Answers
Answer:
Explanation:
Kindly note that I have attached the complete question as an attachment.
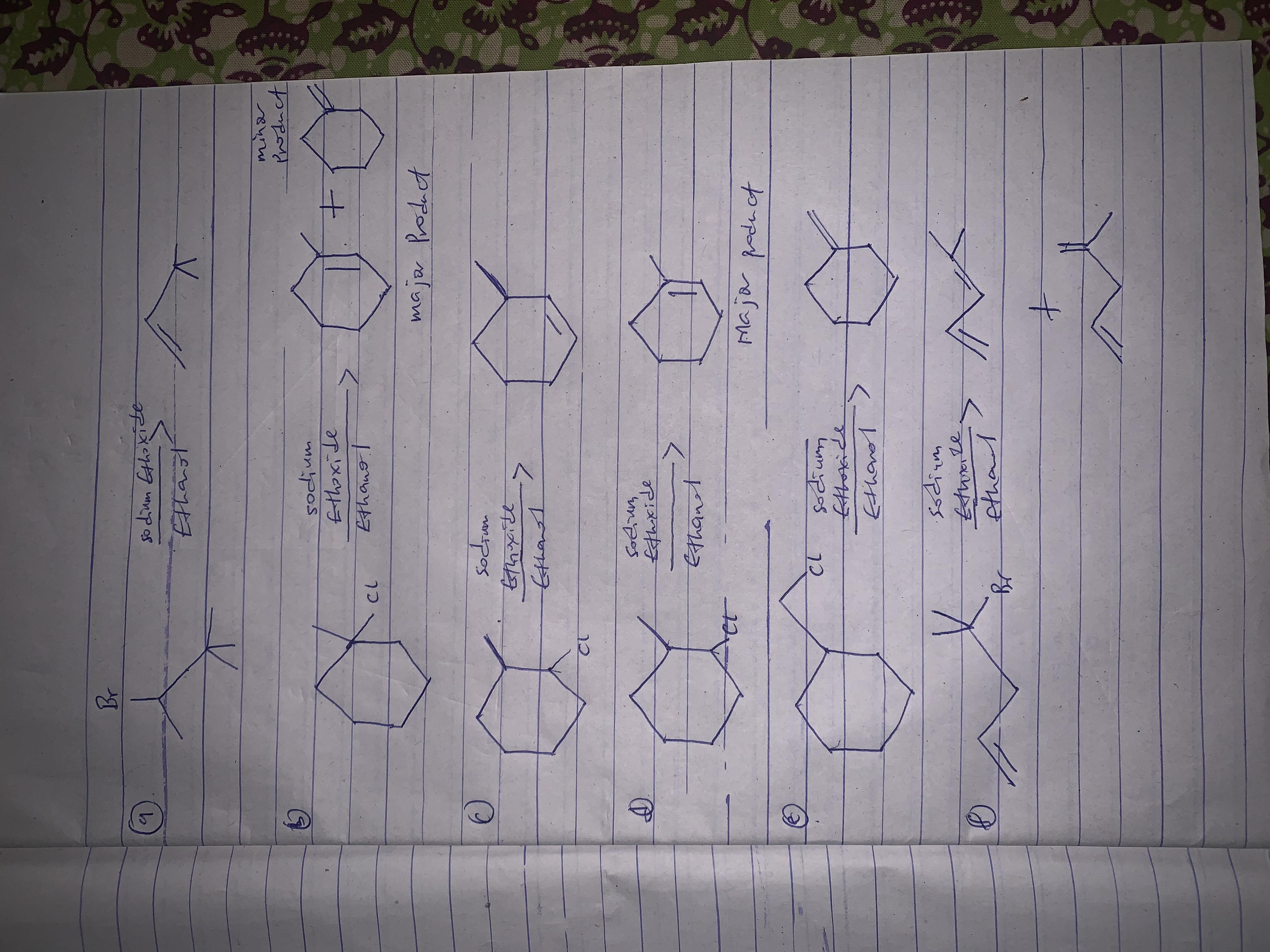
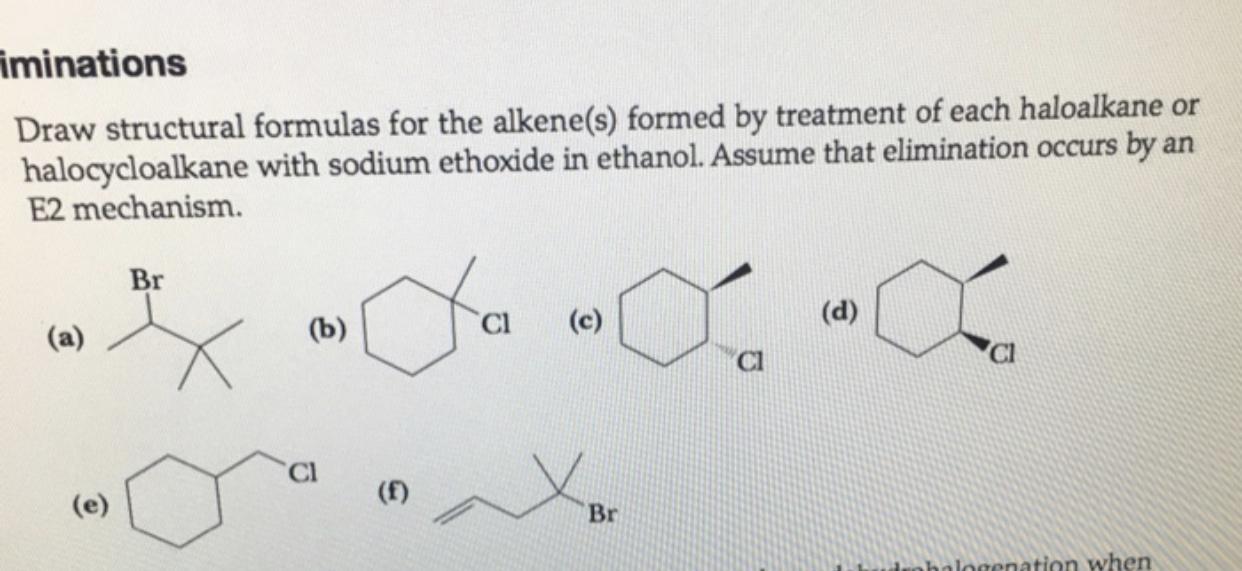
Here, we are told that elimination occurs by an E2 mechanism. What this means is that the hydrogen and the halogen must be above and below for the reaction to proceed.
The possible products are as follows;
Please check attachment for complete equations and diagrams of compounds too.
Related Questions
Questions
Q1.
Use the Periodic Table on page 2 to help you answer this question.
Give the name or symbol of
(a) the element in group 3 and period 4.
Answers
Gallium is the element that belongs to the group 3 and the fourth period. Ga is symbol of Gallium.
The element gallium has an atomic number of 31.While highly pure gallium is covered in a dazzling silvery colour, solid gallium is a blue-grey metal with an orthorhombic crystalline structure.It is a crucial part of numerous semiconductors. Due to its capacity to transform power into light, it is additionally used in red LEDs (light emitting diodes).Because of its high boiling point, it is perfect for recording temperatures that would cause a thermometer to vaporise.From iron pyrites, zinc blende, germanite, and bauxite, this metal can be readily removed as a byproduct.Because gallium is a corrosive chemical, it can cause serious skin and eye burns asse well as significant irritation.Learn more about semiconductors here
https://brainly.com/question/1918629
#SPJ9
A balloon is filled to a volume of 2.20L at a temperature of 25.0*C. The balloon is then heated to a temperature of 51*C. Find the new volume of the balloon
Answers
The new volume of the balloon after heating it to a temperature of 51 °C is approximately 2.39 L.
What is the final volume of the balloon?Charles's law states that "the volume occupied by a definite quantity of gas is directly proportional to its absolute temperature.
It is expressed as;
\(\frac{V_1}{T_1} =\frac{V_2}{T_2}\)
Given that:
Initial temperature of gas T₁ = 25°C = (25.0 + 273.15) = KInitial volume of gas V₁ = 2.2 LFinal temperature T₂ = 51 °C = ( 51 + 273.15 ) = 324.15 KFinal volume V₂ = ?Substituting the given values and solve for V₂:
\(V_1T_2 = V_2T_1\\\\V_2 = \frac{V_1T_2}{T_1} \\\\V_2 = \frac{2.2\ *\ 324.15}{298.15 }\\ \\V_2 = 2.39 \ L\)
Therefore, the final volume is 2.39 litres.
Learn more about Charles's law here: https://brainly.com/question/23122443
#SPJ1
what is it called when the mass of atoms found in nature is determined by measuring the masses of the elements combined in chemical compounds, a large number of atoms must be used to make the measurement.
Answers
Answer:
This process is called mass spectrometry. In mass spectrometry, a sample is ionized, typically by bombarding it with high-energy electrons, and the resulting ions are separated based on their mass-to-charge ratio. The mass of the ions can be measured using a mass spectrometer, and this allows the determination of the atomic masses of the elements present in the sample. To obtain accurate results, it is generally necessary to measure the masses of a large number of atoms, as the precision of the measurement increases with the number of atoms that are measured
Explanation:
What type of energy transformation happens when you boil water
Answers
Answer:
thermal energy.
Explanation:
Thermal energy is produced when the atoms and molecules in a substance vibrate faster due to a rise in temperature.
a 12.4g sample of hydrogen gas occupies a volume of 23.5L at a certain temperature and pressure. What volume does a 56.2 g sample of oxygen gas occupy at these same conditions
Answers
As a result, at the same temperature and pressure as the 12.4 g sample of hydrogen gas, a 56.2 g sample of oxygen gas takes up 39.3 l.
At STP, how much space would 48.0 g of oxygen gas take up?1mol of petrol takes up 22.4 litres at STP. So, 32 grams of oxygen take up 22.4 litres of space. Consequently, 33.6 litres of space are taken up. Hence, 33.6 litres is the volume that 48 g of O2 at STP occupy.
To resolve this issue, we can employ the ideal petrol rule, PV = nRT. If both gases have the same temperature and pressure, we can put up the equation as follows: PV = nRT
For hydrogen gas: n(H2) = m(H2) / M(H2)
= 12.4 g / 2.016 g/mol
= 6.16 mol
Substituting the given values, we get:
(1 atm)(23.5 L) = (6.16 mol)(0.08206 L·atm/mol·K)(T)
Solving for T, we get:
T = (1 atm)(23.5 L) / (6.16 mol)(0.08206 L·atm/mol·K)
T = 283 K
For oxygen gas:
n(O2) = m(O2) / M(O2)
n(O2) = 56.2 g / 31.9988 g/mol
n(O2) = 1.757 mol
Substituting the values, we get:
(P)(V) = (n)(R)(T)
(1 atm)(V) = (1.757 mol)(0.08206 L·atm/mol·K)(283 K)
Solving for V, we get:
V = (1.757 mol)(0.08206 L·atm/mol·K)(283 K) / (1 atm) = 39.3 L
To know more about oxygen visit:-
https://brainly.com/question/14386958
#SPJ1
give me the answer for this multiple choice question

Answers
13) The concentration is 0.376 M.
14) The molecule is bent
15) The s orbital is spherically symmetrical
16) Barium and bromine are most apt to form an ionic bond
17) The enthalpy of reaction is equal to the enthalpy of formation for option C
18) The hybrid set of orbitals is shown by option B
19) Iodine forms the ion in option D
20) The enthalpy is - 44 kJ/mol
21) Carbon excited state is shown by option A
22) Option D does not violate the octet rule (carbon)
23) It is a transition metal
24) The structure is tetrahedral
25) The third ionization energy is shown by option C
26) The element is neon
What is the quantum numberConcentration = Number of moles/ volume
Concentration = 0.5 moles/1.22 L
= 0.376 M
The sulfur dioxide molecule is bent due to the fact that we have lone pairs in the molecule of the compound.
Given the fact that the s orbital do have a spherically symmetrical shape, the orbital quantum number is zero.
ΔH = Bonds broken - Bonds formed
= (614 + 4(413) + 433) - (5(413) + 348 + 328)
= 2698 - 2741
- 44 kJ/mol
Learn more about quantum number:https://brainly.com/question/32116992
#SPJ1
14. Which of the following will result in a chemical change?
A. Melting ice to obtain water
B. Evaporating alcohol into vapor
C. Burning coal in a furnace
D. Drying wood in a shed
Help ASAP
Answers
Answer:
C
Explanation:
a chemical change is when a substance changes into another.
wood burning turns into heat which would be considered a chemical change
The EPA has used the slogan “Ozone: good up high, bad nearby” in some of its publications for the general public. Explain the message
Answers
Answer:
------------------------------
Explanation:
---------------
Hydrogen H2
molecules are kept at 300.0K
in a
cubical container with a side length of 20.0cm.
Assume that you can treat the molecules as though they were moving in a one-dimensional box. (a) Find the ground state energy of the hydrogen molecule in the container. (b) Assume that the molecule has a thermal energy given by kBT/2
and find the corresponding quantum number n
of
the quantum state that would correspond to this thermal energy.
Answers
The corresponding quantum number n of the quantum state would correspond to this thermal energy.
n = 29.9E(1) = 3.17 x 10⁻¹⁸ JWhat is the ground state energy of the hydrogen molecule in the container.?Generally, To solve this problem, we can use the one-dimensional version of the Schrödinger equation to find the ground state energy of the hydrogen molecule in the container. The Schrödinger equation for a particle in a one-dimensional box is given by:
E(n) = (n^2 h²)/(8mL²)
where E(n) is the energy of the nth energy level, h is Planck's constant, m is the mass of the hydrogen molecule, and L is the length of the side of the container.
(a) To find the ground state energy, we need to find the energy of the n=1 energy level. Plugging in the values given in the problem, we find:
E(1) = (1² * (6.626 x 10^-34 J*s)²)/(8 * 1.67 x 10^-27 kg * (20 x 10⁻² m)²)
E(1) = 3.17 x 10^⁻¹⁸ J
This is the ground state energy of the hydrogen molecule in the container.
(b) To find the quantum number n that corresponds to a thermal energy of kBT/2, we need to rearrange the Schrödinger equation to solve for n:
n = √(8mL^2 * E(n)/h^2)
Plugging in the values given in the problem and solving for n, we find:
n = √(8 * 1.67 x 10⁻²⁷ kg * (20 x 10^-2 m)^2 * (1.38 x 10⁻²³ J/K * 300 K)/(6.626 x 10⁻³⁴ J*s)²)
n = 29.9
Thus, the quantum number n that corresponds to the thermal energy of kBT/2 is approximately 30. Note that the value of n will be a positive integer, so the actual value of n may be slightly different from this estimate.
Read more about ground-state energy
https://brainly.com/question/2289096
#SPJ1
An emission spectrum has a line in the blue region. How does this occur in the atom?
A. An electron ABSORBS a photon as it goes from a HIGHER TO LOWER energy level.
B. An electron EMITS a photon as it goes from a HIGHER TO LOWER energy level.
C. An electron EMITS a photon as it goes from a LOWER TO HIGHER energy level.
D. An electron ABSORBS a photon as it goes from a LOWER TO HIGHER energy level.

Answers
B. An electron EMITS a photon as it goes from a HIGHER TO LOWER energy level.
When an electron in an atom drops from a higher energy level to a lower energy level, it releases energy in the form of a photon.
What is Spectrum?
In science, the term "spectrum" is often used to describe the range of colors of visible light, known as the "visible spectrum," which includes all the colors of the rainbow. This spectrum is produced when white light is dispersed into its component colors by a prism or other means.
This energy is specific to the atom and its electron configuration, which is why each atom emits a unique set of wavelengths, creating a distinct emission spectrum.
Since the emission spectrum in the question has a line in the blue region, it means that the energy released by the electron corresponds to a specific frequency or wavelength in the blue region of the visible spectrum.
Learn more about Spectrum from given link
https://brainly.com/question/2261239
#SPJ1
a simply supported beam of diameter d, length l, and modulus of elasticity e is subjected to a fluid crossflow of velocity v, density , and viscosity . its center deflection is assumed to be a function of all these variables. (a) rewrite this proposed function in dimensionless form. (b) suppose it is known that is independent of , inversely proportional to e, and dependent only on v2 , not and v separately. simplify the dimensionless function accordingly. hint: take l, , and v as repeating variables
Answers
a) This proposed function in dimensionless form is
δ / L = f ( L / D , ρVD / μ , E / ρ V² )
b) δE / ρV²L = f( L / D)
a) dimension :
δ = f ( ρ , D , L , E , V , μ)
{L} {M/L³} {L} {L} {M/LT²} {L/T} {M/LT}
n is equal to 7 , j is equals to 3 ,
n - j = 4
b) suppose it is known that δ is independent of μ, inversely proportional to E, and dependent only on ρV² , ρ not and V are separately. the dimensionless function accordingly
δ ∝ 1 / E
δ / L = ρV² / E f ( L / D)
Thus, δE / ρV²L = f( L / D)
To learn more dimensionless here
https://brainly.com/question/14435908
#SPJ4
Can youguys helpplease its for marks and is due tommorow.

Answers
Explanation:
i believe this is right. my bad if its not

Determine the number of moles of air present in 1.35 L at 750 torr and 17.0°C. Which equation should you use? P equals StartFraction n R T over V EndFraction. n equals StartFraction R T over P V EndFraction. n equals StartFraction P V over R T EndFraction.
Answers
Answer:
0.056 moles air
Explanation:
P·V = n·R·T => n = P·V/R·T
P = 750 Torr = 750 mmHg = (750mm/760mm/atm) = 0.9868 Atm
V = 1.35 Liters
R = 0.08206 L·atm/mole·K
T = 17°C = (17 + 273) K = 290K
n = (0.9868atm)(1.35L)/(0.08206L·atm/mole·K)(290K) = 0.056 moles air
Answer:
first part is C
.056 moles
third part is B
Last is 932ml
Explanation:
What is the hydrogen ion concentration of a solution with a pH of 2.0?
Answers
Answer:
[H+] = 10^-2
Explanation:
pH = -log[H+]
2 = -log[H+]
[H+] = 10^-2
In a 0.2000 M solution of a monoprotic weak acid, [H+] = 9.86 × 10–4 M. What is the Ka for this acid?
Answers
To answer the question we need the equation to calculate Ka (the acid dissociation constant).
For any acid we have the following dissociation equilibrium:
\(HA\leftrightarrows H^++A^-\)The Ka equation is the following:
\(Ka=\frac{[H^+][{A^-}{]}^{{}{}{}}}{[HA]}\)So to answer the question we need the concentration of the acid, which is 0.2M, of the protones, which is 9.86x10-4M, and the cation [A+].
As this is s monoprotic acid, [H+] is the same as [A+], which is 9.86x10-4M.
Now we calculate:
\(Ka=\frac{[9.86x10^{-4}M][{9.86x10^{-4}M]}^{{}{}{}}}{[0.2M]}=4.86x10^{-6}M\)So the Ka for this acid is 4.86x10-6 M.
HQ5.40
Homework Answered Due Today, 11:59 PM
The reaction 3H₂(g) + N₂(g) → 2NH3(g) has an enthalpy of reaction of -92.6 kJ/mol. If 1 g of hydrogen and 2 g of nitrogen are
reacted, how much heat is produced (kJ)?
Answers
The amount of heat energy produced when 1 g of hydrogen and 2 g of nitrogen are reacted, is -6.61 KJ
How do i determine the heat energy produced?First, we shall obtain the limiting reactant. Details below:
3H₂ + N₂ -> 2NH₃
Molar mass of N₂ = 28 g/molMass of N₂ from the balanced equation = 1 × 28 = 28 g Molar mass of H₂ = 2 g/molMass of H₂ from the balanced equation = 3 × 2 = 6 gFrom the balanced equation above,
28 g of N₂ reacted with 6 g of H₂
Therefore,
2 g of N₂ will react with = (2 × 6) / 28 = 0.43 g of H₂
We can see that only 0.43 g of H₂ is needed in the reaction.
Thus, the limiting reactant is N₂
Finally, we the amount of heat energy produced. Details below:
3H₂ + N₂ -> 2NH₃ ΔH = -92.6 KJ
Molar mass of N₂ = 28 g/molMass of N₂ from the balanced equation = 1 × 28 = 28 gFrom the balanced equation above,
When 28 grams of N₂ reacted, -92.6 KJ of heat energy were produced.
Therefore,
When 2 grams of N₂ will react to produce = (2 × -92.6) / 28 = -6.61 KJ
Thus the heat energy produced from the reaction is -6.61 KJ
Learn more about heat energy:
https://brainly.com/question/31429264
#SPJ1
what best describes the bonding in the compound ICl?
Answers
Ice has a density of 0.92g/cm3. It will float in water.
Answers
The density of pure water is 1 g/cm^3.
Its density is 0.98 g cm 3 at room temperature, in comparison with the handiest zero.92 g cm 3 for ice, a reality that has to be defined through atomic, and molecular concepts. If ice has been no longer much less dense than water, it might sink, having a devastating impact on lake backside ecosystems. believe it or now not, ice is honestly about 9% much less dense than water. for the reason that water is heavier, it displaces the lighter ice, causing the ice to glide to the pinnacle.
The density of ice is about 90 percent that of water, but that could range because ice can contain air, too. meaning that about 10 percent of an ice cube or iceberg will be above the water line. The density of water is maximum at four∘C, and the density of the ice is much less than the water due to its susceptible intermolecular pressure of attraction. as the density of water is more, it's miles heavier than ice. therefore ice floats on the floor of the water. Ice continually floats due to the fact it's far less dense than everyday water. because frozen water molecules shape a crystal.
Learn more about A density here:-https://brainly.com/question/17780219
#SPJ9
For each of the reactions at constant pressure, determine whether the system does work on the surroundings, the surroundings does work on the system, or essentially no work is performed.
Work done by the system
Work done on the system
No work done
Answer Bank
NaHCO3(s)+HC2H3O2(aq)⟶NaC2H3O2(aq)+H2O(l)+CO2(g)
2H2O2(g)⟶O2(g)+2H2O(g)
N2(g)+3H2(g)⟶2NH3(g)
H2(g)+F2(g)⟶2HF(g)
Answers
The following are the result, which shows on which system work is done under pressure or not:
1. \(NaHCO_{3} (s) + HC_{2} H_{3} O_{2}(aq)\) ⟶ \(NaC_{2} H_{3} O_{2} (aq) + H_{2} O(l) + CO_{2} (g)\)
Answer: Work done by the system
2. \(2H_{2} O_{2} (g)\) ⟶ \(O_{2} (g)\) + \(2H_{2} O(g)\)
Answer: No work done
3. \(N_{2} (g)\) + \(3H_{2} (g)\) ⟶ \(2NH_{3} (g)\)
Answer: Work done on the system
4. \(H_{2} (g) + F_{2} (g)\)⟶ \(2HF(g)\)
Answer: No work done
1. \(NaHCO_{3} (s) + HC_{2} H_{3} O_{2}(aq)\) ⟶ \(NaC_{2} H_{3} O_{2} (aq) + H_{2} O(l) + CO_{2} (g)\)
An aqueous solution of \(NaC_{2} H_{3} O_{3}\), liquid water, and gaseous \(CO_{2}\) is created in this reaction between the solid \(NaHCO_{3}\) and the liquid \(HC_{2} H_{3} O_{2}\). As the reaction generates a gas, it will expand against the environment and alter it. Therefore, the system does work.
2. \(2H_{2} O_{2} (g)\) ⟶ \(O_{2} (g)\) + \(2H_{2} O(g)\)
In this process, hydrogen peroxide gas is broken down into oxygen gas and water vapour. As this is a gas-phase reaction, neither the system nor its surroundings will significantly expand nor contract. As a result, no work is done.
3. \(N_{2} (g)\) + \(3H_{2} (g)\) ⟶ \(2NH_{3} (g)\)
In this process, nitrogen gas and hydrogen gas are used to create ammonia gas. The reaction will cause the system's volume to drop since the reaction reduces the number of gas molecules from 4 to 2 in the first place. The environment will strive to compress the system. As a result, the system is under construction work done by a system.
4. \(H_{2} (g) + F_{2} (g)\)⟶ \(2HF(g)\)
In this process, fluorine gas and hydrogen gas are used to create hydrogen fluoride gas. This reaction does not involve any major expansion or compression of the system or its surroundings since the quantity of gas molecules does not change. As a result, no work is done.
For more questions on Exothermic reaction
https://brainly.com/question/2924714
#SPJ4
Determine the mass of water formed when 12.5 L NH 3 (at 298 K and 1.50 atm) is reacted with 18.9 L of O 2 (at 323 K and 1.1 atm).
4 NH 3( g) + 5 O 2( g) → 4 NO( g) + 6 H 2O( g)
Answers
16.93g of water formed when 12.5 LNH3 (at 298 K and 1.50 atm) is reacted with 18.9 L of O2 (at 323 K and 1.1 atm).
What is water in chemistry?The primary component of Earth's hydrosphere and the fluids in all known living things is water (H2O), an inorganic chemical that is transparent, tasteless, odorless, and almost colorless (in which it acts as a solvent).
Despite not providing food, energy, or organic micronutrients, water is necessary for all known forms of life. Each of its molecules has one oxygen and two hydrogen atoms, which are connected by covalent bonds, as indicated by the chemical formula H2O. At a 104.45° angle, the hydrogen atoms are joined to the oxygen atom.
We will use the idea gas equation to calculate the moles of NH3 and O2
that is Pv= n RT
where; P= pressure,
V= volume,
n = number of moles,
R=gas constant = 0.0821 l .atm/ mol.K
can also be written as
n = PV /RT
The moles of NH3
n= (1.50 atm x 12.5 L) /( 0.0821 L. atm /mol.k x 298 K) = 0.766 moles
The moles of O2
=(1.1 atm x 18.9 L) / ( 0.0821 L. atm/ mol.k x 323 K) = 0.784 moles
Now we will write the reaction between NH3 and O2
4 NH3 + 5 O2 → 4 No +6H2O
from equation above 0.766 moles of NH3 reacted to produce
0.766 x 6/4 =1.149 moles of H2O
0.784 moles of O2 reacted to produce
0.784 x 6/5 = 0.9408 moles of H20
As we can see O2 is totally consumed, O2 is the limiting reagent and therefore the moles of H2O produced = 0.9408 moles
Mass of H2O = moles x molar mass
Molar mass of H2O = (1 x2)+16= 18 g/mol
mass = 18 g/mol x 0.9408 moles
= 16.93 grams
Thusm 16.93g of water formed when 12.5 L NH 3 (at 298 K and 1.50 atm) is reacted with 18.9 L of O 2 (at 323 K and 1.1 atm).
Learn more about water
https://brainly.com/question/1313076
#SPJ9
Draw the structure of phosphatidylserine and discuss its components
Answers
Phosphatidylserine is a type of phospholipid that is mainly found in cell membranes. Its structure is made up of two fatty acid chains, a phosphate group, a serine molecule, and a glycerol molecule.
The fatty acid chains are hydrophobic, meaning they repel water, while the phosphate group and serine molecule are hydrophilic, meaning they attract water.
The glycerol molecule acts as a bridge that connects the two fatty acid chains to the phosphate group and serine molecule.
The structure of phosphatidylserine is important for its function in the cell membrane.
Because of the hydrophobic and hydrophilic components of its structure, phosphatidylserine is able to form a lipid bilayer, which is a barrier that separates the inside of the cell from the outside environment.
The hydrophilic heads of the phosphatidylserine molecules face outward and interact with water, while the hydrophobic tails face inward and repel water.
Phosphatidylserine also plays a role in cell signaling and apoptosis, which is programmed cell death.
It acts as a signaling molecule by binding to proteins that are involved in cellular pathways.
In addition, phosphatidylserine is translocated to the outer leaflet of the cell membrane during apoptosis, which signals to immune cells that the cell is ready to be removed.
In conclusion, the structure of phosphatidylserine is made up of two fatty acid chains, a phosphate group, a serine molecule, and a glycerol molecule. Its hydrophobic and hydrophilic components allow it to form a lipid bilayer in cell membranes, and it also plays a role in cell signaling and apoptosis.
For more such questions on Phosphatidylserine
https://brainly.com/question/16179573
#SPJ8
What did Aristotle believe?
A. That matter did not exist in the physical world
B. That the scientific method should be used to test ideas
C. That all matter was composed of earth, fire, water, and air
D. That all matter was composed of tiny atoms
Answers
Answer:
C.) That all matter was composed of earth, fire, water and air
Explanation:
Just took the test
Write a balanced chemical equation for the reaction of aqueous solutions of lead(II) nitrate and potassium iodide
to produce solid lead (II) iodi de and aqueous potassium nitrate.
Help pleaseee
Answers
Answer: Pb(NO3)2(aq) + 2KI(aq) —> PbI2(s) + 2KNO3(aq)
Explanation:
plz help me... my ppr is gonig on......
In first line of Lyman Series, wave number is
1 point
82.26 x 10^5
97.49 x 10^5
109.678 x 10^5
none
Answers
Answer:
geruow0irghvn3p0unhie0ghik
Explanation:
I don't really think what this question about

Answers
Answer:
A single time the hail stone was drafted up and down in the atmosphere
Explanation:
Using the molecular dipoles/polarity of H2O, NH3, and CH4 explain why does CH4 not mix with H2O ?
Answers
So
CH_4 doesn't mix with water instead it mixes up with non polar solvents like Benzene,Toluene
Which of the following is an intensive property of a sample of water?
A. Mass
B. Molar mass
C. Number of moles
D. Volume
Answers
Molar mass is an intensive property of a sample of water.
The molar mass of a certain chemical entity is its mass per unit quantity (symbol M, SI unit kgmol1). Always identify the chemical in question in accordance with the definition of the mole. A substance's molar mass is the volume of the material that contains one mole of the substance. Its basic definition is the weight of a single mole of a substance. The atomic mass of each substance is multiplied by the subscript of that element in the chemical formula to determine the molar mass.
One mole of a specific compound or material has a mass of 1 molar. We are aware that the number of moles in a compound (n) can be expressed as the ratio of the compound's given mass to its molar mass. This is measured in g mol1, the standard unit. However, the SI unit, kg mol 1, is quite uncommon. The amount of particles in 1 mole of the substance is equal to the number of atoms present in 12g of the 12C isotope.
Learn more about molar mass here:
https://brainly.com/question/837939
#SPJ1
12. How many grams are equal to 3.46 moles of sodium sulfide, Na2S? with work please
Answers
Answer:
\(\boxed {\boxed {\sf About \ 270 \ grams \ of \ Na_2 S}}\)
Explanation:
To convert from moles to grams, the molar mass must be used.
1. Find Molar Mass
The compound is sodium sulfide: Na₂S
First, find the molar masses of the individual elements in the compound: sodium (Na) and sulfur (S).
Na: 22.9897693 g/mol S: 32.07 g/molThere are 2 atoms of sodium, denoted by the subscript after Na. Multiply the molar mass of sodium by 2 and add sulfur's molar mass.
Na₂S: 2(22.9897693 g/mol)+(32.07 g/mol)=78.0495386 g/molThis number tells us the grams of sodium sulfide in 1 mole.
2. Calculate Grams
Use the number as a ratio.
\(\frac{78.0495386 \ g \ Na_2S}{1 \ mol \ Na_2S}\)
Multiply by the given number of moles, 3.46.
\(3.46 \ mol \ Na_2S *\frac{78.0495386 \ g \ Na_2S}{1 \ mol \ Na_2S}\)
The moles of sodium sulfide will cancel.
\(3.46 *\frac{78.0495386 \ g \ Na_2S}{1 }\)
\(3.46 *{78.0495386 \ g \ Na_2S}\)
\(270.051404 \ g \ Na_2 S\)
3. Round
The original measurement of grams, 3.46, has 3 significant figures. We must round our answer to 3 sig figs.
For the answer we calculated, that is the ones place.
The 0 in the tenth place tells us to leave the 0 in the ones place.
\(\approx 270 \ g \ Na_2 S\)
There are about 270 grams of sodium sulfide in 3.46 moles.
Answer:
Mass = 269.8 g
Explanation:
Given data:
Number of moles of Na₂S = 3.46 mol
Mass of Na₂S = ?
Solution:
Formula:
Mass = number of moles × molar mass
Molar mass of Na₂S = 78.0 g/mol
Mass = 3.46 mol × 78.0 g/mol
Mass = 269.8 g
When 3.0 kg of water is warmed from 10 °C to 80 °C, how much heat energy is needed?
Answers
Answer:
THE HEAT NEEDED TO CHANGE 3KG OF WATER FROM 10 C TO 80 C IS 877.8kJ OR 877,800 J.
Explanation:
Mass = 3.0 kg = 3 * 1000 = 3000 g
Initial temperature = 10 C
Final temperature = 80 C
Change in temperature = 80 - 10 = 70 C
Specific heat of water = 4.18 J/g C
Heat needed = unknown
Heat is the amount of energy in joules needed to change a gram of water by 1 C.
Heat = mass * specific heat * change in temperature
Heat = 3000 g * 4.18 J/g C * 70 C
Heat = 877 800 Joules
Heat = 877.8 kJ.
The heat needed to change 3 kg mass of water from 10 C to 80 C is 877,800 J or 877.8 kJ.
How many grams of CO2 is released when 35 grams of C5H12 is burnt in excess oxygen.
Answers
Answer:
The reaction will give off 140 kJ of heat.
Explanation: