Answers
The molecular formula for tripeptide Gly-Gly-His is C10H15N5O4. The structure is attached below in the picture. It has a role as a metabolite.
A tripeptide is created by combining three amino acids, and it is then joined by two or even three peptide bonds. The order of the individual amino acids that make up a peptide dictates how it functions, just like with proteins. The simplest tripeptide is glycine. The most significant tripeptide, according to scientific studies, is glutathione (-L-Glutamyl-L-cysteinylglycine), which has a number of functions in a wide range of life forms.
To learn more about tripeptide click on the given link: https://brainly.com/question/28335208
#SPJ4

Related Questions
With all the steps please!For a 0.2 M aqueous solution of sodium hydroxide, calculate the following:a) pHb) pOHc) [H+]d) [OH-]
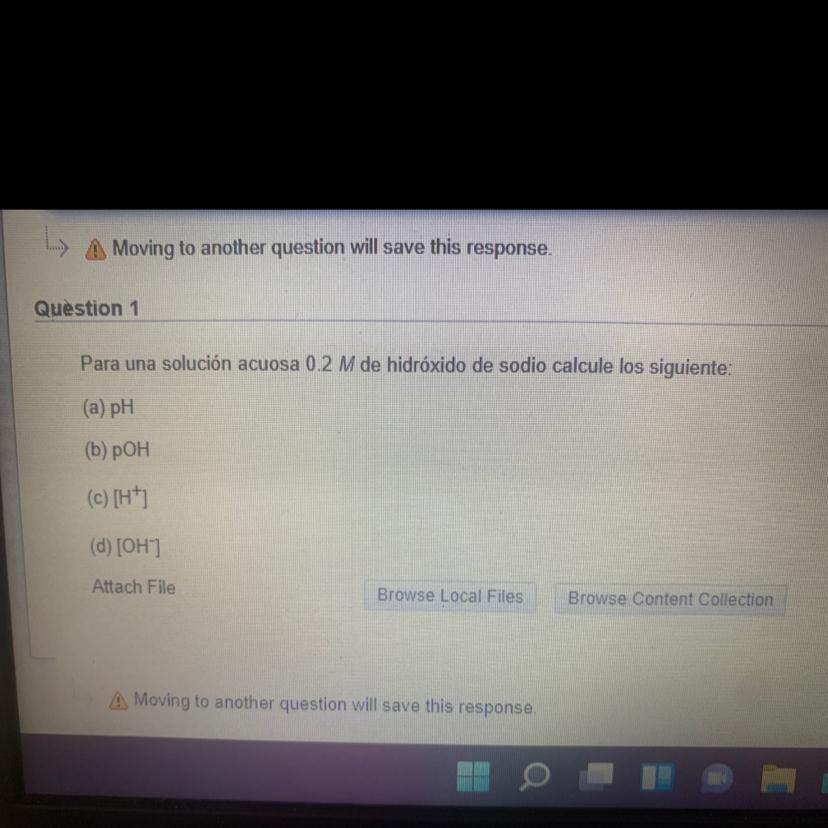
Answers
Answer:
a) pH= 13.3
b) pOH= 0.7
c) [H+]= 5.01*10^-14M
d) [OH-]= 0.2M
Explanation:
The formula of sodium hydroxide is NaOH. In the molecule there are Na+ ions and OH- that dissociates like this:
\(\text{ NaOH}\rightarrow\text{ Na}^++\text{ OH}^-\)That means that 1 mole of NaOH dissociates into 1 mole of Na+ ions and 1 mole of OH-.
So, for a 0.2M solution, 0.2 moles of NaOH will dissociate into 0.2 moles of Na+ ions and 0.2 moles of OH-.
d) [OH-]
From the dissociation of NaOH we know that the concentration of OH- is [OH-]=0.2M.
b) pOH
With the concentration of OH-, we can calulate the pOH:
\(\begin{gathered} pOH=-log\lbrack OH^-] \\ pOH=-log(0.2) \\ pOH=0.7 \end{gathered}\)So, the pOH is 0.7.
a) pH
Knowing the pOH and the following formula, we can calculate the pH of the solution:
\(\begin{gathered} pH+pOH=14 \\ pH+0.7=14 \\ pH=14-0.7 \\ pH=13.3 \end{gathered}\)The pH of the solution is 13.3.
c) [H+]
Now that we know thw pH of the solution, we can calculate the concentration of H+:
\(\begin{gathered} pH=-log\lbrack H^+\rbrack \\ 13.3=-log\lbrack H^+\rbrack \\ 10^{(-13.3)}=\lbrack H^+\rbrack \\ 5.01*10^{-14}=\lbrack H^+\rbrack \end{gathered}\)So, the concentration of H+ is 5.01*10^-14M.
A sealed flask contains 0.50 g of water at 28 ∘C. The vapor pressure of water at this temperature is 28.36 mmHg.
What is the minimum volume of the flask in order that no liquid water be present in the flask?
Answers
This problem is providing us with the mass, 0.50 g, temperature, 28 °C and pressure, 28.36 mmHg, of steam and asks for the volume to not let the steam to condense. At the end, the result turns out to be 18.4 L.
Ideal gasIn chemistry, we can model the pressure-temperature-volume-mole behavior of gases via gas laws with different approaches. However, since this problem involves all these variables, we understand we need to use the ideal gas law:
\(PV=nRT\)
Where we should set the pressure in atmospheres, moles in mol and the temperature in kelvins, as well as solving for volume as it is the required:
\(V=\frac{nRT}{P} \\\\V=\frac{(0.50g*\frac{1mol}{18.02g} )(0.08206\frac{atm*L}{mol*K})(28+273)K}{28.36mmHg*\frac{1atm}{760mmHg} } \\\\V=18.4L\)
Learn more about the ideal gases: https://brainly.com/question/8711877
For this question, we need to use ideal gas law
\(pV=nRT\)
Given :
Mass of water = 0.50g
Temperature = 28°C
Converting to Kelvin Scale,
28 + 273.15 K
=> 298.15 K
Pressure = 28.36 mm of Hg
=> 0.03732 atm
We also know that R = 0.08205 L/atm/mol.K
We want to the Volume
Let's first calculate the number of moles (n) of H2O :
\(n = \frac{m}{M}\), where m is the mass and M is the molar mass of water ( = 18,01528 g/mol )
n = 0.50g / 18,01528 g/mol
n = 0.02775 mol
\(V= \frac{nRT}{p}\)
\(V= \frac{(0.02775mol \times 0.08205l/atm/mol.k \times 298.15k)}{0.03732atm} \)
\( = > V = 18.19L\)
Therefore, the minimum volume = 18.19L
~Benjemin360
Which is the order of these solutions from strong acid to strong base?
Solutions:
household ammonia
battery acid
baking soda
stomach acid
antacid
A-stomach acid household ammonia battery acid antacid baking soda
B-battery acid stomach acid antacid baking soda household ammonia
C-battery acid stomach acid baking soda antacid household ammonia
D-stomach acid battery acid antacid baking soda household ammonia
Answers
Answer:
First is battery acid with a pH of 1, second is stomach acid with a pH of 1.5-3.5 (depends), third is antacid with a pH of 7, fourth is baking soda with a pH of 9, and finally, fifth is household ammonia with a pH of 11.
Here is a picture of the pH levels to better help you understand.

Answer:
b
Explanation:
The term aromatic is a structural term that applies to cyclic conjugated molecules that are planar and lack alkene like reactivity due to enhanced resonance stabilization. Which of the following compounds is not classified as an aromatic compound?
Answers
An aromatic compound is cyclohexadiene
What is an aromatic compound?An aromatic compound is a type of organic compound that contains a ring of atoms with alternating double bonds, known as an aromatic ring or an arene. The most common and well-known example of an aromatic compound is benzene, which has a ring of six carbon atoms with alternating double bonds.
Aromatic compounds are characterized by their unique chemical and physical properties, including their stability. They are know to be planar and lack alkene like reactivity due to enhanced resonance stabilization.
Learn more about aromatic compound:https://brainly.com/question/30630680
#SPJ1
A compound will not be classified as aromatic if it is not cyclic, planar, or, have a continuous ring of pi electrons that follows Hückel's rule.
Aromatic compoundsAn organic compound would not be classified as an aromatic compound if it does not meet the criteria for being an aromatic compound.
The criteria for being an aromatic compound include being:
cyclicplanarhaving a continuous ring of pi electrons that follows Hückel's rule.If an organic compound is not cyclic or planar, or if it does not have a continuous ring of pi electrons that follows Hückel's rule, then it would not be classified as an aromatic compound.
More on aromatic compounds can be found here: https://brainly.com/question/30630680
#SPJ1
Which structure is the Lewis structure for ammonia (NH3)?
A.
A bond line structure of a compound has N H H H. The nitrogen has two dots at its bottom represents a lone pair of electrons.
B.
A bond line structure of a compound has H N H in the linear plane and hydrogen is branching upward, and the compound is H N (H) H.
C.
A bond line structure of a compound has H N H in linear plane and a hydrogen is branching upward, and the compound is H N (H) H. The nitrogen has two dots at its bottom represents a lone pair of electrons.
D.
A bond line structure of a compound has H N H H. The nitrogen has two dots on its top represents a lone pair of electrons.
Answers
Answer: **
H-N-H
|
H
Explanation:
Look at a periodic table to determine how many electrons you need to account for. Hydrogen (H) only has 1 electron, while Nitrogen (N) has 5. We have three Hydrogen atoms and one Nitrogen atom, so the total number of electrons will be 3 * 1 + 5 = 8 e-.
Now, place the center atom, which will be Nitrogen and place the three Hydrogens on three sides of it as above in the answer. You should use single bonds for this. Each single bond is a pair of electrons, so since we have three single bonds so far, we have accounted for 2 * 3 = 6 electrons. However, we need 2 more electrons for the total of 8. We put these electrons in as a lone pair above Nitrogen.
We check to see if everything follows the octet rule: Nitrogen has three single bonds, so that's 6 e-, as well as one lone pair, so that's another 2 e- for a total of 8 e-. Check. Now look at Hydrogen: H is the only element whose full orbital is 2 e-. Each H has a single bond with Nitrogen, so each does have 2 e-.
Thus, we know this is the correct diagram, and we are done.
Explanation:
A bond line structure of a compound has H N H in linear plane and a hydrogen is branching upward, and the compound is H N (H) H. The nitrogen has two dots at its bottom represents a lone pair of electrons. So ,the correct answer is option C.
The correct Lewis structure for ammonia (\(NH_3\)) is option C. It shows a bond line structure with three hydrogen atoms (H) bonded to a central nitrogen atom (N) in a linear plane.
One hydrogen atom branches upward from the plane. Additionally, the nitrogen atom in this structure has two dots at its bottom, indicating a lone pair of electrons. This arrangement follows the octet rule, as nitrogen has formed three covalent bonds with hydrogen, completing its valence shell. The lone pair on nitrogen gives ammonia its characteristic properties.
Thus, option C accurately represents the Lewis structure of ammonia, showing the bonding and lone pair arrangement of its atoms.
To know more about bond line structure:-
https://brainly.com/question/30639285
When 75.5 grams of phosphorus pentachloride react with an excess of water, as shown in the unbalanced chemical equation below, how many moles of hydrochloric acid will be produced? Please show all your work for the calculations for full credit. PCl5 + H2O --> H3PO4 + HC
Answers
Answer:
Explanation: M(PCL5)= 31 + 5(35.5)
=208.5g/mol
M(H20)= 18g/mol
n(PCL5) = 75.5÷208.5
= 0.362mol
n(HCl)/n(PCL5)= 5/1
n(HCl)= 5×0.362
=1.81mol of HCl
How could the age be interpreted in a rock in which the blocking temperature has been reached?
Answers
PLEASE ANSWER QUICKLY!!!!
2KI (aq) + Cl₂(g) → 2KCl(aq) + 1₂(g)
What volume of 12 gas forms when
21 L Cl2 react at STP?
[?] L 12

Answers
The volume of 12 gas forms when 21 L Cl2 react at STP is 21 L.
To determine the volume of 12 gas (I assume you mean I2 gas) formed when 21 L of Cl2 reacts at STP (standard temperature and pressure), we need to use the ideal gas law equation.
The ideal gas law equation is given by:
PV = nRT
Where:
P = pressure
V = volume
n = number of moles
R = ideal gas constant
T = temperature
At STP, the pressure is 1 atm, and the temperature is 273.15 K.
From the balanced equation, we can see that the molar ratio between Cl2 and I2 is 1:1. So, if 21 L of Cl2 reacts, it will produce an equal volume of I2 gas.
Given that the volume of Cl2 is 21 L, we can assume the volume of I2 gas formed will also be 21 L.
Therefore, the volume of I2 gas formed is 21 L.
For more such questions on volume
https://brainly.com/question/1749900
#SPJ8
Select the correct terms to complete this statement about charged particles.
Like charges attract | repel, and opposite charges attract repel. According to Coulomb's law, as the distance between two charged particles decreases, the force between the particles decreases I increases. As the magnitude of the charges decreases, the force decreases | increases.
Answers
Like charges repel each other, while opposite charges attract each other. This principle is one of the fundamental aspects of electrostatics. According to Coulomb's law, the force between two charged particles is directly proportional to the product of their charges and inversely proportional to the square of the distance between them.
As the distance between two charged particles decreases, the force between them increases. This is because the closer the particles are, the stronger the electric field they create, leading to a stronger force of interaction.
On the other hand, as the magnitude of the charges decreases, the force between the particles also decreases. This is because the force is directly proportional to the product of the charges. If one or both of the charges are smaller, the force they exert on each other will be weaker.
In summary, according to Coulomb's law, decreasing the distance between charged particles increases the force between them, while decreasing the magnitude of the charges decreases the force. This understanding of the relationship between charge, distance, and force is crucial in explaining the behavior of charged particles and the interactions between them.
Know more about Coulomb's law here:
https://brainly.com/question/26892767
#SPJ8
A reaction occurs in a calorimeter, resulting in the starting temperature of 38.8 ℃ and final temperature 21.0 ℃. What can you say about the reaction and the enthalpy change (ΔH) during the reaction?
Answers
Answer:
hola comoe stas
Explanation:
gracias x los puntos
i need help asap
A sample of tin goes through a temperature change of -160.56 °C while releasing 36298 joules of heat. The specific heat capacity of tin is 0.227 J/(g.°C). What is the mass of this sample?
A 13.66 mol sample of ammonia absorbs 33834 joules of heat. The specific heat capacity of ammonia is 80.08 J/(mol. °C). By how much did the temperature of this sample change, in degrees Celsius?
A sample of cobalt undergoes a temperature change of -1132.52 °C while releasing 455500 joules of heat. The specific heat capacity of cobalt is 0.4187 J/(g.°C). What is the mass of this sample?
A 372.4 g sample of indium goes through a temperature change of +140.73 K while absorbing
12505 joules of heat. What is the specific heat capacity of indium?
A 4.721 mol sample of molybdenum absorbs 35961 joules of heat. The specific heat capacity of molybdenum is 24.06 J/(mol-°C). By how much did the temperature of this sample change, in degrees Celsius?
A 56.2 g sample of ethanol is subjected to a temperature change of -110.56 K. The specific heat capacity of ethanol is 2.44 J/(g K). How many joules of heat were transferred by the sample?
A 5.774 mol sample of chromium absorbs 38674 joules of heat. The specific heat capacity of chromium is 23.35 J/(mol °C). By how much did the temperature of this sample change, in degrees Celsius?
A 4.9 mol sample of magnesium is subjected to a temperature change of -683.83 K. The specific heat capacity of magnesium is 24.9 J/(mol K). How many joules of heat were transferred by the sample?
A 0.2687 mol sample of tin is subjected to a temperature change of +222.48 K. The specific heat capacity of tin is 27.112 J/(mol K). How many joules of heat were transferred by the sample?
A 1.008 mol sample of neon undergoes a temperature change of -703.43 K while releasing
14738 joules of heat. What is the specific heat capacity of neon?
Answers
Answer:
To solve these problems, we can use the formula:
q = mcΔT
where q is the heat transferred, m is the mass of the substance, c is the specific heat capacity of the substance, and ΔT is the temperature change.
The mass of the sample of tin can be calculated as:
q = mcΔT
36298 J = m × 0.227 J/(g.°C) × (-160.56 °C)
m = 708.2 g
The temperature change of the sample of ammonia can be calculated as:
q = mcΔT
33834 J = 13.66 mol × 80.08 J/(mol.°C) × ΔT
ΔT = 31.7 °C
The mass of the sample of cobalt can be calculated as:
q = mcΔT
455500 J = m × 0.4187 J/(g.°C) × (-1132.52 °C)
m = 27.4 g
The specific heat capacity of indium can be calculated as:
q = mcΔT
12505 J = 372.4 g × c × 140.73 K
c = 0.238 J/(g.°C)
The temperature change of the sample of molybdenum can be calculated as:
q = mcΔT
35961 J = 4.721 mol × 24.06 J/(mol.°C) × ΔT
ΔT = 31.9 °C
The heat transferred by the sample of ethanol can be calculated as:
q = mcΔT
q = 56.2 g × 2.44 J/(g K) × (-110.56 K)
q = -15,585 J
The temperature change of the sample of chromium can be calculated as:
q = mcΔT
38674 J = 5.774 mol × 23.35 J/(mol.°C) × ΔT
ΔT = 27.4 °C
The heat transferred by the sample of magnesium can be calculated as:
q = mcΔT
q = 1.008 mol × 24.9 J/(mol K) × (-683.83 K)
q = -17,134 J
The heat transferred by the sample of tin can be calculated as:
q = mcΔT
q = 0.2687 mol × 27.112 J/(mol K) × 222.48 K
q = 1676.7 J
The specific heat capacity of neon can be calculated as:
q = mcΔT
14738 J = 1.008 mol × c × (-703.43 K)
c = 36.8 J/(mol.°C)
Explanation:
Please finish the sentence for me! giving 20 points for it
In a chemical change the number of atoms in the _________________
has to _________________ the number of atoms in the
___________________ because of the LAW OF CONSERVATION OF
MATTER.
Answers
Answer:
i think In a chemical change the number of atoms in the reactants has to equal the number of atoms in the products because of the LAW OF CONSERVATION OF MATTER.
I WILL MARK BRAINLIEST
Question
Which of the following is an example of a valid experiment?
Placing balls of different masses at the top of a ramp and measuring the distance that they roll.
Asking your family members if they prefer ham or turkey,
Placing two plants in a dark closet
Which of the following should NOT be included in your conclusion?
Responses
A list of materials required for the experiment
A brief explanation of the purpose of your experiment
A scientific explanation for your conclusion,
A statement of whether your hypothesis was correct or not
Which of these is NOT a testable hypothesis?
Responses
Plants that receive more light will grow at a faster rate
Adding fertilizer to plants will cause them to grow at a faster rate
Plants that receive less water will grow at a slower rate,
Plants that receive compost will taste better
Answers
Answer:
1. placing balls
2. list of materials
Explanation:
A sample of ammonia, NH3, has a mass of 78.25 g. Calculate the number of ammonia molecules in the sample.
number of molecules:
Answers
There are approximately \(2.76 * 10^{24\) ammonia molecules in the given sample.
To calculate the number of ammonia molecules in the sample, we need to use Avogadro's number and the molar mass of ammonia.
The molar mass of ammonia \((NH_3)\) can be calculated by adding up the atomic masses of nitrogen (N) and hydrogen (H):
Molar mass of \(NH_3\) = (1 x atomic mass of N) + (3 x atomic mass of H)
= (1 x 14.01 g/mol) + (3 x 1.01 g/mol)
= 14.01 g/mol + 3.03 g/mol
= 17.04 g/mol
Now, we can calculate the number of moles of ammonia in the sample using the formula:
Number of moles = Mass of the sample / Molar mass
Number of moles = 78.25 g / 17.04 g/mol
≈ 4.5865 mol (rounded to four decimal places)
Finally, we can use Avogadro's number, which represents the number of particles (atoms, molecules, etc.) in one mole of a substance. Avogadro's number is approximately \(6.022 * 10^{23\) particles/mol.
Number of ammonia molecules = Number of moles x Avogadro's number
Number of ammonia molecules ≈ 4.5865 mol x (\(6.022 * 10^{23\) molecules/mol)
≈ \(2.76 * 10^{24\) molecules (rounded to two significant figures)
Therefore, the provided sample contains roughly \(2.76 * 10^{24\) ammonia molecules.
Learn more about moles on:
https://brainly.com/question/24748125
The number of ammonia molecules in the sample is approximately 2.764 x \(10^{24}\) molecules.
To calculate the number of ammonia molecules in a given sample, we need to use Avogadro's number and the molar mass of ammonia.
The molar mass of ammonia (NH3) is calculated as follows:
Molar mass of N = 14.01 g/mol
Molar mass of H = 1.01 g/mol
Total molar mass of NH3 = 14.01 g/mol + (3 * 1.01 g/mol) = 17.03 g/mol
Now, we can calculate the number of moles of ammonia in the sample:
Number of moles = Mass of sample / Molar mass of NH3
Number of moles = 78.25 g / 17.03 g/mol = 4.594 moles
Next, we use Avogadro's number, which states that there are 6.022 x \(10^{23}\) molecules in one mole of a substance.
Number of molecules = Number of moles * Avogadro's number
Number of molecules = 4.594 moles * 6.022 x \(10^{23}\) molecules/mol = 2.764 x \(10^{24}\) molecules
Therefore, there are approximately 2.764 x \(10^{24}\) ammonia molecules in the given sample of 78.25 g.
Know more about Avogadro's number here:
https://brainly.com/question/1513182
#SPJ8
In the following experiment, a coffee-cup calorimeter containing 100 mL
of H2O is used. The initial temperature of the calorimeter is 23.0 ∘C
. If 6.60 g of CaCl2 is added to the calorimeter, what will be the final temperature of the solution in the calorimeter? The heat of solution ΔHsoln of CaCl2 is −82.8 kJ/mol
.
Assume that the specific heat of the solution formed in the calorimeter is the same as that for pure water: Cs=4.184 J/g⋅∘C
.
Express your answer with the appropriate units.
Answers
In the following experiment, a coffee-cup calorimeter containing 100 mL of \(H_{ 2} O\) is used. The initial temperature of the calorimeter is 23.0 ∘C. If 6.60 g of \(CaCl_{2}\) is added to the calorimeter, Final temperature of the solution in the calorimeter = 11.
The first step in solving this problem is to calculate the number of moles of \(CaCl_{2}\\\) added to the calorimeter.
Moles of \(CaCl_{2}\) = mass of \(CaCl_{2}\) / molar mass of \(CaCl_{2}\)
Moles of\(CaCl_{2}\) = 6.60 g / 110.98 g/mol (molar mass of \(CaCl_{2}\)
Moles of\(CaCl_{2}\) = 0.0594 mol
We can use the equation for heat transfer to find the change in temperature of the solution. q = mCsΔT, where q is the heat transferred, m is the mass of the solution, Cs is the specific heat of the solution, and ΔT is the change in temperature.
We know that the initial temperature of the calorimeter is 23.0 ∘C and the mass of the solution is 100 g (since the density of water is 1 g/mL). We can solve for ΔT: ΔT = q / mCs
To find q, we can use the enthalpy change of solution (ΔHsoln) and the number of moles of\(CaCl_{2}\)added: q = ΔHsoln x moles of\(CaCl_{2}\)
q = -82.8 kJ/mol x 0.0594 mol
q = -4.92 kJ
Now we can solve for ΔT: ΔT = (-4.92 kJ) / (100 g x 4.184 J/g⋅∘C)
ΔT = -11.8 ∘C
We can find the final temperature of the solution by adding the change in temperature to the initial temperature: Final temperature = 23.0 ∘C - 11.8 ∘C =11 ∘C.
Learn more about calorimeter here:
https://brainly.com/question/4802333
#SPJ1
List all possible values of the magnetic quantum number ml for a 1s electron.
Answers
The magnetic quantum number (ml) represents the orientation of the orbital in three-dimensional space the only possible value of the magnetic quantum number ml for a 1s electron is 0.
What is a quantum ?Quantum is the smallest possible unit of a physical quantity, such as energy or momentum. It is a fundamental concept in quantum mechanics, which is the branch of physics that deals with the behavior of matter and energy at the atomic and subatomic level.
The idea of quantization was first proposed by Max Planck in 1900, when he discovered that energy is emitted and absorbed in discrete units called "quanta" when studying the behavior of light and blackbody radiation. Later, this idea was extended to other physical quantities, such as the momentum and position of particles.
According to quantum mechanics, the behavior of particles and systems cannot be fully described using classical mechanics, which assumes that particles have definite positions and velocities at all times. Instead, the behavior of particles and systems is described using wave functions, which represent the probability of finding a particle at a given position and time.
The principles of quantum mechanics have important applications in many areas of physics, including atomic and molecular physics, condensed matter physics, and particle physics. They are also the basis.
To know more about quantum visit :
https://brainly.com/question/16746749
#SPJ1
An unknown liquid has a heat of vaporization of 5.48 kJ/mole. If the vapor pressure of this liquid at -170 degrees C is 117 torr, what is the normal boiling point of this liquid in degrees C? HINT: Normal boiling point occurs when the vapor pressure of the liquid is the same as atmospheric pressure (1 atm or 760 mm Hg).
Answers
The normal boiling point of the unknown liquid is 57.4°C.
The normal boiling point occurs when the vapor pressure of the liquid is equal to the atmospheric pressure. At normal boiling point, the temperature of the liquid is called the boiling point.
Using the Clausius-Clapeyron equation:
ln(P₂/P₁) = -(ΔHvap/R) * (1/T₂ - 1/T₁)
where P₁ is the vapor pressure at the given temperature T₁, P₂ is the vapor pressure at the boiling point temperature T₂, ΔHvap is the heat of vaporization, R is the gas constant.
At -170°C, the vapor pressure of the liquid is given as P₁ = 117 torr. At normal boiling point, the vapor pressure of the liquid is P₂ = 760 torr.
Converting all units to SI units, we have:
P₁ = 15.47 Pa
P₂ = 101325 Pa
ΔHvap = 5480 J/mol
R = 8.314 J/(mol*K)
Plugging in the values, we get:
㏑(101325/15.47) = -(5480/8.314) * (1/T₂ - 1/103.15)
Solving for T₂, the boiling point is found to be:
T₂ = 57.4°C
As a result, the unknown liquid's usual boiling point is 57.4°C.
To know more about the Pressure, here
https://brainly.com/question/14748171
#SPJ1
When Sam plays online video games with other people he makes sure to avoid offensive language play by the rules his gaming group established and help other players have a good time. Sam is being a good digital citizen by following a code of
Answers
"Sam is being a good digital citizen by following a code of conduct.
A code of conduct is a set of rules that govern how people should behave online. It is important to follow a code of conduct because it helps to create a safe and respectful environment for everyone.
Here, Sam is following the code of conduct for his gaming group. This code of conduct may include rules about using offensive language, cheating, and griefing. By following these rules, Sam is helping to create a positive experience for everyone in his gaming group.
Therefore, Sam is following the code of conduct.
Learn more on code of conduct :https://brainly.com/question/30093328
#SPJ1
How many chlorine atoms are found in 8.3 moles of chlorine?
Answers
Answer:
5*10²⁴ chlorine atoms are found in 8.3 moles of chlorine.
Explanation:
Avogadro's Number or Avogadro's Constant is called the number of particles that make up a substance (usually atoms or molecules) and that can be found in the amount of one mole of said substance. Its value is 6.023*10²³ particles per mole. Avogadro's number represents a quantity without an associated physical dimension, so it is considered a pure number that allows describing a physical characteristic without an explicit dimension or unit of expression. Avogadro's number applies to any substance.
Then you can apply the following rule of three: if 1 mole of the compound contains 6.023 * 10²³ atoms, 8.3 moles of the compound how many atoms does it have?
\(amount of atoms=\frac{8.3 moles*6.023*10^{23}atoms }{1 mole}\)
amount of atoms≅ 5*10²⁴ atoms
5*10²⁴ chlorine atoms are found in 8.3 moles of chlorine.
What has to be true in order for a single replacement reaction to happen?

Answers
Answer:
I think it's the second one.
a sample of gas has a mass of 0.560 g . Its volume is 125 mL at a temperature of 85 ∘C and a pressure of 757 mmHg . Find the molar mass?
Answers
The gas's molar mass is 126 g/mol.
What is pressure?A gas, liquid, or solid's force per unit area exerted on surfaces it is in touch with is known as pressure. It is the amount of force delivered per unit area, to put it another way. The impact of the gas molecules with the container walls causes the pressure of a gas.
How do you determine it?The ideal gas law, which links the pressure (P), volume (V), temperature (T), and number of moles (n) of a gas, may be used to get the molar mass of the gas:
PV = nRT
where R is the gas universal constant.
The temperature will first be converted to kelvin (K) as follows:
T = 85 °C + 273.15 = 358.15 K
Let's now convert the pressure into atm:
757 mmHg = 757/760 atm + 0.996 atm
The ideal gas law may now be rearranged to account for the number of moles:
n = PV/RT
n= (0.996 atm)(0.125 L)/(0.0821 Latm/molK)(358.15 K)
n =0.00445 mol
Using the mass (m) and number of moles (n), we can finally determine the molar mass (M) as follows:
M = m/n
M = 0.560 g/0.00445 mol
M = 126 g/mol
As a result, the gas's molar mass is around 126 g/mol.
To know more about pressure, visit:
brainly.com/question/18107358
#SPJ1
Electrophilic nitration of benzoic acid gives almost exclusively 1,3-nitrobenzoic acid. By drawing the appropriate resonance forms of the intermediate cations resulting from attack of [NO2]+, explain this result.
Answers
The electrophilic nitration of benzoic acid involves the attack of the nitronium ion ([NO2]+) on the benzene ring of the benzoic acid. The intermediate formed is a positively charged arenium ion, which can resonate between different resonance forms.
One of the resonance forms of the intermediate cation is shown below:
O O
// //
/C+ <-----> /C
\ \
\ O-
In this resonance form, the positive charge is delocalized over the carbon atom and the adjacent oxygen atom. The resulting resonance hybrid is stabilized by the delocalization of the positive charge over the ring, which lowers the overall energy of the system.
The nitronium ion can attack at the meta position, which leads to the formation of 1,3-nitrobenzoic acid. The attack at the ortho or para positions would lead to the formation of other isomeric products.
The reason for the selective formation of 1,3-nitrobenzoic acid is due to the resonance stabilization of the intermediate cation. The meta position is less hindered than the ortho and para positions, and the resonance form shown above places the positive charge at the meta position. Therefore, the attack of the nitronium ion at the meta position is favored over the other positions. Additionally, the resonance form shown above places the negative charge of the carboxylate group at the para position, which makes it less favorable for the nitronium ion to attack at this position.
Overall, the resonance stabilization of the intermediate cation favors the selective formation of 1,3-nitrobenzoic acid in the electrophilic nitration of benzoic acid.
For more questions on electrophillic nitration-
brainly.com/question/14803468
#SPJ4
8. The substance made as a result of a chemical reaction is called the
A. reactant
D. product
Which ONE????
Answers
Answer:
product
Explanation:
its the result of the reaction.
How much water has to be evaporated from 250 mL of 1 M Ca(OH)2 to make it 3 M?
Answers
Approximately 166.67 mL of water needs to be evaporated from 250 mL of 1 M Ca(OH)2 to make it 3 M.
To find the amount of water that needs to be evaporatedThe relationship between the initial and final concentrations and volumes must be taken into account.
Given: Initial concentration \((C^1) = 1 M Initial volume (V^1) = 250 mL\)
\((C^2) = 3 M final concentration\)
We can use the equation:
\(C^1 * V^1 = C^2 * V^2\)
Where:
\(V^2\)is the final volume of the solution
Rearranging the equation to solve for V2:
\(V^2 = (C^1 * V^1) / C^2\)
Substituting the given values:
\(V^2 = (1 M * 250 mL) / 3 M\)
\(V^2 = 250 mL / 3\)
\(V^2\) ≈ \(83.33 mL\)
To find the amount of water that needs to be evaporated, we subtract the final volume from the initial volume:
Amount of water to be evaporated = \(V^1 - V^2\)
Amount of water to be evaporated = 250 mL - 83.33 mL
Amount of water to be evaporated ≈ 166.67 mL
Therefore, approximately 166.67 mL of water needs to be evaporated from 250 mL of 1 M Ca(OH)2 to make it 3 M.
Learn more about Initial concentration here: brainly.com/question/30720317
#SPJ1
The bright-line spectra of four elements, G,J, L, and M, and a mixture of at
least two of these elements are given below.
Which elements are present in the mixture?
M
Mixture
750
750
G and J
G and L
M, J, and G
M, J, and L
700
700
650
650
Bright-Line Spectra
600
600
550 500
550
Wavelength (nm)
500
450
450
400
400
.
Answers
Based on the given bright-line spectra and the observed wavelengths in the mixture's spectrum, the elements G and J are the ones present in the mixture.
From the given bright-line spectra and the spectrum of the mixture, we can determine the elements present in the mixture by comparing the specific wavelengths observed. Examining the bright-line spectra, we can identify that G has a distinct wavelength at 650 nm, J at 600 nm, L at 550 nm, and M at 500 nm.
Looking at the spectrum of the mixture, we can observe two prominent wavelengths, 650 nm and 600 nm. These correspond to the wavelengths of G and J, respectively. Since the spectrum of the mixture does not exhibit the wavelengths specific to L (550 nm) or M (500 nm), we can conclude that only G and J are present in the mixture.
Therefore, based on the given bright-line spectra and the observed wavelengths in the mixture's spectrum, the elements G and J are the ones present in the mixture.
This analysis relies on the principle that each element has characteristic wavelengths at which they emit light. By comparing the observed wavelengths in the mixture's spectrum with those of the individual elements, we can determine the elements present in the mixture.
Know more about wavelengths here:
https://brainly.com/question/10750459
#SPJ8
The activation energy, Ea, for a particular reaction is 13.6 kJ/mol. If the rate constant at 475 K is 0.0450 1/min, then what is the value of the rate constant at 769 K? (R = 8.314 J/mol • K)
Answers
At 769 K, the rate constant equals 2.22 1/min.
When the reaction's EA is zero, what is the reaction's rate constant equal to?The final expression is either k=A or k=A. This implies that the response rate will be equal to the value of the collision frequency rather than the temperature when the activation energy is zero.
The Arrhenius equation, which connects the rate constant (k) to the activation energy (Ea) and temperature (T), can be used to solve this issue:
\(A = * exp (-Ea / (R * T))\)
With the rate constant (k) at 475 K, we can utilize this knowledge to calculate the pre-exponential factor (A) as follows:
0.0450 1/min = A * exp(-13.6 kJ/mol / (8.314 J/mol•K * 475 K))
\(A = 5.74 x 10^9 min^-1\)
The rate constant (k) at 769 K can now be calculated using the Arrhenius equation once more as follows:
\(k = 5.74 x 10^9 min^-1\) * exp(-13.6 kJ/mol / (8.314 J/mol•K * 769 K))
k = 2.22 1/min (rounded to two significant figures)
To know more about rate constant visit:-
https://brainly.com/question/20305871
#SWPJ1
What are the charges of the ions in an ionic compound containing cobalt(III) and fluoride ions?
Write the formula for the compound.
Answers
The charge on the ions in an ionic compound containing cobalt(III) and fluoride ions is Co³⁺ and F⁻¹ and the formula of the compound is CoF₃.
Ionic compounds are a type of chemical compound where the oppositely-charged ions of a metal and a nonmetal are attracted to each other to form an ionic bond.
The compound formed from the bonded ions will have very different properties from the elements that make up the compound.
While atoms are neutral because they have an equal number of protons and electrons, ions have a net charge and result when an atom loses or gains electrons.
Learn more about Ionic compound, here:
https://brainly.com/question/3222171
#SPJ1
Please help and try to explain if you can!

Answers
Answer: 4.76 * 10^2
476.
you bring the ending decimal to the first number and it takes you two hops to get the first number so that's what you put in the box after the 10
If 4.0 L of a 4.6 M SrCl2 solution is diluted to 45 L , what is the molarity of the diluted solution
Answers
If 4.0 L of a 4.6M SrCl2 solution is diluted to 45L, the molarity of the diluted solution is 0.41M.
How to calculate molarity?The molarity of a diluted solution can be calculated using the following formula:
M1V1 = M2V2
Where;
M1 = initial concentrationM2 = final concentrationV1 = initial volumeV2 = final volumeAccording to this question, 4.0 L of a 4.6M SrCl2 solution is diluted to 45L, the molarity of the diluted solution is calculated as follows:
4.6 × 4 = 45 × M2
18.4 = 45M2
M2 = 18.4/45
M2 = 0.41M
Therefore, if 4.0 L of a 4.6M SrCl2 solution is diluted to 45L, the molarity of the diluted solution is 0.41M.
Learn more about molarity at: https://brainly.com/question/2817451
2. For each of the ionic compounds in the table below, name the compound and explain the rule that you
used in formulating your name for the compound.
Name:
Rule for naming compound:
-PbF4
-NH4NO3
-Li2S
Answers
Answer:
2
Explanation:
Lead(|V) fluoride
Ammonium Nitrate
Lithium sulfide
For the rules, I don't know what you were taught. I just do it intuitively since I have done so much chemistry.
The first one the roman numerals represents the charge of the lead which much match the 4- charge from the 4 fluorides.
The second one is just two polyatomic ions which you just have to remember.
The last one is the typical ionic compound naming technique i guess.