Answers
The carbocation intermediate in step 1 of the SN1 mechanism is an sp2 hybridized carbon is the 2-ol -3 Methyl hexene formed ftom the 2cholro 3 Methyl hexene in the presence of methanol.
Its molecular geometry is trigonal planar, consequently taking into consideration unique factors of nucleophilic attack, left and rightbranch of chemistry that offers with the spatial association of atoms and organizations in molecules. : the spatial association of atoms and organizations in a compound and its relation to the residences of the compound.
Stereochemistry is the department of chemistry that involves “the have a look at of the unique spatial preparations of atoms in molecules”. Stereochemistry is the systematic presentation of a particular subject of technological know-how and generation that historically calls for a quick initial tour into history.
Read more about stereochemistry;
https://brainly.com/question/213277
#SPJ4
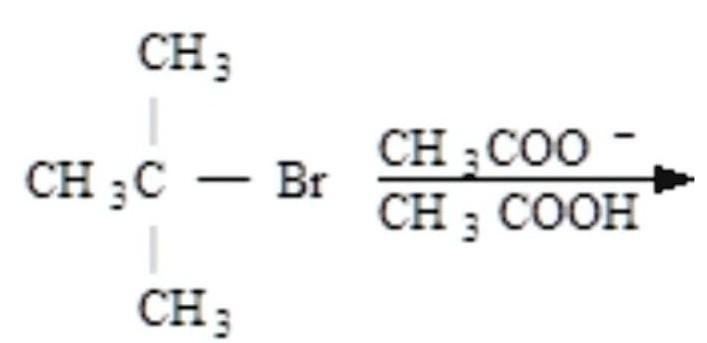
Related Questions
HELPPPPPPPP i need help!!!!!!!!!!!!!

Answers
Answer:
C!
Explanation:
when water is boiling the water particles will give each other more space and will then be turned into a gas :)
Write a balanced nuclear equation for the beta decay of 234/90Th

Answers
The balanced nuclear equation for the beta decay of 234/90Th can be represented as follows: ^234/90Th --> ^234/91Pa + e^0/-1β
In this equation, the nucleus of thorium-234 (234/90Th) undergoes beta decay. During beta decay, a neutron within the nucleus is converted into a proton, resulting in the emission of an electron (beta particle). As a result, the thorium-234 nucleus is transformed into protactinium-234 (234/91Pa) by gaining one proton.
The beta particle emitted during the decay process is represented as e^0/-1β, where the superscript 0 denotes that the electron has no charge (neutral), and the subscript -1 indicates that the electron carries a negative charge of -1.
It is important to note that in a nuclear equation, the total atomic mass and atomic number on both sides of the equation must be equal to maintain a balanced equation and conserve mass and charge.
For more such questions on balanced nuclear equation visit:
https://brainly.com/question/31505524
#SPJ8
-Convert 6.02 x 1020 formula units of MgCl₂ to mol of MgCl₂:
Answers
6.02 x \(10^{20\) formula units of MgCl₂ is equal to 0.1 moles of MgCl₂.
To convert formula units of MgCl₂ to moles of MgCl₂, we need to use Avogadro's number, which relates the number of formula units to the number of moles.
Avogadro's number (NA) is approximately 6.022 x 10^23 formula units per mole.
Given that we have 6.02 x 10^20 formula units of MgCl₂, we can set up a conversion factor to convert to moles:
(6.02 x 10^20 formula units MgCl₂) * (1 mol MgCl₂ / (6.022 x 10^23 formula units MgCl₂))
The formula units of MgCl₂ cancel out, and we are left with moles of MgCl₂:
(6.02 x 10^20) * (1 mol / 6.022 x 10^23) = 0.1 mol
Therefore, 6.02 x 10^20 formula units of MgCl₂ is equal to 0.1 moles of MgCl₂.
It's important to note that this conversion assumes that each formula unit of MgCl₂ represents one mole of MgCl₂. This is based on the stoichiometry of the compound, where there is one mole of MgCl₂ for every one formula unit.
Additionally, this conversion is valid for any substance, not just MgCl₂, as long as you know the value of Avogadro's number and the number of formula units or particles you have.
For more such questions on MgCl₂ visit:
https://brainly.com/question/26311875
#SPJ8
1. When an object wants to stay at rest or wants to stay in motion unless
an outside force comes and disturbs it.*
Answers
for liquid chromatography, gas chromatography and capillary electrophoresis, explain which terms of the van deemter equation contribute to the separation, which do not, and why.
Answers
Van Deemter's equation is represented by the notation A + B/u + Cu, where A represents eddy diffusion, B represents longitudinal molecular diffusion, and C represents mass transfer, contribute to separation.
One of the most crucial components of the van Deemter equation is the stationary phase particle size. The plate number (Nth) and the column packing's particle size have an inverse relationship for a given column length. The plate number and separation power increase with particle size. In order for the distinct components to be gathered in their purest form during chromatography, the components in solution must be sufficiently separated. The gap between the bands for each component increases, making it simpler to accomplish this.
To learn more about chromatography click here https://brainly.com/question/26491567
#SPJ4
Most of the energy used in the United States comes from
Answers
Answer:
Energy in the United States comes mostly from fossil fuels: in 2010, data showed that 25% of the nation's energy originates from petroleum, 22% from coal, and 22% from natural gas.
Explanation:
It is really self explanitory
What is the boiling point in °C of a 0.32 molal aqueous solution of NaCl?
BP (water) = 100.00 °C Kb (Water) = 0.512 °C/m
Answers
Answer:
the boiling point of solution at 3 decimal point is 100.329०C Ans.
Explanation:
given data -
molality of Nacl = 0.321 m
molal boiling point elevation constant (Kb) =0.512०C/m
# formula of change of boiling point of sample =
∆ Tb =i × Kb × m
Kb = molal boiling point of elevation constant
m = molality
i = vont's hoff factor.
Nacl is strong electrolyte and its 100% dissociate so the value of i for Nacl is 2
put value in the formula
∆ Tb = 2 × 0.512 ०C/m × 0.321m
= 0.3287
= 0.329०C
∆Tb = T'b - Tb
T'b = boiling point of solution
Tb= boiling point of solvent( water)
0.329०C = T'b - 100०c ( boiling point of water = 100०C)
T'b = 0.329०C + 100०C
= 100.329०C
hope this helps
Wich stament describes to organ systems working together to get rid of waste played by cells

Answers
Answer:
C. Kidneys filter wastes from the bloodstream and produce urine
Explanation:
The distance between two adjacent peaks on a wave is called the wavelength. (2pts) a. The wavelength of ultraviolet light is 255nm. What is the wavelength in meters? b. The wavelength of a beam of red light is 683nm. What is its wavelength in angstroms?
Answers
Answer:
a.2.55e-7
b.6830
Explanation:
PLEASE HELP: For the chemical reaction
2 NaOH + H2SO4 -> Na2SO4 + 2H2O
How many miles of sodium sulfate will be produced from 28.1 g of sodium hydroxide?
Miles of sodium sulfate: (blank) mol
Answers
Answer:
Calculate the number of moles of NaOH:
molar mass of NaOH = 23.0 g/mol (Na) + 16.0 g/mol (O) + 1.0 g/mol (H) = 40.0 g/mol
moles of NaOH = mass / molar mass = 28.1 g / 40.0 g/mol = 0.7025 mol
Use stoichiometry to determine the number of moles of Na2SO4 produced:
From the balanced chemical equation, we see that 2 moles of NaOH react to form 1 mole of Na2SO4, so:
moles of Na2SO4 = 0.7025 mol NaOH × (1 mol Na2SO4 / 2 mol NaOH) = 0.35125 mol Na2SO4
Therefore, 28.1 g of sodium hydroxide will produce 0.35125 mol of sodium sulfate.
Explanation:
Chemistry Lab Determination of the Universal Gas Constant (R)
SHOW ALL WORK
Given:
Initial mass of butane lighter: 54.24g
Final Mass of Butane Lighter: 54.01g
Temperature of water: 23.0°C
Volume of gas collected: 100.0mL
FIND:
Barometric pressure of room: 766.86 mmHg CONVERTED TO atm
Vapor pressure of water at room temperature(PH2O) (IN atm)
FIND:
Mass difference if butane lighter in grams
Moles of Butane gas collected in moles of C4H10
Partial pressure if butane gas in atm
Converted temperature of water in Kelvin
Converted volume of gas collected in Liters
Experimental value of R in Latm/molk
Accepted value of R in Latm/molk
Percent error in experimental value of R in %
CONCLUSION QUESTIONS:
1. List at least 3 factors that either did it could contribute to the percent error
2. Should the value of R go up or down if the gas had not been corrected for the partial pressure of water. Why?
3. How could this experiment be repeated to increase the accuracy, or in other words, decrease the percent error?
NOTE: LET ME KNOW IF YOU WANT A PICTURE OF THE LAB INSTRUCTIONS TO HELP SOLVE
ALSO SHOW ALL WORK PLS
Answers
To solve this problem, I'll need some additional information related to the molar mass of butane (C4H10). Please provide the molar mass of butane so that I can proceed with the calculations.
Differences between voltage, current and resistance?
Answers
Answer:
Voltage is the measure of electric potential energy per unit charge, current is the flow of electric charge through a circuit, and resistance is the property of a material that opposes the flow of electric current.
Ohm's Law relates these three concepts by stating that current is directly proportional to voltage and inversely proportional to resistance.
Hope this helps!
How is a magnetic field produced?
O when an electromagnetic field interacts with a magnet
O when a current runs through a conductor
O when an object has an electric charge
O when electrons move through a circuit
Answers
Answer:
A magnetic field is a vector field that describes the magnetic influence on moving electric charges, electric currents, and magnetic materials. ... Magnetic fields are produced by moving electric charges and the intrinsic magnetic moments of elementary particles associated with a fundamental quantum property, their spin.
Answer: when an electromagnetic field interacts with a magnet
Explanation:
Hydrogen iodide is not produced by the same method as for hydrogen chloride.why??
Answers
Answer:
Using Phosphoric acid will work perfectly for producing Hydrogen halides because its not an Oxidizing agent. ...
Using an ionic chloride and Phosphoric acid
H3PO4 + NaCl ==> HCl + NaH2PO4
H3PO4 + NaI ==> HI + NaH2PO4
H2SO4 + NaCl ==> HCl + NaHSO4
This method(Using H2So4) will work for all hydrogen hydrogen halide except Hydrogen Iodide and Hydrogen Bromide.
The Sulphuric acid won't be useful for producing Hydrogen Iodide because its an OXIDIZING AGENT. Whist producing the Hydrogen Iodide... Some of the Iodide ions are oxidized to Iodine.
2I-² === I2 + 2e-
Explanation:
Please I need help thank you

Answers
Answer:
its sodium hydroxide
Explanation:
Please help me with thi
Write a molecular equation, name of atom and number used in the chemical reaction for Iron(III) nitrate and
sodium thiosulfate reaction.
Answers
Answer:
Fe3+(aq) + 2S2O32–(aq)→ [Fe(S2O3)2(H2O)2]–(aq)
One mole of Iron(III) nitrate and two mole of sodium thiosulfate are used for a balanced equation
Explanation:
Molecular formula of sodium thiosulfate is S2O32-
Molecular formula of Iron(III) nitrate - Fe2NO3
The chemical equation
Fe3+(aq) + 2S2O32–(aq)→ [Fe(S2O3)2(H2O)2]–(aq)
One mole of Iron(III) nitrate and two mole of sodium thiosulfate are used for a balanced equation
name the chemical compound

Answers
Answer:
hydrochloric acid
Explanation:
chemical compound any substance composed of identical molecules consisting of atoms
Which sequence represents the relationship between temperature and volume as explained by the kinetic-molecular
theory?
higher temperature → less kinetic energy
higher temperature → more kinetic energy→more space between particles → higher volume
less space between particles → higher volume
higher temperature → more kinetic energy less space between particles → lower volume
higher temperature → less kinetic energy→more space between particles lower volume
Answers
The correct sequence is higher temperature→ more kinetic energy→ more space between particles→ higher volume.
According to the postulates of Kinetic gas theory:
Postulate 1: It states that the average kinetic energy of gas particles is proportional to the absolute temperature of the gas.
Which states that the higher temperature, the higher the kinetic energy of the molecules, which is the start of the sequence of the relationship between temperature and volume.
Postulate 2: Average kinetic energy is proportional to the square of the speed, it shows that at higher the kinetic energy the faster the molecules will move.
Postulate 3: As the particles move faster, the particles will collide more frequently so they will move away from each other, occupying more space.
Postulate 4: More space between the molecules results in more volume.
So, the complete and correct sequence is higher temperature → higher kinetic energy (higher speed) → more space → more volume.
Learn more about the Kinetic Gas Theory here:
https://brainly.com/question/7563496
#SPJ10
Which particles in atoms have a negative electric charge?
Neutrons
Nuclei
Protons
Electrons
Answers
Answer:
Electrons
Explanation:
Atoms both have negative and positive particles
Protons are positive
Electrons are negative
Neutrons are neutral
Nuclei is where you find both neutrons and protons in an atom
Answer:
c electrons
Explanation:
can plants stop soil erosion
Answers
Answer:
yes. they can block the wind which causes top soil erosion and they also soak up some the water in the soil
Please help , I’m stuck

Answers
Answer:
a) I believe it has a volume of 25 ml. I found the value by subtracting 40 from 65.
Hope this Helps!
-kiniwih426
The numbers in front of the chemical formulas are called? 2 H2 + O2 --> 2 H20
Answers
Answer:
They are called coefficients.
Identify the possible issues if a sample in a spectrophotometer gives no reading. Select one or more: The wrong wavelength may be set. There may be an issue with the spectrophotometer. The sample may be placed impro
Answers
The question is incomplete; the complete question is;
Identify the possible issues if a sample in a spectrophotometer gives no reading. Select one or more:
The wrong wavelength may be set.
The sample may be placed improperly in the cuvette holder.
There may be an issue with the composition of the sample.
There may be an issue with the spectrophotometer
Answer:
The wrong wavelength may be set.
There may be an issue with the spectrophotometer
Explanation:
Substances do not absorb radiation at all wavelengths. The proper wavelength at which a substance absorbs must be used for a reading to be obtained from the spectrophotometer. If this is not done, no reading is obtained from the spectrophotometer.
Generally, if the spectrophotometer has an issue, it may display no reading until the machine is fixed.
The possible issues if a sample in a spectrophotometer gives no reading are:
The sample may be placed improperly in the cuvette holder. The wrong wavelength may be set.According to the given question, we are asked to identify the possible reasons why a spectrophotometer would give no reading when a sample is used on it.
As a result of this, it is important to note that it is possible that the sample may not have been placed correctly on the cuvette holder or the wrong wavelength may be set which would cause the no reading error to show.
Read more here:
https://brainly.com/question/13998813
A farmer used too much pesticide (bug spray) and it washed down into the pond environment. What could happen to the Biotic AND Abiotic factors in the pond?
Answers
In a pond, the biotic factors include:
fishescrabssnailsplanktonalgaeThe abiotic factors are:
sunlighttemperaturerainfalldissolved mineralsWhat are the biotic and abiotic factors in a pond?Biotic factors are the living components of an environment whose activities affect the environment.
Biotic factors include plants, animals, bacteria, fungi, etc,
Abiotic factors are the non-living components of an environment that affect the environment.
Biotic and abiotic factors interact with each other.
In a pond, the biotic factors include:
fishescrabssnailsplanktonalgaeThe abiotic factors include:
sunlighttemperaturerainfalldissolved mineralsLearn more about biotic and abiotic factors at: https://brainly.com/question/1322838
#SPJ1
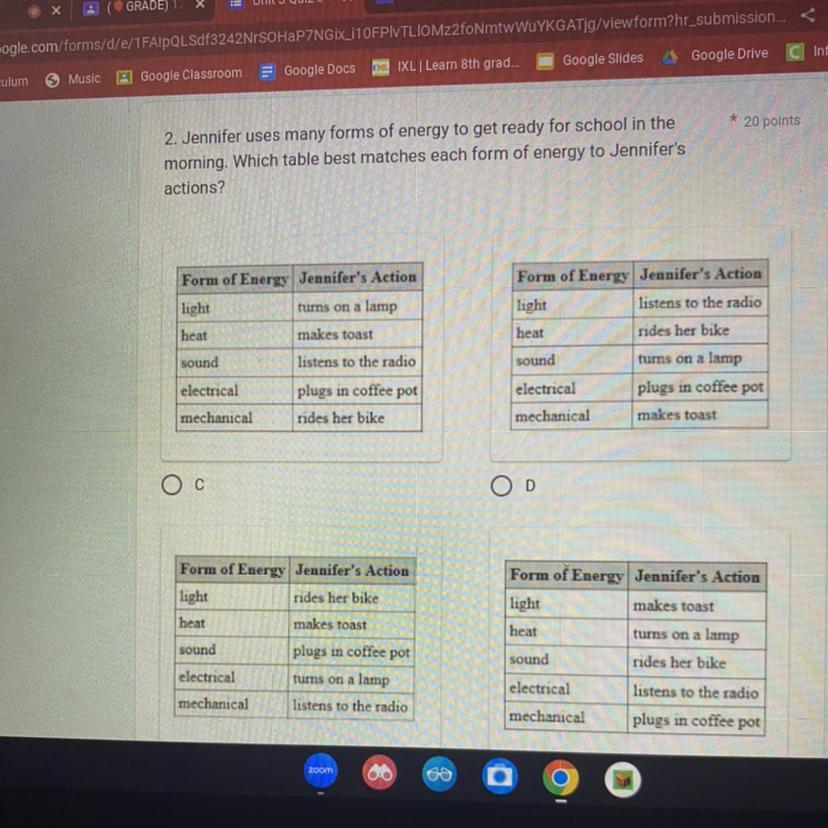
2. Jennifer uses many forms of energy to get ready for school in the
morning. Which table best matches each form of energy to Jennifer's
actions?
Form of Energy Jennifer's Action
light
turns on a lamp
makes toast
listens to the radio
plugs in coffee pot
rides her bike
JOSTAGENS
sound
O C
E
electrical
mechanical
Form of Energy Jennifer's Action
Light
rides her bike
makes toast
plugs in coffee pot
turns on a lamp
listens to the radio
sound
electrical
mechanical
S
Form of Energy Jennifer's Action
Light
listens to the radio
heat
rides her bike
sound
turns on a lamp
electrical
plugs in coffee pot
mechanical
makes toast
O D
* 20 points
Form of Energy Jennifer's Action
light
heat
sound
electrical
mechanical
makes toast
turns on a lamp
rides her bike
listens to the radio
plugs in coffee pot
Answers
The table best matches each form of energy to Jennifer's actions is A.
What is energy?Energy is defined as the capacity to labor long hours or be extremely busy without becoming exhausted. Red blood cells, which carry oxygen in the blood throughout the body, are created by vitamin B12 in the body. Your body's cells use the oxygen once it gets there to produce energy.
Electrical energy, chemical energy obtained from fuels, food, and energy derived from the sun are the major types of energy used in our homes. Everyday appliances convert electrical energy into a variety of forms, including mechanical/kinetic, sound, heat, light, and other types of electromagnetic radiation.
Thus, the table best matches each form of energy to Jennifer's actions is A.
To learn more about energy, refer to the link below:
https://brainly.com/question/1932868
#SPJ1
what major organic product would you expect to obtain when acetic anhydride reacts with ch3ch2ch2oh nh3
Answers
The major product of the reaction between acetic anhydride and CH3CH2CH2OH is CH3COOCH2CH2CH3. While, the major product of the reaction between acetic anhydride and NH3 is CH3CONH2.
What is acetic anhydride?Acetic anhydride is the compound (CH3COO)2. It is a precursor for the synthesis of many organic compounds.
Now let us consider the use of acetic anhydride in synthesis. The major product of the reaction between acetic anhydride and CH3CH2CH2OH is CH3COOCH2CH2CH3. While, the major product of the reaction between acetic anhydride and NH3 is CH3CONH2.
Learn more about acetic anhydride: https://brainly.com/question/14356798
The eye can see colors because:
a. the cones in the retina send signals to the brain.
b. the rods in the cornea send signals to the brain.
c. the optic nerve determines the color of the object and sends signals to the brain
d. None of the choices are correct.
Answers
Answer:
The answer is A, I think.
Answer:
the answer is A
Explanation:
The human eye and brain together translate light into color. Light receptors within the eye transmit messages to the brain, which produces the familiar sensations of color. Newton observed that color is not inherent in objects. Rather, the surface of an object reflects some colors and absorbs all the others.
when the temperature of an ideal gas is increased from 27C to 927C then kinetic energy increases by
Answers
Answer:
The rms speed of its molecules becomes. (T) has become four times. Therefore, v_(rms) will become two times,...
A heterogeneous ore mixture contains 35.0 % In2O3 by mass. How many tons of the ore must be mined to provide 325.0 kg of indium metal? (2000 lb = 1 ton, assume exact)
Answers
Answer:
1.2375 ton of ore
Explanation:
Mass of indium = 325
Formula = In2O3
Molar mass = 277.64 g/mol
Molar mass of indium = (2*114.8)g
229.6 indium is in 277.64 of In2O3
Mass of In2O3 required = 277.64/229.6 x 325
= 392.94kg
35 % In2O3 in ore
For 35 kg = 100 kg required
For 392.94,
100/35 x 392.94
= 1122.686 kg
1122.68 x 2.2
= 2469.9 pounds
2469.9/200
= 1.235 tons of ore
Based on a Kc value of 0.250 and the given data table, what are the equilibrium concentrations of XY, X, and Y , respectively?
Answers
From the solution that we have in the question;
The concentration of X and Y is 0.28 MThe concentration of XY is 0.32 MWhat is the equilibrium constant?The equilibrium constant, denoted as K, is a value that quantitatively represents the ratio of the concentrations of products to reactants at equilibrium in a chemical reaction.
It is a fundamental concept in chemical equilibrium.
The value of the equilibrium constant provides valuable information about the position of equilibrium and the relative concentrations of species involved in a chemical reaction.
Kc = [X] [Y]/[XY]
\(0.25 = (0.1 + x)^2/(0.5 - x)\)
\(0.25(0.5 - x) = (0.1 + x)^2\)
\(0.125 - 0.25x =0.01 + 0.2x + x^2\\ x^2 + 0.45x - 0.115 = 0\)
x = 0.18 M
The equilibrium amount of X and Y= 0.28 M and the equilibrium concentration of XY = 0.32 M
Learn more about equilibrium constant:
https://brainly.com/question/29253884
#SPJ1
Based on the answer to the question that we have;
A 0.28 M concentration of X and Y exists at equilibriumXY's concentration at equilibrium is 0.32 M.The equilibrium constantThe ratio of the product to reactant concentrations in a chemical reaction at equilibrium is represented quantitatively by the equilibrium constant, abbreviated as K.
It is a cornerstone of the theory of chemical equilibrium.
A chemical reaction's equilibrium position and the relative concentrations of the species involved can both be learned from the equilibrium constant's value.
Kc = [X][Y]/[XY]
\(0.25 = (0.1 + x)^2/(0.5 - x)\\0.25(0.5 - x) = (0.1 +x)^2\\0.125 - 0.25x = 0.01 +0.2x +x^2\\= 0.18 M\)
The equilibrium concentration of;
XY =0.5 - 0.18
=0.32 M
Then the equilibrium amount of
X and Y is
0.1 + 0.18= 0.28 M.
Learn more about equilibrium:brainly.com/question/29253884
#SPJ1
