Draw the exo and endo product for the reaction of cyclopentadiene and maleic anhydride. Which one will be favored?
Answers
The endo product is the favored product in the reaction between cyclopentadiene and maleic anhydride.When cyclopentadiene reacts with maleic anhydride, it undergoes a Diels-Alder reaction to form two different products, the exo and endo products.
The exo product is formed when the two substituents on the diene and dienophile are on the opposite sides of the newly formed ring. On the other hand, the endo product is formed when the two substituents are on the same side of the ring.
The endo product is typically favored in this reaction because it is more stable than the exo product. This is because the endo product has a more favorable overlap between the orbitals involved in the formation of the new sigma bond.
In conclusion, the Diels-Alder reaction between cyclopentadiene and maleic anhydride forms both exo and endo products, but the endo product is typically favored due to its greater stability.
Sub-heading: Drawing Exo and Endo Products
Step 1: Identify the reactants
- Cyclopentadiene: C5H6, a 5-membered ring with two adjacent double bonds.
- Maleic anhydride: C4H2O3, a cyclic molecule with an anhydride functional group.
Step 2: Determine the Diels-Alder reaction
- The reaction is a Diels-Alder reaction, which involves a conjugated diene (cyclopentadiene) reacting with a dienophile (maleic anhydride) to form a cyclic compound.
Step 3: Draw the exo product
- In the exo product, the two carbonyl oxygen atoms of maleic anhydride point away from the cyclopentadiene ring.
- To draw the exo product, connect one double bond of cyclopentadiene to one double bond of maleic anhydride, and the other double bond to the remaining double bond in maleic anhydride. Ensure the carbonyl oxygen atoms are pointing away from the cyclopentadiene ring.
Step 4: Draw the endo product
- In the endo product, the two carbonyl oxygen atoms of maleic anhydride point towards the cyclopentadiene ring.
- To draw the endo product, follow the same steps as for the exo product but make sure the carbonyl oxygen atoms are pointing towards the cyclopentadiene ring.
Favored Product
Step 5: Determine the favored product
- The endo product is favored in this reaction due to secondary orbital interactions that stabilize the transition state.
In conclusion, the endo product is the favored product in the reaction between cyclopentadiene and maleic anhydride.
To know more about Exo and Endo product refer to
https://brainly.com/question/29738690
#SPJ11
Related Questions
Which two events will happen if more H2 and N2 are added to this reaction after it reaches equilibrium?
3H2 + N2 to 2NH3
Answers
If more \(H_{2}\) and \(N_{2}\) are added to the reaction 3\(H_{2}\) + N2 → 2\(NH_{3}\) after it reaches equilibrium, two events will occur Shift in Equilibrium and Increased Yield of \(NH_{3}\)
1. Shift in Equilibrium: According to Le Chatelier's principle, when additional reactants are added, the equilibrium will shift in the forward direction to consume the added reactants and establish a new equilibrium. In this case, more \(NH_{3}\) will be produced to counteract the increase in \(H_{2}\) and \(N_{2}\).
2. Increased Yield of \(NH_{3}\): The shift in equilibrium towards the forward reaction will result in an increased yield of \(NH_{3}\). As more \(H_{2}\) and \(N_{2}\) are added, the reaction will favor the production of \(NH_{3}\) to maintain equilibrium. This will lead to an increase in the concentration of \(NH_{3}\) compared to the initial equilibrium state.
It is important to note that the equilibrium position will ultimately depend on factors such as the concentrations of \(H_{2}\), \(N_{2}\), and \(NH_{3}\), as well as the temperature and pressure of the system. By adding more reactants, the equilibrium will adjust to achieve a new balance, favoring the formation of more \(NH_{3}\).
Know more about Le Chatelier's principle here:
https://brainly.com/question/2943338
#SPJ8
An atom with the electron configuration 2-8-8-2 has an incomplete
Answers
Answer:
3rd principal energy level
Explanation:
Principle energy that explains the energy of the electrons and positions of electrons of a particular atom. There are four energy levels fist to fourth that are made of sublevels and that is made up of s, p, d and f orbitals.
First principal energy level - 2
Second principal energy level - 8
Third principal energy level - 18
Fourth principal energy level - 32
3rd energy level has 9 orbitals, which has 18 electrons
2 in the s orbital,
6 in the three p orbitals, and
10 in the five d orbitals.
So, here there are only 8 electrons in the third principal energy level.
Terbium-147 undergoes positron emission to become a stable atom. what is that stable atom? a. b. c. d. e. f.
Answers
Terbium-147 undergoes positron emission to become a stable atom, the stable atom is ₆₄Gd¹⁴⁷.
What are radioactive decay series?Series (or family) of natural radioactive decay is the set of elements with unstable nuclei, which follow an orderly sequence of spontaneous decay, that is, they emit alpha and beta particles, until a stable lead nucleus is created.
In this case, natural radioactive decay occurs when the nucleus of the atom of some chemical element is unstable and then it "breaks", releasing electromagnetic radiation and disintegrating.
See more about radioactive decay at brainly.com/question/1770619
#SPJ4
If a car traveling at 75 mph west, changes its direction to east, does its acceleration change?
yes or no (1pt)
and explain why you chose the answer
Answers
Answer:
No because it changes velocity, instead of accelerating
Explanation:
and yes, I am in K12 ツ
Answer:
no
Explanation:
it changes velocity, instead of accelerating
calculate the heat of reaction delta h for the following reaction: ccl4(g) h2o(g) -> chcl3(g) hcl(g)
Answers
The heat of reaction (ΔH) for the given reaction is 180.4 kJ/mol. To calculate the heat of reaction (ΔH) for the given reaction:
CCl₄(g) + H₂O(g) -> CHCl₃(g) + HCl(g)
You would need the standard enthalpies of formation for each compound involved in the reaction. The standard enthalpy of formation (ΔHf) is the enthalpy change when one mole of a compound is formed from its elements in their standard states.
Here are the standard enthalpies of formation for the compounds involved:
ΔHf[CCl₄(g)] = -135.5 kJ/mol
ΔHf[H₂O(g)] = -241.8 kJ/mol
ΔHf[CHCl₃(g)] = -104.7 kJ/mol
ΔHf[HCl(g)] = -92.3 kJ/mol
To calculate ΔH for the reaction, you need to sum up the enthalpies of formation of the products and subtract the sum of the enthalpies of formation of the reactants:
ΔH = ΣΔHf(products) - ΣΔHf(reactants)
ΔH = [ΔHf[CHCl₃(g)] + ΔHf[HCl(g)]] - [ΔHf[CCl₄(g)] + ΔHf[H₂O(g)]]
ΔH = [(-104.7 kJ/mol) + (-92.3 kJ/mol)] - [(-135.5 kJ/mol) + (-241.8 kJ/mol)]
ΔH = -196.9 kJ/mol - (-377.3 kJ/mol)
ΔH = 180.4 kJ/mol
Therefore, the heat of reaction (ΔH) for the given reaction is 180.4 kJ/mol.
To know more about enthalpy:
https://brainly.com/question/29145818
#SPJ4
As a sound source approaches someone, the frequency of the sound wave
Answers
Answer:
The Doppler effect is a change in the frequency of sound waves that occurs when the source of the sound waves is moving relative to a stationary listener. As the source of sound waves approaches a listener, the sound waves get closer together, increasing their frequency and the pitch of the sound.
Explanation:
help me with this ?
i need to git this done for school
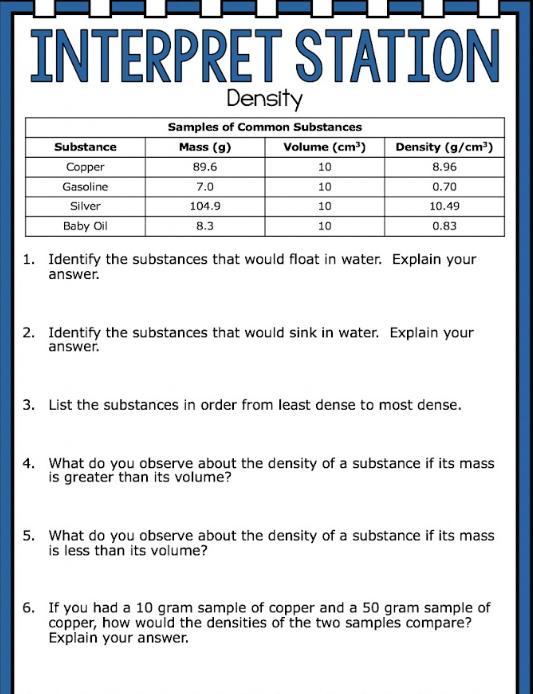
Answers
1) Gasoline and baby oil will float in water
2) Copper and silver will sink in water
3) Silver, Copper, Baby oil Gasoline
4) If the mass is less than volume the density will be less than one
5) If the mass is greater than the volume the density is not less than 1
6) The densities of the substances would be the same.
What is the density?Density is a physical property of matter that measures how much mass is contained in a given volume. It is calculated as the ratio of mass to volume, and is typically expressed in units of grams per cubic centimeter (g/cm³) or kilograms per cubic meter (kg/m³).
The formula for calculating density is:
Density = Mass / Volume
Where Mass is the amount of matter in an object or substance, and Volume is the amount of space that it occupies. Density is an intensive property, which means that it is independent of the amount of substance being measured. For example, the density of a material will be the same regardless of whether you measure it in grams or kilograms.
Learn more about density:https://brainly.com/question/29775886
#SPJ1
Which of the following best describes your choice? It’s my best explanation for now, but I really would have to test my idea to be sure. It's a well-tested explanation for a large number of observations. It's a well-tested description of what happens, but it doesn't explain why it happens.
Answers
I can deduce here that the best that describes my choice is: It’s my best explanation for now, but I really would have to test my idea to be sure.
What is choice?Choice actually refers to the act of choosing between two or more things. It has to do with going with the option, idea, decision, product or something that one prefers.
When we make choices, it depicts what we actually want. Some people still make wrong choices. The choices people make can either make or mar them in the present or future.
Looking at the given question, my choice is actually the first option.
It's clear that the idea hasn't been tested in order to be sure though it's has the best explanation.
Learn more about choice on https://brainly.com/question/6947486
#SPJ1
how does the double bond influence the dispersion forces that can form between the hydrocarbon chains of fatty acid
Answers
A double bond in a hydrocarbon chain of a fatty acid can influence the dispersion forces that can form between the hydrocarbon chains by decreasing the strength of the dispersion forces.
Dispersion forces, also known as van der Waals forces, are the weakest type of intermolecular forces that occur between molecules. These forces are caused by temporary dipoles that form when electrons in the molecules move around.
In a hydrocarbon chain with only single bonds, the chain is able to pack closely together, allowing for stronger dispersion forces between the chains. However, when there is a double bond present in the chain, it creates a kink or bend in the chain, preventing it from packing as closely with other chains. This decreases the strength of the dispersion forces between the chains.
Therefore, the presence of a double bond in the hydrocarbon chain of a fatty acid can decrease the strength of the dispersion forces between the chains, leading to weaker intermolecular forces and potentially different physical properties, such as a lower melting point.
know more about hydrocarbon chain here
https://brainly.com/question/30270057#
#SPJ11
the process of transmission of heat in air is??A:conduction B:convention C:radiation D:oxidation
Answers
Answer:
C:Radiation
Explanation:
Pluto
Answer:
i hope this help
Explanation:
Radiation is a method of heat transfer that does not rely upon any contact between the heat source and the heated object as is the case with conduction and convection. Heat can be transmitted through empty space by thermal radiation often called infrared radiation. This is a type electromagnetic radiation.
what is the melting point of astatine
Answers
Answer:
575.6 F
Explanation:
A educated guess tbh
Answer:
575.6 F
Explanation:
Astatine is a chemical element with the symbol At and atomic number 85. It is the rarest naturally occurring element in the Earth's Crust, occurring only as the decay product of various heavier element. All of astatine's isotopes are short-lived; the most stable is astatine-210, with a half-life of 8.1.
what are function of lipids
Answers
Hope this helped :)
what does SnCl⁴ equals ?
Answers
Answer:
Stannic chloride
Explanation:
How do you calculate relative atomic mass from mass number and percent abundance?
Answers
Change each percent abundance into decimal form by dividing by 100. Multiply this value by the atomic mass of that isotope. Add together for each isotope to get the average atomic mass.
The ratio of the average mass of atoms of a chemical element in a given sample to the atomic mass constant is known as relative atomic mass, also referred to by the outdated term atomic weight. Since the ratio's two components are masses, the final value has no dimensions, which is why it is referred to as relative. The weighted arithmetic mean of the masses of the individual atoms (including their isotopes) that are present in the sample is what determines the relative atomic mass of a certain element for a specific sample.
To know more about relative atomic mass, visit;
brainly.com/question/25698972
#SPJ4
Which property of matter is conserved in chemical reactions and shown by balanced equations?
Answers
The property of matter that is conserved in chemical reactions and shown by balanced equations is known as the Law of Conservation of Mass. According to this law, mass can neither be created nor destroyed in a chemical reaction; it can only be transformed from one form to another.For instance, when two substances are combined, they react and form a new substance.
The products that are formed contain the same number of atoms as the reactants, but in different configurations. To keep track of the number of atoms on either side of the equation, we use coefficients, which indicate the number of molecules or atoms of each substance in the reaction. However, when a chemical equation is written, it must adhere to the law of conservation of mass.The law of conservation of mass is critical in chemical reactions because it ensures that the amount of reactants that go into a reaction equals the amount of products that come out of it. This means that the total mass of reactants must equal the total mass of the products. As a result, the balanced chemical equation must reflect this law.For example, consider the reaction between hydrogen gas and oxygen gas, which forms water. The balanced chemical equation is as follows:2H2 + O2 → 2H2OIn this reaction, two molecules of hydrogen gas react with one molecule of oxygen gas to produce two molecules of water. The coefficients in the balanced chemical equation indicate that two molecules of hydrogen and one molecule of oxygen combine to form two molecules of water, obeying the law of conservation of mass.In conclusion, the Law of Conservation of Mass is a fundamental principle in chemistry that is used to balance chemical equations. It is critical in chemical reactions because it ensures that the total mass of reactants equals the total mass of products, allowing scientists to accurately predict the outcome of a chemical reaction.For such more question on chemical reaction
https://brainly.com/question/11231920
#SPJ8
The volume of the milk included in a school lunch is 0.500 pints (1/2 pint). How many milliliters is this?
Answers
Answer:
236.588
Explanation:
Multiply the # of pints value by 473.2 to find milliliters.
anyone may I get help please

Answers
Answer:
-pneumonoultramicroscopicsilicovolcanoconiosis.ogg
Match these items.
No links please!
Definitions:
1. Built the first American A.C. generator.
2. Found a relationship between electricity and magnetism.
3. Associated with gas and oil.
4. First workable electric light bulb.
5. Developed the equations E=mc^2
6. Provides majority of the energy in the United States.
7. Splitting heavy atoms
8. Produces plutonium
9. Powered by falling water or stream pressure
10. Thin silicon slices
11. Using heat from the earth
Term names:
a. Oersted
b. Geothermal
c. Edison
d. Einstein
e. Salt dome
f. Turbine
g. Fission
h. Westinghouse
i. Solar cells
j. Gas and oil
k. Breeder
Answers
\(\huge{\mathbb{\tt { QUESTION↓}}}\)
1. Built the first American A.C. generator.
2. Found a relationship between electricity and magnetism.
3. Associated with gas and oil.
4. First workable electric light bulb.
5. Developed the equations E=mc^2
6. Provides majority of the energy in the United States.
7. Splitting heavy atoms
8. Produces plutonium
9. Powered by falling water or stream pressure
10. Thin silicon slices
11. Using heat from the earth
\(\huge{\mathbb{\tt {ANSWER↓}}}\)
\(\color{black}{\tt {1.) \: h. \: Westing \: house}}\)
Built the first American A.C. generator\(\color{black}{\tt {2.) \: Oersted}}\)
Hans Christian Oersted discovered the relationship between electricity and magnetism in 1820.\(\color{black}{\tt {3.) \: j. Gas \: and \: oil}}\)
Associated gas refers to the natural gas found in association with oil within the reservoir. There are also reservoirs that contain only natural gas and no oil, this gas is termed non-associated gas.\(\color{black}{\tt {4.) \: c.Edison}}\)
By January 1879, at his laboratory in Menlo Park, New Jersey, Edison had built his first high resistance, incandescent electric light. It worked by passing electricity through a thin platinum filament in the glass vacuum bulb, which delayed the filament from melting. Still, the lamp only burned for a few short hours.\(\color{black}{\tt { 5.) \: d. Einstein}}\)
E = mc2, equation in German-born physicist Albert Einstein's theory of special relativity that expresses the fact that mass and energy are the same physical entity and can be changed into each other.\(\color{black}{\tt {6.)i.solar cells}}\)
Energy Sources in the United StatesPetroleum (crude oil and natural gas plant liquids): 28% Coal: 17.8% Renewable energy: 12.7% Nuclear electric power: 9.6%"\(\color{black}{\tt { 7.) \: g. fission}}\)
Nuclear fission is a process by which the nucleus of an atom is split into two or more smaller nuclei, known as fission products. The fission of heavy elements is an exothermic reaction, and huge amounts of energy are released in the process.\(\color{black}{\tt { 8.) Sorry \: but \: I \: don't \: know \: the \: answer}}\)
\(\color{black}{\tt { 9.) Sorry \: but \: i \: don't \: know \: the \: answer}}\)
\(\color{black}{\tt { 10. ) \: Sorry \: \: But \: I \: don't \: know \: the \: answer }} \)
\(\color{black}{\tt {11.) \: b. Geothermal}}\)
Geothermal/GSHP systems take advantage of nature's underground temperatures by exchanging heat with the earth using an underground network of water (or refrigerant) filled pipes. In the winter, the liquid pulls heat from the ground and transfers it to the house through an exchanger.#CarryOnLearning
#LetsEnjoyTheSummer
→XxKim02xXthe following fatty acid, in which the indicated carbon atom is radiolabeled as 14c, is fed to an experimental mammal: 14ch3(ch2)15cooh after allowing sufficient time for fatty acid oxidation, the 14c label would be found temporarily in which one of the following compounds? a) beta-hydroxy butyryl-coa d) malonyl-coa b) acetyl-coa e) bicarbonate c) propionyl-coa
Answers
The labeled carbon in the fatty acid, 14CH₃(CH₂)15COOH, is located at the omega (ω) end of the molecule, which is the last carbon atom.
Therefore, upon oxidation of this fatty acid, the labeled carbon would not enter the citric acid cycle directly, but rather undergoes beta-oxidation to yield acetyl-CoA. During beta-oxidation, two-carbon units are cleaved from the fatty acid chain and converted to acetyl-CoA. Thus, the 14C label would eventually end up in acetyl-CoA.
The other compounds listed (beta-hydroxybutyryl-CoA, malonyl-CoA, propionyl-CoA, and bicarbonate) are not intermediates in beta-oxidation and would not contain the radiolabeled carbon from the fatty acid.
To learn more about fatty acid refer to:
brainly.com/question/1837143
#SPJ4
When a plant experiences heat, what part of a plant allows for the response to take place?
Answers
SOMEONE PLS HELP ME WITH MY HOMEWORK!!
It’s fill in the blanks and I really need help

Answers
Which are signs of Chemical Change (pick all that apply)
Color Change
Gas Formation
Solid Formation
Liquid Formation
Energy Change
Answers
might be gas change or colour change
(06. 01 LC)
It was observed that the particles of an unknown substance slide past one another. What is the state of matter of the substance? (1 point)
Solid
Liquid
Gas
Plasma
Answers
The liquid is the state of matter of the substance. subsequently, liquid is correct.
A “state of matter” is a manner to describe the behaviour of atoms and molecules in a substance.
In a liquid state of depend, particles are much less tightly packed as compared to solids. liquids take the shape of the box in which they may be kept. liquids are difficult to compress as debris have much less space between them to move. liquids have constant volume but no fixed shape.
To know more about state of matter click here
https://brainly.com/question/9402776
#SPJ4
assign oxidation numbers to each atom in rh(c2o4)2
Answers
The oxidation numbers for each atom in Rh(C2O4)2 are: Rh = +4(each), C = +2 (each), and O = -2 (each).
Assign oxidation numbers to each atom in Rh(C2O4)2:
1. Identify the oxidation numbers of the known atoms: In this compound, we know that the oxidation number of oxygen (O) is always -2 (except in peroxides).
2. Analyze the oxalate ion (C2O4)^2-: Since there are two oxygen atoms (each with an oxidation number of -2), their combined oxidation number is -4.
To balance the charge of the ion, the two carbon atoms must have a combined oxidation number of +4. Each carbon atom, therefore, has an oxidation number of +2.
3. Determine the oxidation number of Rh: In the compound Rh(C2O4)2, there are two oxalate ions, each with a charge of -2, resulting in a total negative charge of -4. To balance this charge, the oxidation number of Rh must be +4.
In summary, the oxidation numbers for each atom in Rh(C2O4)2 are: Rh = +4, C = +2 (each), and O = -2 (each).
To know more about oxidation number:
https://brainly.com/question/30770473
#SPJ11
PLEASE HELP
Fossil fuels are burnt in power plants to produce electricity. Which one of the
following sequences show the correct order of energy transformation?

Answers
Answer:
a
Explanation:
Chemical then thermal because the carbon emissions produce heat
Then kinetic because there is energy transfer then electric because that's the finished result
If 0.45 moles of h2o are produced how many moles of co2 are also produced
Answers
To determine the number of moles of CO2 produced, we need to know the balanced chemical equation for the reaction that produces both H2O and CO2.
Assuming that we're talking about the combustion of a hydrocarbon fuel (like methane, for example) in the presence of oxygen, the balanced chemical equation for this reaction is: CH4 + 2O2 --> CO2 + 2H2O
This equation tells us that for every one mole of methane (CH4) that reacts with two moles of oxygen (O2), we get one mole of carbon dioxide (CO2) and two moles of water (H2O) produced.
From the balanced equation, you can see that for every 2 moles of H2O produced, 1 mole of CO2 is produced. Therefore, if 0.45 moles of H2O are produced, you can determine the moles of CO2 by dividing the moles of H2O by 2: 0.45 moles H2O × (1 mole CO2 / 2 moles H2O) = 0.45/2 = 0.225 moles of CO2.
To know more about reaction visit:
https://brainly.com/question/30464598
#SPJ11
3. In which state of matter do molecules interact to create surface tension? *
A. gas
B. solid
C. liquid
Answers
help with this question

Answers
Answer:
2.5 mole of O2
Explanation:
From the equation we see that we need 5 molecules of oxygen to react with 2 molecules of C2H2.
This means that 5 moles of oxygen molecules are needed to completely react with 2 moles of C2H2, because a mole is a unit of particle count.
From that we can infer that to react with 1 mole of C2H2 we need half of 5 moles of oxygen - so 2.5 mole of oxygen molecules.
when 50.0 g of silicon dioxide is heated with an excess of carbon, 32.2 g of silicon carbide is produced SiO2(s)+3C(s)→SiC(s)+2CO(g).
A. What is the percent yield of this reaction?
B. How many grams of CO gas is evolved?
Answers
In the reaction
A. Percent yield of the reaction is 86.98%.
B. 46.68 g of CO gas is evolved
A. Percent yield of the reactionThe given reaction is as follows:
SiO2(s) + 3C(s) → SiC(s) + 2CO(g)
The molar mass of SiO2 is 60.08 g/mol.
Mass of SiO2 = 50 g
Moles of SiO2 = (50 g) / (60.08 g/mol) = 0.832 mol
According to the balanced equation, 1 mol of SiO2 produces 1 mol of SiC.
So, the moles of SiC produced = 0.832 mol
According to the balanced equation, 1 mol of SiO2 produces 2 mol of CO.
So, the moles of CO produced = 2 × 0.832 = 1.664 mol
Molar mass of CO = 28.01 g/mol
Mass of CO produced = (1.664 mol) × (28.01 g/mol) = 46.68 g
Theoretical yield of SiC = mass of SiC produced = 32.2 g
Percentage yield of the reaction = (Actual yield / Theoretical yield) × 100= (32.2 g / 37.02 g) × 100= 86.98%
So, the percentage yield of the reaction is 86.98%.
B. Mass of CO gas evolved
According to the balanced equation, 1 mol of SiO2 produces 2 mol of CO.
So, the moles of CO produced = 2 × 0.832 = 1.664 mol
Molar mass of CO = 28.01 g/mol
Mass of CO produced = (1.664 mol) × (28.01 g/mol) = 46.68 g
So, the mass of CO gas evolved is 46.68 g.
For more such questions on Percentage yield.
https://brainly.com/question/30579872#
#SPJ11
Do positively charged ions gain or lose electrons
Answers
Answer:
Atoms often get electrons or lose. The atom lacks or earns a "negative" load. Then these electrons are known as ions. Positive ion - If the molecule lacks an electron, it gets more protons than ions.
Explanation:
Positively changed ions are called Cations. Main group elements that are metals usually form cations which have a positive charge by losing one or more electrons.