During an experiment, the percent yield of calcium chloride from a reaction was
80. 34%. Theoretically, the expected amount should have been 115 grams. What was
the actual yield from this reaction? (5 points)
CaCO3 + HCI - CaCl2 + CO2 + H2O
1) 90. 1 grams
2) 92. 4 grams
3) 109. 2 grams
4) 115. 3 grams
Answers
The actual yield from the reaction was 92.4 grams. The answer is 2)
To find the actual yield of calcium chloride from the reaction, we can use the percent yield formula:
Percent Yield = (Actual Yield / Theoretical Yield) x 100%
We know that the theoretical yield of calcium chloride is 115 grams, and the percent yield is 80.34%. Rearranging the formula to solve for actual yield, we get:
Actual Yield = (Percent Yield / 100%) x Theoretical Yield
Plugging in the given values, we get:
Actual Yield = (80.34% / 100%) x 115 grams
Simplifying and solving for actual yield, we get:
Actual Yield = 92.4 grams
Therefore, the actual yield from the reaction was 92.4 grams, which is the second option in the given choices, i.e., option 2.
To know more about actual yield, refer here:
https://brainly.com/question/21925098#
#SPJ11
Related Questions
A gas’s pressure is 765 mm Hg at 23°C. At what temperature in celsius will the pressure be 560 mm Hg?
Answers
Answer:
216 K
Explanation:
T2=T1P2/P1
Change C to K
What is correct about solubility of
substances?

Answers
What type of front is depicted here? (60 Points)
Cold
warm
stationary
Occluded

Answers
Answer:
warm
Explanation:
Please help me ........

Answers
->>
16) Which of the following single-replacement reactions will result in NO REACTION?
A. Na(s) + Mg(NO3)2(aq)
B. Na(s) + Al(NO3)2(aq) ->
C. Na(s) + Cu(NO3)2(aq) ->>
D. Na(s) + Fe(NO3)2(aq) →>>
E. Na(s) + Ba(NO3)2(aq)
Answers
A sample of an unidentified compound contains 29.84% sodium, 67.49% chromium, and 72.67% oxygen. What is the compound's empirical formula? If the molar mass of the molecular formula is 523.96 g/mol, find the molecular formula.
Empirical Formula:
Molecular Formula:
Answers
Answer:
i would help i just dont know sorry
Explanation:
What causes tectonic plates to move slowly over time?
A. Convection currents in the asthenosphere carry
them.
B. Ocean currents push them in different directions.
C. Winds blow steadily on them in different directions.
D. Volcanoes move them from north to south.
Answers
Answer:
A.
Explanation:
because im smart
why are webmo ir frequencies particularly inaccurate for oh and c=o
Answers
WebMO's IR frequencies for OH and C=O functional groups can be inaccurate due to limitations in empirical force fields, inadequate consideration of hydrogen bonding effects, and challenges in selecting appropriate levels of theory and basis sets.
IR frequencies calculated using the WebMO software package can be particularly inaccurate for OH (hydroxyl) and C=O (carbonyl) functional groups. This can be attributed to a few factors. Firstly, WebMO, like many other software packages, relies on empirical force fields to calculate molecular vibrations. These force fields are based on predetermined parameters and assumptions about bond lengths, angles, and force constants. While they work well for many functional groups, the complex nature of OH and C=O vibrations may not be accurately captured by these force fields.
Secondly, OH and C=O groups exhibit strong hydrogen bonding interactions, which can significantly affect their vibrational frequencies. However, the empirical force fields used by WebMO may not fully account for these hydrogen bonding effects, leading to inaccuracies in the calculated IR frequencies.
Furthermore, the accuracy of IR frequency calculations can also depend on the level of theory chosen and the basis set used in the calculations. Different levels of theory and basis sets have varying degrees of accuracy and computational cost, and choosing the appropriate combination for accurate OH and C=O vibrational frequencies can be challenging.
In conclusion, the inaccuracies in WebMO's IR frequencies for OH and C=O functional groups can be attributed to the limitations of empirical force fields, the inadequate consideration of hydrogen bonding effects, and the challenges in selecting appropriate levels of theory and basis sets.
Know more about Frequencies here:
https://brainly.com/question/29739263
#SPJ11
How many moles are on a 7.0 cm x 10.0 cm sheet of 1.0 mm thick aluminum foil? The density of the material is 2.702 g/mL.
Answers
The number of mole present in the aluminum foil, given that the foil has a thickness of 1.0 mm is 0.7 mole
How do I determine the number of mole?We'll begin by obtaining the mass of the aluminum foil. Details below:
Density of aluminum = 2.702 g/mLDimension = 7 cm × 10 cm × 1 mm = 7 cm × 10 cm × 0.1 cmVolume of aluminum = 7 cm × 10 cm × 0.1 cm = 7 cm³ = 7 mLMass of aluminum =?Density = mass / volume
Cross multiply
Mass = Density × Volume
Mass of aluminum = 2.702 × 7
Mass of aluminum = 18.914 g
Finally, we shall determine the number of mole present. Details below:
Mass of aluminum = 18.914 gMolar mass of aluminum = 27 g/mol Number of mole of aluminum =?Mole = mass / molar mass
Number of mole of aluminum = 18.914 / 27
Number of mole of aluminum = 0.7 mole
Thus, the number of mole is 0.7 mole
Learn more about mole:
https://brainly.com/question/13314627
#SPJ1
During the evaporation process, what separates the liquid component of the solution from the solid component?
Answers
During the evaporation process, liquid components separates from the solid components
Evaporation process means the change liquid water to gaseous water called evaporation and during evaporation process the soluble solid can be separated from its liquid component by allowing the liquid component to evaporate either by its own or by heating and also during evaporation the liquid component is lost to the atmosphere and the solid remains behind
Know more about liquid components
https://brainly.com/question/12214360
#SPJ1
write a detailed lab procedure to produce five sucrose solutions ranging from 10% down to 1%.
Answers
Lab Procedure for Preparing Five Sucrose Solutions Ranging from 10% to 1% includes the materials and procedures.
How to write out a lab procedure?Materials:
5 beakers or graduated cylinders
5 different sizes of plastic or glass pipettes
Distilled water
Sucrose
Graduated cylinder
Digital balance
Stirring rod or spoon
Measuring spoon
Lab label
Procedure:
Label five beakers or graduated cylinders with the desired sucrose concentration (10%, 9%, 8%, 7%, and 1%) and set them aside.
Using a graduated cylinder, measure out 100 mL of distilled water and pour it into the beaker labeled 10%.
Weigh the appropriate amount of sucrose needed to make a 10% solution, using a digital balance. For example, for 100 mL of water, 10 g of sucrose is needed.
Add the weighed sucrose to the beaker with distilled water and stir until it dissolves. Repeat this process for each successive concentration (9%, 8%, 7%, and 1%) using smaller and smaller amounts of sucrose and making sure to stir until each is fully dissolved.
For each solution, measure 10 mL using a different size of pipette and transfer it to a new labeled beaker.
Repeat the transfer of 10 mL of each solution to new labeled beakers until you have 5 beakers with 10 mL of each solution.
Note: To accurately measure the volume of the solutions, it is important to use calibrated pipettes. Make sure to label each beaker with the corresponding sucrose concentration, date, and your name for proper identification.
This lab procedure will produce five solutions with sucrose concentrations ranging from 10% to 1% in increments of 1%. The solutions can be used for various experiments or assays that require different sucrose concentrations.
Fond out more on lab procedures here: https://brainly.com/question/15125278
#SPJ1
Using the phase diagram for H₂O, what phase is water in at 1 atm pressure
and -5°C?
A. It is at its melting point.
B. It is in the gas phase.
C. It is in the liquid phase.
D. It is in the solid phase.
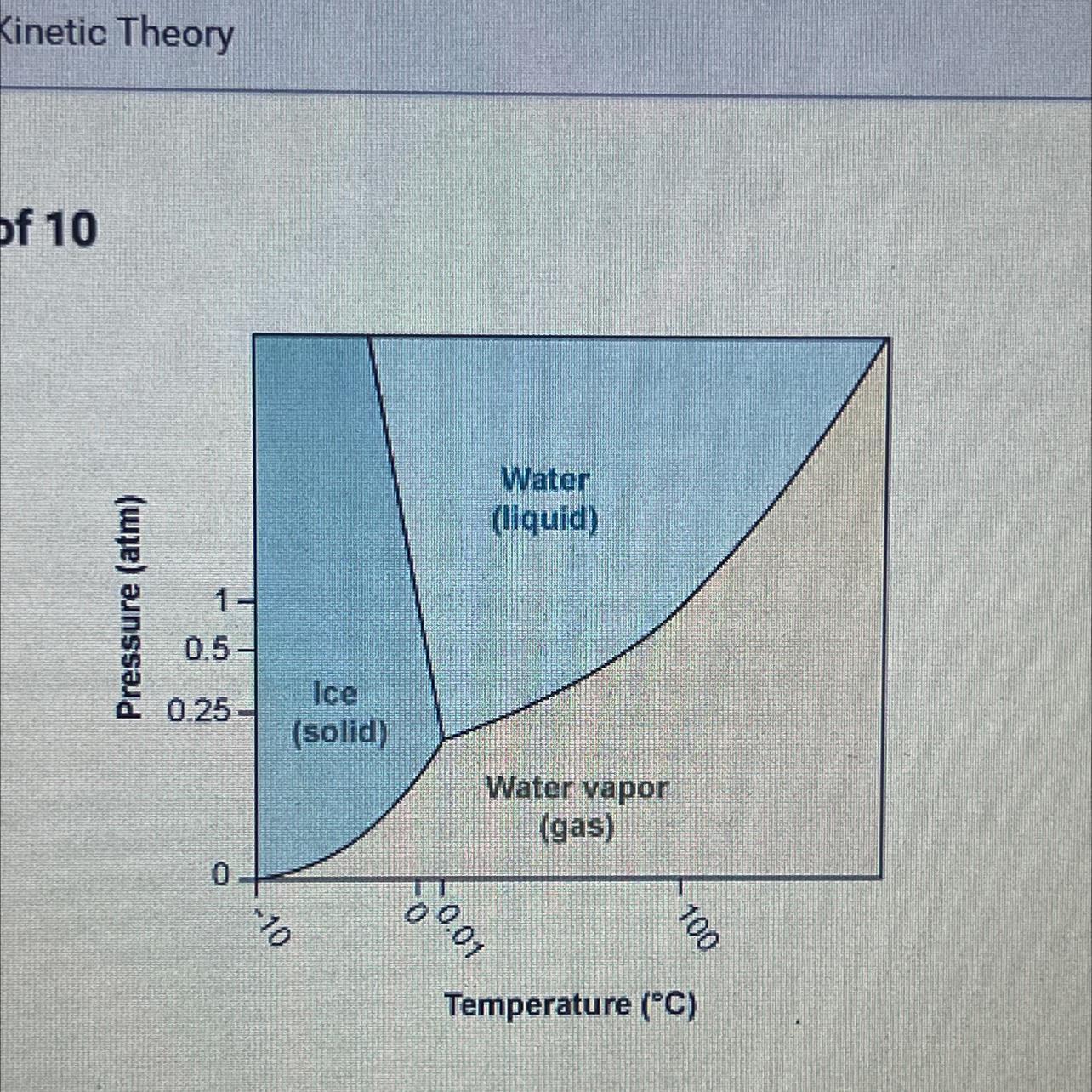
Answers
The number of phases that exist in equilibrium in a system depends upon the variables like temperature, pressure and composition. Here at 1 atm pressure water is in solid phase. The correct option is D.
What is phase diagram?The phase equilibria studies are made simpler by the use of plots which show how the various equilibria depend on the temperature, pressure and composition variables. These diagrams are called phase diagrams.
Here the pressure 1 atm on y-axis coincides with the temperature -5°C on x-axis. The water system is a one component system. So the water is in solid phase.
Thus the correct option is D.
To know more about phase diagrams, visit;
https://brainly.com/question/16945664
#SPJ9
Some one help pls on number 2 don’t mind the tv

Answers
Answer:
No, I believe it's not balanced, srry if I got it wrong for you
1.the study of blood serology
2.the development of digital storage and technology for use in bioscience applications biochemistry
3.the study of poisons genetics
4.the study and use of biological structures and processes for industrial purposes bioinformatics
5.the scientific study of heredity epigenomics
6.the study of the effects and interaction of the expression of genes toxicology 7.the study of the chemistry of organisms biotechnology
8.the study of decomposition of bodies after death anthropology
Answers
The various field of science include the following;
1. Serology.
2. Bioinformatics.
3. Toxicology.
4. Biotechnology.
5. Genetics.
6. Epigenomics.
7. Biochemistry.
8. Taphonomy.
"Fields of Science"Science is the interest and application of information and understanding of the normal and social world taking after a precise strategy based on prove.
1. Serology: The study of blood.
2. Bioinformatics: The development of digital storage and technology for use in bioscience applications.
3. Toxicology: The study of poisons.
4. Biotechnology: The study and use of biological structures and processes for industrial purposes.
5. Genetics: The scientific study of heredity.
6. Epigenomics: The study of the effects and interaction of the expression of genes.
7. Biochemistry: The study of the chemistry of organisms.
8. Taphonomy: The study of decomposition of bodies after death.
Learn more about "Fields of Science" :
https://brainly.com/question/9570229?referrer=searchResults
Does melting sea ice in the Arctic increase sea level directly? Why or why not? How would melting over Antarctica be different?
Answers
Explanation:
The ice melting would make more water because ice is water and if it melts it make water.
hope this helps :)
What must occur before a newly made polypeptide is secreted from a cell?.
Answers
Answer: Your answer is Its signal sequence must target it to the ER, after which it goes to the Golgi.
Hope this helps!
how many number of atoms does these have?

Answers
Answer:
Explanation:
16. 12
17. 8
18. 9
19. 10
20. 5
21. 15
22.8
23. 24
24. 12
25. 3 i guess( some one comment for 25th pls)
26. 2
How much of a 0.230 g radioactive sample with a half-life of 8 hours would remain after a period of 2.50 days?
Answers
The amount of radioactive sample after 2.5 days is 0.00127 g.
What is half-life?Half-life is the time require for half the sample of a radioactive material to decay
To calculate the amount of radioactive sample left after 2.5 days, we use the formula below.
Formula:
R' = R/\(2^{t/n}\)................... Equation 1⇒ Where:
R' = Amount of radioactive sample leftR = Original amount of samplet = Total timen = Half-lifeFrom the question,
⇒ Given:
R = 0.230 gt = 2.5 days = (2.5×24) hours = 60 hoursn = 8 hoursSubstitute these values into equation 1
R' = 0.230/(\(2^{60/8}\))R' = 0.230/\(2^{7.5}\)R' = 0.230/181.02R' = 0.00127 g.Hence. the amount of radioactive sample after 2.5 days is 0.00127 g.
Learn more about half-life here: https://brainly.com/question/25750315
#SPJ1
1. A neutral atom has a mass number of 128 and an atomic number of 52. Use this information to fill in the table below.
Question Answer
How many protons are in this atom? How do you know?
How many electrons does the atom have? How do you know?
Calculate the number of neutrons – show your work.
If this atom becomes an ion with a +4 charge, how many electrons would the ion have?
This atom has an isotope with one extra neutron – what is its mass number?
Answers
1) p=52
2)p=e=52
52-4=48
128
You graph the concentration of reactant A versus time and get a linear set of data with a slope of -1.2 x10-4. NOTE for describing graph... [A] vs. time; y vs. x; y=[A] and x=timeRate = k[A]m[B]n[C]pWhat additional information is required to determine the necessary units for the rate constant, k?Separate your answers with "and" : This and That and ThoseWhat is the reaction order with respect to reactant A?Zeroth OrderHalf OrderFirst OrderSecond Order
Answers
Answer:
Units of the concentration of the reactant and unit of time.
The reaction order with respect to reactant A is m.
Explanation:
The information from the exercise is:
• The axes of the graph:
• The rate formula:
\(Rate=k*[A]^m*[B]^n*[C]^p\)From the rate equation we can see that the reaction order with respect to reactant A is m.
So, the additional information required is the units of the concentration of the reactant and the unit of time, because the constant k is the slope of the graph.

How much water is needed to dissolve 30g or Pb(NO3)2 at 40.0C answer the question? NO BOTS ALLOWED
Answers
Answer:
10 must be added
Explanation:
The vapor pressure of a given molecular substance is affected by changes in ___ and by the strength of the ___ forces for the substance.
Answers
Vapor pressure is defined as the amount of pressure that is exerted by a vapor present over a liquid or solid.
It is determined by the number of gas particles that are present over the surface of the liquid or solid. When the number of gas particles increases, the vapor pressure increases.
It increases with the increase in temperature and decreases with the decrease in temperature.
The strength of the intermolecular forces for a substance is another important factor that influences the vapor pressure of the substance.
Stronger intermolecular forces result in less vapor pressure, while weaker intermolecular forces result in more vapor pressure.
For example, if we compare two different molecular substances with each other, one has strong intermolecular forces, while the other has weak intermolecular forces. The substance with stronger intermolecular forces will have a lower vapor pressure than the substance with weaker intermolecular forces.
Therefore, the vapor pressure of a given molecular substance is affected by changes in temperature and by the strength of the intermolecular forces for the substance.
learn more about Vapor pressure on
https://brainly.com/question/29640321
#SPJ11
How much heat is released when 5.0 g of hydrogen peroxide decomposes at constant pressure when enthalpy change is given as -196kj?
Answers
the letter x replaces the element symbol. the top value represents mass number and the bottom value represents atomic number. 1. ) how many neutrons does this element have? 2. ) how many neutrons does this element have? 3. ) and are these two elements isotopes? (yes or no) 4. 3. ) and are these two elements isotopes? (yes or no)
Answers
Chemical chemical X. The silicon is available. This information is not given here. Therefore, we shall use the atomic number as the amount of protons. So, the solution is 17.
What do the protons in atoms do?A proton, a quasiparticle, is found in the nucleus of every atom.. The particle has an electrical charge that is positive and opposite to the electron's. A single proton would weigh just 1.673? 10-27 kilos if it were isolated, which is only a little bit less than a neutron.
Protons and electrons: what are they?A subatomic particle with a negative charge is an electron. A proton is an unit of matter with the a positive charge. Protons are bound together in an atom's nucleus by the potent nuclear energy. The neutron is a type of subatomic particle sans charge (they are neutral).
To know more about protons visit:
https://brainly.com/question/29602282
#SPJ4
What do you notice when you get into a car that has been sitting in the sun for a while?
Answers
When you get into a car that has been sitting in the sun for a while, there are several noticeable things that may occur. Here are some of the common observations:
1. Heat: One of the first things you'll notice is the intense heat inside the car. This is because the sun's rays have been absorbed by the car's exterior and trapped inside, creating a greenhouse effect. The temperature inside the car can become significantly higher than the temperature outside.
2. Hot Surfaces: The surfaces inside the car, such as the seats, dashboard, steering wheel, and metal parts, can become extremely hot to the touch. This is due to the absorption of heat from the sun. It's important to be cautious and avoid direct contact with these hot surfaces to prevent burns or discomfort.
3. Odor: The interior of the car may have a distinct smell when it has been sitting in the sun for a while. This is often referred to as the "hot car smell." It is caused by the combination of materials, such as upholstery, plastic, and carpet, heating up and emitting a specific odor.
4. Fading or Discoloration: Prolonged exposure to sunlight can cause fading or discoloration of materials inside the car. For example, the upholstery, dashboard, and other surfaces may gradually lose their original color and become faded or discolored over time.
5. Glare: When you first enter a car that has been sitting in the sun, you may notice a strong glare from the sunlight reflecting off the windshield and other glass surfaces. This glare can make it difficult to see clearly and may require the use of sunglasses or adjusting the sun visors to minimize the brightness.
It's important to note that these observations may vary depending on factors such as the intensity of the sunlight, the duration the car has been in the sun, and the materials used in the car's interior. Regular maintenance and taking precautions, such as using sunshades or parking in shaded areas, can help minimize some of these effects.
to know more about the greenhouse effect here:
brainly.com/question/31595505
#SPJ11
How does purifying a compound by distillation work with the principles of green chemistry in particular comment principles 6 and 7.
Answers
By first vaporising the component and then condensing the vapour back into a liquid form, distillation purifies a compound.
The fundamental idea underpinning distillation is that different liquid mixtures may be distinguished by the difference in their boiling points. When a liquid's vapour pressure reaches atmospheric pressure, that temperature is known as the boiling point. This technique divides liquids into volatile and non-volatile categories.
It is then boiled after being placed in the RB flask. The more volatile or lower boiling point component evaporates more quickly and is collected in a different container. Condensation is accelerated with the aid of a condenser.
For instance, distillation can be used to separate a chloroform and aniline mixture. Aniline has a boiling point of 189°C while chloroform has a boiling point of 60°C. Thus, a mixture of chloroform and aniline can be separated by distillation.
To know more about purifying a compound from the link
brainly.com/question/13226101
#SPJ4
What is an astronomical unit?
Answers
Answer:
a unit of measurement equal to 149.6 million kilometers, the mean distance from the center of the earth to the center of the sun.
Explanation:
Answer:
a unit of measurement equal to 149.6 million kilometers, the mean distance from the center of the earth to the center of the sun.
Explanation:
HELP ASAP DUE TODAY

Answers
Answer:
4. c) Lose only 3 electrons
5. d) Nitrogen.
Your answers are correct
Using Molarity to Find Solute Mass | Chemistry
Answers
The molarity equation is as follows: Molarity is defined as moles of solute/liters of solution.
You can calculate the number of moles of solute if you know the molarity (concentration) of a solution and the total volume of the solution (be sure it is in liters). Afterward, you might need to convert the solute's moles to grams.
By percent by mass is another way for estimating the solute concentration in a solution. To do this, an equation of the form
The formula for mass percent is (mass of solute/mass of solution) X 100.
Percent by volume is a third technique for estimating the solute concentration in a solution. The formula for this is:
(Volume of Solute/Volume of Solution) X 100% is the formula for percent by volume.
Learn more about Molarity here:
brainly.com/question/29568864
#SPJ4
how many joules is transferred and what is the mass of the water the question is seen in the photo below

Answers
The granite block transferred 2052.88 joules of energy and the mass of the water is 19.84 grams.
Apply the idea of energy conservation to calculate the amount of energy that was transferred from the granite block to the water. The energy obtained by the water will be equivalent to the energy lost by the granite block.
Firstly, determine the energy lost by the granite block:
\(\rm \Delta Q_{granite} = mass_{granite} \times specific\ heat_{granite} \times \Delta T_{granite}\)
In which:
\(mass_{granite}\) = 126.1 grams (mass of the granite block)
\(\rm specific\ heat_{granite}\) = 0.795 joules/gram degree Celsius (specific heat capacity of granite)
\(\rm T_{granite}\) = final temperature - initial temperature
Given:
initial temperature = 92.6°C
final temperature = 51.9°C
ΔT = 51.9°C - 92.6°C = -40.7°C
ΔQ = 126.1 g × 0.795 J/g°C × (-40.7°C)
ΔQ = -2052.88 J
The negative sign represent that the granite block loses energy.
Due to the conservation of energy, the energy received by the water will be equal to that lost by the granite block in magnitude but will be of the opposite sign:
\(\rm \Delta Q_{water}\)= - \(\rm \-\Delta Q_{granite}\)
\(\rm \Delta Q_{water}\) = 2052.88 J
Thus, the granite block transferred 2052.88 joules of energy.
To determine the mass of the water, use the following equation:
\(\rm \Delta Q_{water}\) = mass of water × specific heat of water × ΔT of water
In which:
mass of water = to find
specific heat of water = 4.186 joules/gram degree Celsius (specific heat capacity of water)
ΔT of water = final temperature of water - initial temperature ofwater
initial temperature = 24.7°C
final temperature = 51.9°C
ΔT of water = 51.9°C - 24.7°C = 27.2°C
Substitute the values:
2052.88 J = mass of water × 4.186 J/g°C × 27.2°C
To solve for mass of water:
mass of water = 2052.88 J / (4.186 J/g°C × 27.2°C)
mass of water = 19.84 grams
Thus, the mass of the water is 19.84 grams.
Learn more about energy, here:
https://brainly.com/question/30007427
#SPJ1