Answers
The aldol self-condensation of 3-phenyl-2-propenal (also known as cinnamaldehyde) would result in the formation of an enone product. Here is the reaction:
mathematica
Copy code
H+
|
H-C=C-CH=CH-CHO + H-C=C-CH=CH-CHO
| |
CHO CHO
The product of this reaction would be 3-phenyl-2-cyclohexen-1-one, which is an enone compound. The reaction involves the formation of an aldol intermediate, which undergoes dehydration to form the enone product.
The structure of the enone product is shown below:
mathematica
Copy code
H
|
H-C=C-C(=O)-C=C-C6H5
|
Ph
The double bond between the C-2 and C-3 carbons has been converted into a carbonyl group (C=O) and a C=C double bond, resulting in the formation of the enone.
Related Questions
helpp plz I’ll mark brainiest
choose all statements that describe acids

Answers
what is chromatography
Answers
Answer:
Chromatography is the method of seperating of colour/pigments from a solution.
Explanation:
It is based on a principle that, "speed of different sized particles in a same medium is different."
Chromatography are of many types like paper chromatography, Adsorption chromatography, Gas liquid portion chromatography.
How is heat transferred from one object to another? A. Heat moves from warmer objects to cooler objects. B. Heat moves from cooler objects to warmer objects. c. Heat moves between objects of the same temperature. D. Heat moves back and forth between two objects.
Answers
Answer: I believe the answer is A, heat moves from warmer objects to cooler objects. I know for sure it isn’t C or D though so A
The heat is transferred from one object to another as heat moves from warmer objects to cooler objects. The correct option is A.
What is the transfer of heat?There are three ways to transfer heat. They are conduction, convection, and radiation. Heat travels from one body to another body. If the temperature of two objects is different, then the heat travels from higher temperature to lower temperature.
Conduction is the transfer of energy when two objects ate in contact with each other. Convection is a transfer between object and environment. Radiation is when transferred by emission of electromagnetic radiation.
Thus, the correct option is A. Heat moves from warmer objects to cooler objects.
Learn more about the transfer of heat, here:
https://brainly.com/question/13433948
#SPJ5
Which is the balanced version of the half-reaction below?
H2S → S+H+
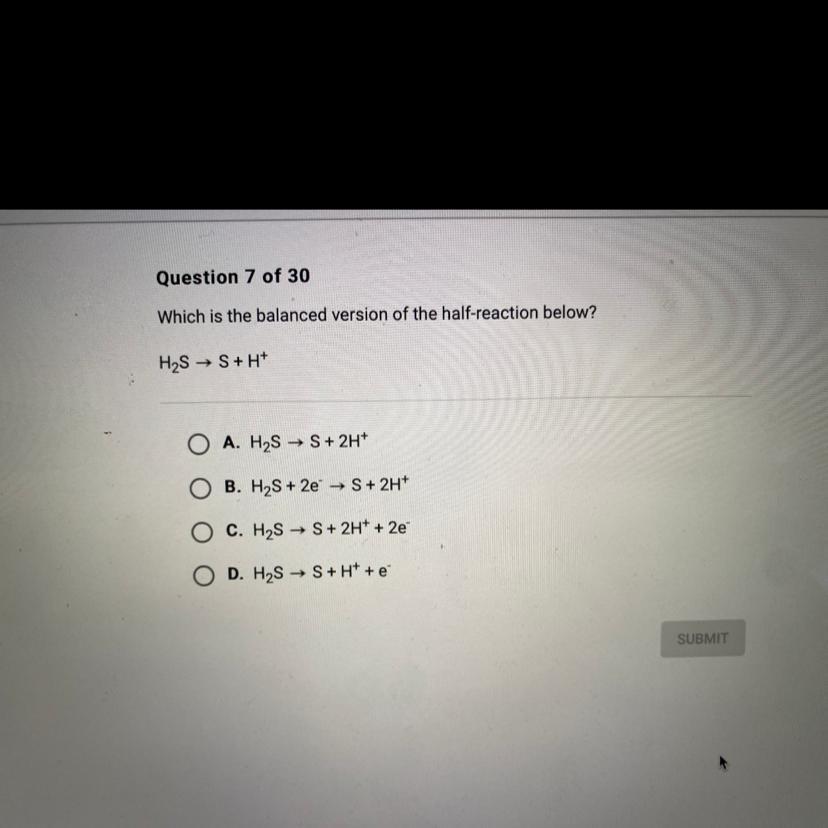
Answers
Answer:
C. \(H_2S\rightarrow S+2H^++2e^-\)
Explanation:
Hello there!
In this case, according to the given chemical reaction, it turns out possible to realize there is one sulfur atom on each side of the chemical equation but two hydrogen atoms on the left and one on the right, which means the latter must be balanced in agreement to the law of conservation of mass.
In such a way, by setting a 2 on H⁺, the reaction will be balanced:
\(H_2S\rightarrow S+2H^+\)
Now, we count the transfer electrons for sulfur from -2 to 0 as 2e⁻ on the right, which will match with the option C.
\(H_2S\rightarrow S+2H^++2e^-\)
Regards!
In using the Haber process in the formation of ammonia, what mass of hydrogen is needed to produce 51.0 grams of ammonia? 3 H₂(g) + N2 (g) → 2 NH3(g).
Answers
The mass of hydrogen needed to produce 51.0 grams of ammonia is ≈ 9.07 grams.
To determine the mass of hydrogen required to produce 51.0 grams of ammonia (NH3) using the Haber process, we need to calculate the stoichiometric ratio between hydrogen and ammonia.
From the balanced chemical equation:
3 H₂(g) + N₂(g) → 2 NH₃(g)
We can see that for every 3 moles of hydrogen (H₂), we obtain 2 moles of ammonia (NH₃).
First, we need to convert the given mass of ammonia (51.0 grams) to moles. The molar mass of NH₃ is 17.03 g/mol.
Number of moles of NH₃ = Mass / Molar mass
= 51.0 g / 17.03 g/mol
≈ 2.995 moles
Next, using the stoichiometric ratio, we can calculate the moles of hydrogen required.
Moles of H₂ = (Moles of NH₃ × Coefficient of H₂) / Coefficient of NH₃
= (2.995 moles × 3) / 2
≈ 4.493 moles
Finally, we can convert the moles of hydrogen to mass using the molar mass of hydrogen (2.02 g/mol).
Mass of H₂ = Moles × Molar mass
= 4.493 moles × 2.02 g/mol
≈ 9.07 grams
Therefore, approximately 9.07 grams of hydrogen is needed to produce 51.0 grams of ammonia in the Haber process.
Know more about the mass of hydrogen here:
https://brainly.com/question/14083730
#SPJ8
Ethanol (C2H5OH) is produced from the fermentation of sucrose (table sugar) C12H22O11 in the presence of enzymes.
The balanced equation for this reaction is: C12H22O11 (aq) + H2O(g) ---> 4C2H5OH(l) + 4CO2(g)
a. What is the theoretical yield of ethanol when 648g of sucrose undergo fermentation? Show mass to mass conversion setup with units and correct sigfigs in the final answer.
b. If the actual yield is 339g, what is the percent yield? Show % yield formula setup with units and the final answer in correct sigfigs.
Answers
The enzymes invertase and zymase, which are produced by Saccharomyces cerevisiae, are in charge of the alcoholic fermentation of sucrose, which generates ethanol and CO2.
Which specific enzyme induces the fermentation of sugar?The transformation of sugar into ethanol and carbon dioxide is catalyzed by an enzyme complex known as zymase. It is naturally found in yeast. Zymase activity varies across yeast strains.
Glucoseamylase enzymes convert the flour's starch into maltose and fermentable sugars. The dough rises as a result of the yeast fermentation process. Moreover, these enzymes are used to create glucose, which Saccharomyces cerevisiae then ferments to produce ethanol.
The typical temperature for this to happen is around 30°C. Sugar produced from plants is a renewable resource.
learn more about enzymes invertase
https://brainly.com/question/20732474
#SPJ1
You are studying a reaction where A is consumed and B is produced. If the reaction starts with 200 mM A, after 1 minute 100 mM A remains, and after another 45 seconds 25 mM remains, what is the order of the reaction and what is the rate constant?
Answers
The order of the reaction is zero and the rate constant is 1.67.
How to calculate the rate?From the information given, the rate law expression is illustrated thus:
Rate = k.[A]x
Time (sec) Concentration (mM)
0 200
60 100
105 B25
After 60 sec,
Rate = -d[A]/dt
= -[100 - 200]/(60 - 0)
= 100/60 = 1.67
After 105 sec,
Rate = -d[A]/dt
= -[25 - 100]/(105 - 60)
= 75/45 = 1.67
As we can see, the rate is constant. This is constant with respect to change in the concentration of A.
Learn more about rate on:
https://brainly.com/question/25537936
#SPJ1
Molecules can be composed of
__. Select all that apply. ...
two of more of the same atoms
two or more smaller molecules
two or more different atoms
one or more particle
Answers
Answer:
Molecules can be composed of two or more of the same atom
Explanation:
If you are measuring the mass of a liquid on a balance scale, what do you have to take into consideration to accurately find the mass?
Answers
Answer:
make sure the scale is at 0 before putting the liquid
How much heat is gained by nickel when 31.4 g of nickel is warmed from 27.2 °C to 64.2 °C? The specific heat of nickel is 0.443 J/g · °C.
Answers
Explanation:
To calculate the heat gained by nickel, we can use the formula:
q = m * c * ΔT
where q is the heat gained, m is the mass of the nickel, c is the specific heat of nickel, and ΔT is the change in temperature.
Given:
- Mass of nickel, m = 31.4 g
- Specific heat of nickel, c = 0.443 J/g · °C
- Change in temperature, ΔT = 64.2 °C - 27.2 °C = 37.0 °C
Substituting the values into the formula, we get:
q = (31.4 g) * (0.443 J/g · °C) * (37.0 °C)
Simplifying the calculation, we get:
q = 584 J
Therefore, the heat gained by nickel when 31.4 g of nickel is warmed from 27.2 °C to 64.2 °C is 584 J.
in a 52 weeks high and low, if the numbers show difference between each other, there is less risk of loss - but there is also less opportunity for gain, either.
Answers
Smaller numbers mean less risk of losing, but less chance of gain, Volatility.
What is gain?In electronics, gain is a measure of the ability of a two-terminal circuit (often an amplifier) to increase the power or amplitude of a signal from its input. Adjusting the gain control sets the amount of distortion in the sound no matter how loud the final volume is set. This means that the gain setting determines how clean or dirty the sound is, regardless of the master volume setting.Gain is simply the amplification factor, i.e. the parts of the output power to input power. Wins are often expressed in percentages, especially for small wins. Example 3% of ratio signifies to a power gain of 1.03.to learn more about gain from the given link:
https://brainly.com/question/28891489
#SPJ1
Can you have a pH thats in decimals? For example .3 or .4?
Answers
A pH of 4 is ten times more acidic than a pH of 5.
Yes, pH can have decimal values. In fact, pH values can range from 0 to 14 and can have any value between them including decimals. pH is a measure of the acidity or alkalinity of a solution, and it is determined by the concentration of hydrogen ions (H+) in the solution.A solution with a pH of 7 is neutral, which means that it has an equal concentration of hydrogen ions (H+) and hydroxide ions (OH-). An acidic solution has a pH below 7 and a high concentration of H+ ions. On the other hand, an alkaline solution has a pH above 7 and a low concentration of H+ ions.A pH that is less than 7.0 indicates acidity. pH less than 7.0 is acidic while pH greater than 7.0 is alkaline. Each number on the pH scale represents a ten-fold change in the acidity/alkalinity of the solution. For example, a pH of 5 is 10 times more acidic than a pH of 6, and 100 times more acidic than a pH of 7.A pH of 0 indicates a very strong acidic solution while a pH of 14 indicates a very strong alkaline solution. It's worth noting that pH is a logarithmic scale, meaning that a change of one pH unit corresponds to a ten-fold change in hydrogen ion concentration.
for more questionson acidic
https://brainly.com/question/31110544
#SPJ8
A student diluted 10.0 milliliters (mL) of a 9.00 Molar (M) HCl solution in order to prepare a 0.50 M solution for his organic extraction analysis.What quantity of water, in mL, was added to make the 0.50M HCl solution?
A: 150
B: 160
C: 170
D: 180
Answers
The quantity of water in mL that was added to make the solution is 170 mL (option C)
How to determine the volume of the diluted solution Volume of stock solution (V₁) = 10 mL Molarity of stock solution (M₁) = 9 MMolarity of diluted solution (M₂) = 0.5 MVolume of diluted solution (V₂) =?M₁V₁ = M₂V₂
9 × 10 = 0.5 × V₂
90 = 0.5 × V₂
Divide both side by 0.5
V₂ = 90 / 0.5
V₂ = 180 mL
How to determine the volume of the water Volume of stock solution (V₁) = 10 mL Volume of diluted solution (V₂) = 180 mL Volume of water added =?Volume of water added = V₂ – V₁
Volume of water added = 180 – 10
Volume of water added = 170 mL
Learn more about dilution:
https://brainly.com/question/15022582
Based on Chromium's position on the periodic table, which statement describes the element
chromium (Cr), atomic number 24?
Answers
Answer:
C. chromium is a metal that is less reactive than sodium.
Explanation:
Hello.
Given the options:
A. chromium is a nonmetal and therefore a good conductor of heat and electricity .
B. chromium is a metal that is more reactive than potassium .
C. chromium is a metal that is less reactive than sodium .
D. chromium is a noble gas that is not reactive.
In this case, since chromium is in period 4 group VIB we infer it is a transition metal which slightly reacts with acids and poorly reacts with oxygen and other oxidizing substances. Thus, in comparison with both sodium and potassium which are highly reactive even with water as they get on fire, we can say that it is less reactive than both potassium and sodium, therefore, answer is: C. chromium is a metal that is less reactive than sodium.
Best regards.
A nonapeptide was determined to have the following amino acid composition: (Arg)2, (Gly)2, (Phe)2, His, Leu, Met. 1-fluoro-2,4-dinitrobenzene (FDNB) reacts with free amino (but not amido or guanidino) groups in proteins to produce dinitrophenyl (DNP) derivatives of amino acids. The native peptide was incubated with 1-fluoro-2,4-dinitrobenzene (FDNB) and then completely hydrolyzed; 2,4-dinitrophenylhistidine was identified by HPLC. What does this tell you about the peptide sequence? When the native peptide was exposed to cyanogen bromide (CNBr), an octapeptide and free glycine were recovered. What does this tell you about the peptide sequence? Incubation of the native peptide with trypsin gave a pentapeptide, a tripeptide, and free Arg.The peptides were separated and treated with FDNB. 2,4-Dinitrophenyl-histidine was recovered from the pentapeptide, and 2,4-dinitrophenylphenylalanine was recovered from the tripeptide.What does this tell you about the peptide sequence? Digestion with the enzyme pepsin produced a dipeptide, a tripeptide, and a tetrapeptide. The tetrapeptide was composed of (Arg) 2, Phe, and Gly. What does this tell you about the peptide sequence? What is the sequence of the nonapeptide?
Answers
The information provided tells us that the peptide sequence contains a histidine residue at some position.
This information indicates that the peptide sequence contains a histidine residue, which is covalently modified by FDNB to form 2,4-dinitrophenylhistidine. The fact that the histidine residue is modified by FDNB suggests that it is in the free amino form, rather than being part of an amide or guanidino group.
This is because FDNB reacts with free amino groups, but not with amido or guanidino groups. The presence of a histidine residue in the peptide sequence can provide information about the functional and structural properties of the peptide, as histidine is known to play important roles in protein structure and function.
Learn more about peptide sequence brainly.com/question/30505255
#SPJ4
How many moles of O2 are made from 4 moles of H2O
Answers
Based on its location on the periodic table, how many electrons does nitrogen have in its outer energy level?
Answers
Answer:
its 5 got it right
Which of the following would cause an increase in the magnetic force
between two magnets?
A. Decreasing the separation between the two magnets
B. Increasing the separation between the two magnets
C. Decreasing the amount of excess charge on the first magnet
D. Increasing the amount of excess charge on the first magnet
Answers
Decreasing the separation between the two magnets will result in increase in the magnetic force between two magnets.
What is a Magnet?This is referred to a substance which produces magnetic field and result in the attraction and repulsion of certain types of things.
Increase in the separation will reduce the magnetic force and vice versa which is why option A was chosen.
Read more about Magnet here https://brainly.com/question/14997726
#SPJ1
What is the molarity 10.0g of Cr(NO3)3 in 325 mL of solution
Answers
Answer:
Explanation:
molar mass Cr(NO3)3 = 238 g/mol
Convert 325 ml to liters: 325 mls x 1 L / 1000 mls = 0.325 L
Convert 10.0 g to moles: 10.0 g x 1 mol / 238 g = 0.0420 moles
Molarity = moles/liters = 0.0420 moles / 0.325 L = 0.129 M (3 sig. figs.)
The most reactive metal is one with
O 4 valence electrons
1 valence electron
O 2 valence electrons
o 3 valence electrons
Help me!
Answers
1 valence electron
Explanation:
It is because the metal having one valence electron can readily lose single electron from its outermost shell.
Answer: the answer is 1 valence electron
Explanation: just did a test with this quest thx for the points good luck :D
The reactant concentration in a zero-order reaction was 8.00×10−2 M
after 140 s and 4.00×10−2 M after 400 s
. What is the rate constant for this reaction?
Answers
The rate constant for the reaction is either 7.14×10−3 s−1 or 2.50×10−3 s−1, depending on which rate was used to calculate it.
Determining the rate constantThe rate of the reaction is given by the equation:
Rate = -k[A]
where k is the rate constant and [A] is the concentration of the reactant.
Rate at t=140 s:
Rate = (8.00×10−2 M - 0 M) / (140 s - 0 s)
= 5.71×10−4 M/s
Rate at t=400 s:
Rate = (4.00×10−2 M - 0 M) / (400 s - 0 s)
= 1.00×10−4 M/s
Since this is a zero-order reaction, the rate of the reaction is constant, and we can use either rate to calculate the rate constant:
k = Rate / [A]
Using the rate at t=140 s:
k = 5.71×10−4 M/s / 8.00×10−2 M = 7.14×10−3 s−1
Using the rate at t=400 s:
k = 1.00×10−4 M/s / 4.00×10−2 M
= 2.50×10−3 s−1
The rate constant for the reaction is either 7.14×10−3 s−1 or 2.50×10−3 s−1.
Learn more on zero-order reaction https://brainly.com/question/21663229
#SPJ1
Is rusting of an iron exothermic or endothermic event if the nail is the system?
exothermic or endothermic?
Answers
Answer:
exotherimc reaction
Explanation:
:)
Answer:
???????????????????????????
Vhat does a very dark soil sample most likely indicate about soil quality?
A. high salinity
B. a low amount of nutrients
C. a large amount of nutrients
D. low salinity
Answers
Macmillan Learning
Consider the mechanism.
step 1:
step 2:
overall:
Which species is an intermediate?
A
AB
AC
B
Which species is a catalyst?
AC
B
AB
A
Answers
Considering the mechanism -
Step 1: A + B → AB
Step 2: AB + C → AC + B
Overall: A + C → AC
Here AB is the intermediate and B is a catalyst.
AB is an intermediate of the reaction because it is formed and completely utilized in the reaction mixture by itself.
A catalyst is a type of species which is added to the reaction mixture but it remains unchanged as it do not participate in the overall reaction. A catalyst helps the reaction to form the products at a faster rate.
Intermediate is formed from the reactants which interacts to produce immediately visible products of a chemical reaction, while catalyst results in speeding up of a chemical reaction and it is not consumed in the reaction.
Complete question is -
Macmillan Learning
Consider the mechanism.
Step 1: A + B → AB
Step 2: AB + C → AC + B
Overall: A + C → AC
Which species is an intermediate?
A
AB
AC
B
Which species is a catalyst?
AC
B
AB
A
To learn more about mechanism,
brainly.com/question/14208373
#SPJ1
A 0.563 M solution of the salt NaA has a pH of 11.56. Calculate the Ka value for the acid HA. Record your answer in scientific notation to 3 sig figs.
Answers
Answer:
\(\displaystyle K_a = 4.24\times 10^{-10}\)
Explanation:
Write the base reaction of NaA with water:
\(\displaystyle \text{A}^-_\text{(aq)}+\text{H$_2$O}_\text{($\ell$)}\rightleftharpoons \text{HA}_\text{(aq)} + \text{OH}^-_\text{(aq)}\)
Hence, the equilibrium constant expression for the reaction is:
\(\displaystyle K_b = \frac{[\text{OH}^-][\text{HA}]}{[\text{A}^-]}\)
Thus, to find Ka, we can find Kb and use the fact that Ka × Kb = Kw.
From the reaction and initial concentration of NaA, create an ICE chart:
\(\begin{tabular}{llllll} & A^- &\text{H$_2$O} & \rightleftharpoons & HA & OH^- \\I & 0.563 M & \---- & & 0 M & 0 M \\C & -\text{ $ x$} & \---- & & +\text{ $x$ M} & + \text{$x$ M} \\E & \text{(0.563 - $x$) M} & \---- & & \text{$x$ M} & \text{$x$ M} \end{tabular}\)
Find [OH⁻] from the given pH:
\(\displaystyle \begin{aligned} \text{pH} +\text{pOH} & = 14.00 \\ \\ \text{pOH} & = 14.00 - \text{pH} \\ \\ & = 14.00 - (11.56) \\ \\ & = 2.44 \\ \\ -\log[\text{OH}^-] & = 2.44 \\ \\ [\text{OH}^-] &= 10^{-2.44} \\ \\ & =0.00363 \text{ M}= 3.63\times 10^{-3} \text{ M} = x\text{ M}\end{aligned}\)
Solve for all species concentrations at equilibrium from the found x value:
\(\displaystyle [\text{HA}] = [\text{OH}^-] = 3.63\times 10^{-3} \text{ M}\)
And:
\(\displaystyle \begin{aligned} \ [\text{A}^-] & = 0.563 - 3.63\times 10^{-3} \text{ M}\\ \\ & = 0.559\text{ M}\end{aligned}\)
Find Kb:
\(\displaystyle \begin{aligned} \displaystyle K_b &= \frac{[\text{OH}^-][\text{HA}]}{[\text{A}^-]} \\ \\ & = \frac{(3.63\times 10^{-3})(3.63\times 10^{-3})}{(0.559)}\\ \\ & = 2.36\times 10^{-5}\end{aligned}\)
Find Ka:
\(\displaystyle \begin{aligned} K_a\cdot K_b & = K_w \\ \\ K_a & = \frac{K_w}{K_b} \\ \\ & = \frac{(1.00 \times 10^{-14})}{(2.36\times 10^{-5})} \\ \\ &= 4.24\times 10^{-10} \end{aligned}\)
In conclusion:
\(\displaystyle K_a = 4.24\times 10^{-10}\)
HELP
____________ sweat by water from the leaf cells __________________ into the air, which pulls _______________________ from the leaf into the air.
Answers
Answer:
Water sweated by water from the leaf cells evaporating into the air, which pulls water and nutrients from the leaf into the air.
if there are more products than reactants, does that mean there is an increase in the forward or backward reaction? And if there are more reactants that products, is there an increase in the forward or backward reaction?
Answers
Answer:
If there are more products than reactants, that means the reaction has shifted towards the left, which is the backward direction. If there are more reactants than products, that means the reaction has shifted towards the right, which is the forward direction.
Dau coroana va roggg!!!!!!

Answers
Answer:
it's very easy and simple answer u can't do it
What is the molarity of a solution made from dissolving 3.0 moles of NaCl in 250 mL of solution?
3.0 M NaCl
0.012 M NaCl
750 M NaCl
12 M NaCl
Answers
Answer:
Molarity = 12 M
Explanation:
Given data:
Molarity of solution = ?
Number of moles of NaCl = 3.0 mol
Volume of solution = 250 mL (250/1000 = 0.25 L)
Solution:
Molarity is used to describe the concentration of solution. It tells how many moles are dissolve in per litter of solution.
Formula:
Molarity = number of moles of solute / L of solution
Molarity = 3.0 mol / 0.25 L
Molarity = 12 M
Answer:
\(\boxed {\boxed {\sf 12 \ M \ NaCl}}\)
Explanation:
Molarity, which tells us the concentration of a solution, is found by dividing the moles of solute by the liters of solution.
\(M=\frac{moles \ of \ solute}{liters \ of \ solution}\)
1. Define Values
There are 3.0 Moles of NaCl. This is the moles of solute.
There are 250 milliliters of solution, but we need the liters.
a. Convert mL to L
1 milliliter is equal to 0.001 liters. We can multiply the given number of milliliters (250) by 0.001.
250 mL * 0.001 L/mL= 0.25 L
\(moles \ of \ solute = 3.0 \ mol \ NaCl \\liters \ of \ solution= 0.25 \ L\)
2. Calculate Molarity
Substitute the values into the formula.
\(M=\frac{3.0 \ mol \ NaCl}{0.25 \ L}\)
Divide.
\(M= 12 \ mol \ NaCl/L\)
3. Define Units
1 mole per liter is equal to 1 molar.
Our answer of 12 mol NaCl/ L is equal to 12 M NaCl
The molarity is 12 M NaCl
The rate of reaction was measured during a chemical reaction. After the first 3 seconds, the rate of reaction was 1.8 x10−6 M/s. Which of the following would you expect after another 3 seconds?
Answers
The question is incomplete, the complete question is;
The rate of reaction was measured during a chemical reaction. After the first 3 seconds, the rate of reaction was 1.8 x10−6 M/s. Which of the following would you expect after another 3 seconds? a
The rate would be higher, and the concentration of reactants would be lower.
b
The rate would be higher, and the concentration of reactants would be higher.
c
The rate would be lower, and the concentration of reactants would be lower.
d
The rate would be lower, and the concentration of reactants would be higher.
Answer:
The rate would be lower, and the concentration of reactants would be lower.
Explanation:
The rate of reaction refers to how quickly or slowly the reactants disappear or the products appear in a given reaction. The rate of reaction depends on the concentration of the reactants. Thus, as concentration decreases with time, the rate of reaction decreases accordingly.
Therefore, reaction rates tend to decrease with time since the concentration of the reactants decrease with time as the reactants are being converted into products. Thus after three seconds, the rate would be lower, and the concentration of reactants would be lower. Hence the answer above.
Answer:
The rate would be lower, and the concentration of reactants would be higher.
Explanation:
I took the test and i think i got it right