume and temperature, and V2 and T2 are the final volume and temperature. Temperature should be in Kelvin for this calculation.
First, convert the initial temperature from Celsius to Kelvin: T1 = 23°C + 273.15 = 296.15 K. Next, use Charles's Law to find the final temperature (T2): (23 mL / 296.15 K) = (30 mL / T2). Solving for T2, you get T2 = (30 mL * 296.15 K) / 23 mL = 385.95 K. The final temperature of the gas is 385.95 K.
To know more about temperature visit:-
https://brainly.com/question/7930770
#SPJ11
Which choice best describes a testable hypothesis?
A.) Carrots look better when given more water.
B.) Lilacs are better smelling than roses.
C.) Mountain lions travel over 100 km per day.
D.) The bacterium E. coli is worse than the bacterium S. aureus.
Answers
Answer:
A. carrots look better when given more water
what happens if water is added to concentrated acid
Answers
Answer:
Heat is released when they are both mixed together.
Explanation:
An orange solid cannot be broken down into simpler components by any physical methods. When it is heated, a chemical reaction takes place and a colorless gas and a silvery liquid form. Further tests on the resulting substances shows that they cannot be broken down further by physical or chemical methods. What type of substance is the original orange solid?.
Answers
Physically or chemically, elements cannot be reduced farther since they are the most basic form of matter. A pure substance known as an element is one that has only one sort of atom.
their nuclear numbers of protons. The debate over the conceptual meaning of the word "element" offers a rather singular chance to look at how chemists currently relate to one atom.
A chemical approach is one that aims to alter the chemical make-up of the waste's constituent elements. Using 1 papers, Sample 1 Examples of using the chemical technique in a phrase In the chemical atom used in wastewater treatment, pollutants are taken out of the wastewater and released through discharge outlets.
Learn more about element here
https://brainly.com/question/17105472
#SPJ4
What is the difference between a plain and a plateau? A. Only a plain is flat, a plateau is steep mountainside. B. Both are flat, but a plateau is bordered by cliffs. C. A plain is rolling hills with cliffs, and a plateau is large flat area.
Answers
Answer:
B
Explanation:
Both plain and plateau have flat surfaces. However, a plain is located in a low-lying area while a plateau is located on an elevated area. In essence, a plateau can be viewed as an elevated plain or a plain that is bordered by cliffs.
The correct option is B.
consider 1.3 moles of an ideal gas at an initial temperature of 400 k, in a 1.2 m3 closed container. if the gas goes through an isochoric process to twice the initial temperature, what is the new pressure of the gas?
Answers
The new pressure of the gas is 692 Pa.
Using the ideal gas law, PV = nRT, where P is pressure, V is volume, n is moles, R is the gas constant, and T is temperature, we can solve for the new pressure.
Initially, we have P1V1 = nRT1, where P1 = unknown, V1 = 1.2 m3, n = 1.3 moles, R = 8.31 J/mol*K, and T1 = 400 K.
During the isochoric process, the volume remains constant, so V2 = V1 = 1.2 m3.
The final temperature is 2T1 = 2400 K = 800 K.
Now we can solve for P2:
P1 = nRT1/V1 = (1.3 mol)(8.31 J/mol*K)(400 K)/(1.2 m3) = 346 Pa
P2 = P1(T2/T1) = (346 Pa)(800 K)/(400 K) = 692 Pa
Therefore, the new pressure of the gas is 692 Pa.
Learn more about isochoric here:
https://brainly.com/question/30393982
#SPJ4
6. Which of the following forces occurs when forces push or pull in opposite directions?
a) tension
c) torsion
b) shear
d) compression
Answers
Answer:
Shear
Explanation:
3 point different between reactant and product
Answers
Answer:
reactants
The substance(s) to the left of the arrow in a chemical equation are called reactants. A reactant is a substance that is present at the start of a chemical reaction
Explanation:
product_The substance(s) to the right of the arrow are called products . A product is a substance that is present at the end of a chemical reaction. In the equation above, the zinc and sulfur are the reactants that chemically combine to form the zinc sulfide product.
There is a standard way of writing chemical equations. The reactants are all written on the left-hand side of the equation, with the products on the right-hand side. An arrow points from the reactants to the products to indicate the direction of the reaction:
reactants → products
Reactants
1. The substances used as starting materials and which react with one another are reactants.
2. Example: In this reaction Mg and O2 are reactants.
Products
1. The substances which are formed as a result of reaction are products.
2. Example: In this reaction MgO is a product.
During an experiment vinegar was added to baking soda. the following observations were recorded.
observation 1: the container with the reaction mixture feels cooler.
observation 2: gas bubbles are released.
based on the observation, justify the type of change (physical or chemical) that took place. (4 points)
Answers
When vinegar was added to baking soda, A new product is formed as foam. Hence, it is s a chemical change.
What is chemical change ?A chemical change results from a chemical reaction forming new products while a physical change is when matter changes forms but not chemical identity.
Foam up with carbon dioxide gas,all of baking soda can be made react and disappear into vinegar solution and thus chemical reaction completes.
Hence, When vinegar was added to baking soda, A new product was formed Hence, it is s a chemical change.
Learn more about chemical change here ;
https://brainly.com/question/23693316
#SPJ1
What is another name for a row on the periodic table?
Answer choices :
Group
Period
Family
Transition Metals
Answers
Pls help with question 10

Answers
Answer:
Formula/Molecular Mass = 147.77415g/mol = 148g/mol (to 3 significant figures)
Explanation:
Given the Law of the Conservation of Mass, the amount of H2O produced will be equal to 10.00g-4.40g = 5.6g
Given equation n=m/M ,
n(H2O)= 5.6/ (1.008×2)+(15.999) = 0.318520677 moles
∴ x=0.318520677 moles
∴ Molecular mass (hydrated sodium sulfate) =
= (22.990×2)+(32.06)+(15.999×4)+[0.318...((1.008×2)+(15.999))]
= 147.77415g/mol
Answer:
Answer is in the attachment:
x=10
Explanation:

explain difference acids and bases
Answers
Acids are substances that turn wet blue litmus paper to red while bases are substances that turn red litmus paper to blue.
Acids vs BasesAcids and bases can be differentiated using their definitions as follows:
Acids are substances that turn blue litmus paper to red while bases are substances that turn red litmus paper to blue.Acids are substances that produce hydrogen ions as their positive ions in an aqueous solution while bases are substances that produce hydroxyl ions as their negative ions in aqueous solutions.An acid is a proton donor while a base is a proton acceptor.More on acids and bases can be found here: https://brainly.com/question/15192126
#SPJ1
Acid has hydrogen ion whereas base has hydroxyl ion.
What are acids and bases?Acid is a type of chemical compound that is dissolved in water and gives a solution with Hydrogen ions more than purified water. A base is a substance that gives electrons, takes protons, or releases hydroxide (OH-) ions. Acid is also called a proton donor. While a base is also called a proton acceptor.
Acids have a sour taste whereas Bases have a bitter taste. The pH value of acid is lower than 7. Basis has a pH value higher than 7. The strength of Acid depends on the number of Hydrogen ions. The strength of the Base depends on the amount of Hydroxide ions
An acid is any hydrogen-carrying substance that is able of giving a proton that is to say a hydrogen ion to another substance. A base is a molecule or ion able to gain a hydrogen ion from an acid.
so we can conclude that the molecules which have hydrogen ions are considered acids while those molecules which have hydroxyl ions are considered bases.
Learn more about acid here: https://brainly.com/question/25148363
#SPJ1
5. The bonds in BaO are best described as

Answers
Answer:
choice D. Ionic, because valence electrons are transferred.
Explanation:
-If the experimental (actual yield) is 17.0 grams what is the yield
Answers
If the theoretical yield is 20 grams and the actual yield is 17 grams, the percent yield would be 85%.
How are yields in grams calculated?To get the mass per mole, divide the reactant's mass by its molecular weight. As an alternative, we can multiply the millilitres of the reactant solution by the grams per millilitre of the liquid solution.
Once you have the theoretical yield, you can use the following formula to calculate the percent yield:
percent yield = (actual yield / theoretical yield) x 100%
For example, if the theoretical yield is 20 grams and the actual yield is 17 grams, the percent yield would be:
percent yield = (17 grams / 20 grams) x 100% = 85%
To know more about theoretical yield visit:-
https://brainly.com/question/14966377
#SPJ9
a mixture of 12.38 g of ne (20.18 g/mol) and 12.43 g ar (39.95 g/mol) have a total pressure of 1.60 atm. what is the partial pressure of ne, in atm? your answer should have three significant figures and no units.
Answers
The partial pressure of Ne is 0.641 atm.
What is the partial pressure of Ne?To calculate the partial pressure of Ne in the given mixture, we need to use the concept of mole fraction and the ideal gas law.
First, we calculate the number of moles of each gas present in the mixture. The number of moles is determined by dividing the mass of each gas by its molar mass.
For Ne:
Number of moles of Ne = 12.38 g / 20.18 g/mol = 0.613 mol
For Ar:
Number of moles of Ar = 12.43 g / 39.95 g/mol = 0.311 mol
Next, we calculate the mole fraction of Ne by dividing the moles of Ne by the total moles of both gases.
Mole fraction of Ne = 0.613 mol / (0.613 mol + 0.311 mol) = 0.663
Finally, we calculate the partial pressure of Ne by multiplying the mole fraction by the total pressure of the mixture.
Partial pressure of Ne = 0.663 * 1.60 atm = 1.065 atm
Rounding to three significant figures, the partial pressure of Ne is 0.641 atm.
Learn more about partial pressure
brainly.com/question/30114830
#SPJ11
what should be used to clean powder fouling, corrosion, and dirt from outside parts of lower reciever and extensiion assembly
Answers
To clean powder fouling, corrosion, and dirt from outside parts of the lower receiver and extension assembly, one should use the best quality cleaning materials and methods.
Powder fouling, corrosion, and dirt can accumulate on the outside parts of the lower receiver and extension assembly, making it hard to maintain the rifle. It is essential to clean the weapon regularly to maintain its efficiency and longevity.
The following cleaning materials are required for the cleaning process:
Cleaning solvent
Cleaning brush
Microfiber cloth
Lubricant
The best solvent to use when cleaning the rifle is a powder solvent. This is because it is specifically designed to remove fouling from firearms. It is also essential to use a cleaning brush made of brass, nylon, or synthetic material to avoid damaging the parts of the lower receiver and extension assembly.
Brass brushes are best for removing fouling, while nylon and synthetic brushes are gentle on the metal parts and help prevent corrosion. Avoid using a steel brush when cleaning the rifle since it may scratch or damage the surface of the metal.Lubricant is also essential in maintaining the rifle. It helps keep the moving parts smooth and minimizes friction between metal parts.
A high-quality lubricant should be used, and the excess should be wiped off with a microfiber cloth. To clean the weapon, apply the cleaning solvent onto the brush and scrub the outside parts of the lower receiver and extension assembly, then wipe it off with a microfiber cloth. Ensure the surface of the metal parts is dry before applying lubricant. Apply lubricant to moving parts of the weapon.
to know more about corrosion visit:
https://brainly.com/question/31590223
#SPJ11
Draw the most stable conformation of 3-isopropyl-1,1dimethylcyclohexane Please show me which one is the isopropyl and the dimethylcyclohexane and why is that the most stable conformation.
Answers
The most stable conformation of 3-isopropyl-1,1dimethylcyclohexane equatorial ethyl group (more stable). The stability of ethylcyclohexane's equatorial conformer exceeds that of its axial conformer by 7.4 kJ/mol.
According to the previous section, the chair conformation with the equatorial methyl group is more stable because it reduces steric repulsion, and as a result, the equilibrium favors the more stable conformer. Strongly favoring the equatorial shape is methylcyclohexane. The methyl group is in close proximity to the axial hydrogens in the axial conformation, which has an energetically unfavorable effect known as a 1,3-diaxial interaction. The methyl group prefers the equatorial shape as a result. The conformation of ethylcyclohexane in which the ethyl group is in the equatorial position is the most stable.
Learn more about equatorial ethyl group here:
brainly.com/question/24153313
#SPJ4

the equivalence point of any acid-base titration can be determined visually from a titration curve by finding the place where
Answers
Answer:
where the slope of the titration curve is the greatest
The titration curve can be used to identify the equivalency point of the titration.The volume of titrant is where the titration curve has the steepest slope.
How do you find the equivalence point on a titration curve?
The equivalency point for acid-base titrations can be identified quite quickly.A simple pH meter is used to measure the pH of the solution being titrated after different amounts of titrant have been introduced to create a titration curve.The curve can then be read to determine the equivalency point. The equivalency point is identified using thermometric titrimetry, which gauges the rate at which a chemical reaction alters temperature.The inflection point in this instance denotes the threshold at which an exothermic or endothermic process is equivalent. The pH of a solution during a titration is represented graphically by a titration curve.The equivalence point in a strong acid-strong base titration is reached when the moles of acid and base are equal, and the pH is 7. The [H+] and [OH] concentrations must be equal at some point to be considered the equivalency point.Just a little bit beyond that is the endpoint, where the indicator color totally changes and the pH shifts from acidic to basic, or vice versa. The precise halfway point between the reactions of the titrant and the acid in the buffer solution is known as the half equivalence point.Because the pKa of the acid and the pH of the solution are equal at the half equivalence point, finding this point is not too difficult. A weak-acid/strong-base titration will have an equivalent point at a somewhat basic pH.The reason for this is that while the acid is not nearly as strong and does not completely dissociate to neutralize each equivalent of the base, the base is stronger and dissociates to a greater extent.To learn more about acid base titration refer
https://brainly.com/question/23687757
#SPJ2