Draw one enantiomer of the major product of the diels–alder reaction shown. Use wedged and hatched bonds to indicate the orientation of substituents in the product. Chirality centers must contain four bonds in order to be graded correctly. Hydrogen atoms on chirality centers must be shown.
Answers
The chirality centers have four bonds as shown. Hatched bonds point into the plane of the paper, and wedged bonds point out of the plane of the paper. Enantiomer of the Major Product is shown in the below figure:
[image]
The hydrogen atoms are displayed on the chirality centers. The resulting product is chiral because it has a stereogenic center. Diels-Alder reaction is a cycloaddition reaction between a diene and a dienophile that creates a cyclic product with a new double bond. The reaction between cyclopentadiene and maleic anhydride to form cis-Norbornene-5,6-endo-dicarboxylic anhydride is an example of a Diels-Alder reaction. The cis-Norbornene-5,6-endo-dicarboxylic anhydride is an unsymmetrical molecule, thus it is chiral. The presence of chirality centers makes it challenging to determine the absolute configuration of the enantiomer.
The major product of the Diels–Alder reaction, cis-Norbornene-5,6-endo-dicarboxylic anhydride, has a single stereogenic center, making it chiral. The enantiomers may be obtained by simple reflection.
To know more about Enantiomer visit:
brainly.com/question/30035010
#SPJ11
Related Questions
Calculate the mass of ammonium sulfide (NH4)2S in 3.00 L of a 0.0200 M solution.
Answers
The mass of (NH4) 2S in the solution is : Mass = 0.0600 mol × 60.08 g/mol = 3.60 g.
The given molarity and volume of the solution can be used to calculate the number of moles of ammonium sulfide (NH4)2S.Then, the number of moles can be converted to mass using the molar mass of (NH4)2S.Mass of ammonium sulfide (NH4)2S in 3.00 L of a 0.0200 M solution is given by : Mass = moles × molar mass.The number of moles of (NH4)2S can be found using the equation:Molarity = Number of moles / Volume.Rearranging this equation, we get:Number of moles = Molarity × Volume Number of moles of (NH4)2S = 0.0200 M × 3.00 L.Number of moles of (NH4)2S = 0.0600 mol.The molar mass of (NH4)2S can be calculated by summing the molar masses of ammonium (NH4) and sulfide (S) ions.Molar mass of (NH4)2S = (2 × Molar mass of NH4) + Molar mass of S= (2 × 14.01 g/mol) + 32.06 g/mol= 60.08 g/mol.
For more question on mass
https://brainly.com/question/1838164
#SPJ8
Which element has a 2p sublevel that contains 4 electrons?
-Praseodymium
-Oxygen
-Chromium
-Sulfur
Answers
Answer: Oxygen
Explanation: Oxygen has electron configuration 1s²2s²2p^4.
Other element have 2p^6
Fill in each blank with the word that best completes the statement

Answers
2) hypothesis
3) experiment
Hope this helped! :>
Which equation correctly describes the dissociation of calcium nitrate into ions in an aqueous media?
Answers
The dissociation of calcium nitrate in aqueous media leads to the formation of calcium ions and nitrate ions, which play significant roles in various chemical and biological processes.
In aqueous media, calcium nitrate (Ca(NO3)2) dissociates into ions through a process known as ionization or dissociation. The correct equation that represents this dissociation is:
Ca(NO3)2 → Ca^2+ + 2NO3^-
In this equation, the solid calcium nitrate (Ca(NO3)2) breaks apart in water to form calcium ions (Ca^2+) and two nitrate ions (2NO3^-). The (aq) notation represents the aqueous state of the ions, indicating their presence in a water solution.
The dissociation of calcium nitrate occurs because water molecules surround the ions and exert electrostatic forces, causing the compound to separate into its constituent ions. Calcium cations (Ca^2+) are formed when water molecules interact with calcium atoms, attracting away two valence electrons. Nitrate anions (NO3^-) result from the interaction between water molecules and the nitrate group.
It's important to note that the dissociation of calcium nitrate is complete in aqueous solutions, meaning that all molecules of calcium nitrate separate into individual ions. This complete dissociation is due to the high solubility of calcium nitrate in water.
The resulting calcium ions and nitrate ions are then free to participate in various chemical reactions or interact with other ions present in the solution. Calcium ions are involved in processes such as precipitation, complexation, and biological functions, while nitrate ions are essential for plant nutrition and can partake in redox reactions.
Overall, the dissociation of calcium nitrate in aqueous media leads to the formation of calcium ions and nitrate ions, which play significant roles in various chemical and biological processes.
learn more about calcium nitrate here
https://brainly.com/question/5346392
#SPJ11
Ethers have the same number and kinds of atoms as alcohols. However, they have very different physical properties and physiological effects. In the left box below, draw the structural formula for ethyl alcohol. Then construct a model. Next, take the model apart and rearrange it in the only other possible configuration to create dimethyl ether. Lastly, draw the structural formula for dimethyl ether in the right box below. Ethyl alcohol Dimethyl ether _________ ________Which three atoms in what particular arrangement do you think are specific to ethers?
Answers
The structural formula of ethyl alcohol can be represented as \(CH_3CH_2OH\). The resulting structural formula for dimethyl ether can be represented as \(CH_3OCH_3\). The three atoms in dimethyl ether that are specific to ethers are the two carbon atoms and the oxygen atom that form the ether linkage (-O-).
Ethers and alcohols are two types of organic compounds that contain the same number and kinds of atoms. However, despite their similar chemical compositions, they have different physical and physiological properties.
Ethyl alcohol, also known as ethanol, is a common type of alcohol that contains two carbon atoms, six hydrogen atoms, and one oxygen atom. Its structural formula can be represented as \(CH_3CH_2OH\). Ethanol is a colorless, volatile liquid that has a distinctive odor and is used as a solvent, fuel, and recreational drug.
To construct a model of ethanol, we would need to assemble two carbon atoms, six hydrogen atoms, and one oxygen atom in a specific arrangement. The carbon atoms should be bonded together by a single covalent bond, and each carbon atom should be bonded to three hydrogen atoms and one oxygen atom. The oxygen atom should be bonded to one carbon atom and one hydrogen atom. The resulting model should resemble a T-shape with the oxygen atom at the top of the T and the carbon atoms and hydrogen atoms forming the stem of the T.
Dimethyl ether, on the other hand, is an ether that has the same number and types of atoms as ethanol but a different arrangement. To create dimethyl ether from ethanol, we would need to take apart the ethanol model and rearrange its atoms in the only other possible configuration. In this case, we would need to remove the hydroxyl (-OH) group from the ethanol molecule and replace it with a methyl (\(-CH_3\)) group. We would need to do this for both carbon atoms in the ethanol molecule to create dimethyl ether.
The resulting structural formula for dimethyl ether can be represented as \(CH_3OCH_3\). This compound has the same number and types of atoms as ethanol, but it is an ether instead of an alcohol. Ethers have different physical and physiological properties than alcohols because they have a different arrangement of atoms. Specifically, the three atoms in dimethyl ether that are specific to ethers are the two carbon atoms and the oxygen atom that form the ether linkage (-O-). This linkage gives ethers their characteristic physical and chemical properties, which are different from those of alcohols.
For more such questions on Ethers and alcohols.
https://brainly.com/question/30434200#
#SPJ11
what is the mass in grams of 0.250 mol of the common antacid calcium carbonate?
Answers
Answer:That's 25.8 g of calcium carbonate In 100 g of the sample.
Explanation:
brainliest pls
Which statements are true concerning elements in the same group of the periodic table? Select all that apply.
A. They have similar periodic properties.
B. They are all metals or nonmetals, but not both.
C. They are either all solids or all liquids or all gases.
D. They have the same number of shells of electrons.
E. They have the same number of inner core electrons.
F. They have the same outer shell electron configuration.
Answers
The statements A and F are true concerning elements in the same group of the periodic table.
To select all that apply, we need to evaluate each statement.
A. They have similar periodic properties.
This statement is true. All the elements in the same group will have similar chemical and physical properties due to the electron configuration of their outer shell. For example, the alkali metals group (Li, Na, K, Rb, Cs, and Fr), has a valence electron configuration of s¹ (in the outer shell), which gives them the tendency to react vigorously with water, as well as other properties.
B. They are all metals or nonmetals, but not both.
This is false. If we take a look at the p-block of the periodic table, we can see that the groups in this block are conformed by nonmetals, metals, and metalloids. For example, the icosagens group is formed by metalloids (B) and metals (Al, Ga, In, Tl).
C. They are either all solids or all liquids or all gases.
This is false. In some groups, all the elements are solids (alkaline earth metals) or gases (group of noble gases), but in others, the groups are conformed by gases with solids (pnictogens group) or by gases with liquids (halogens group).
D. They have the same number of shells of electrons.
This is false. In a group, the number of shells increases from top to bottom in the periodic table. For example, the electron configuration of the elements in the alkali metals is:
H: 1s¹ Li: [He]2s¹Na: [Ne]3s¹K: [Ar]4s¹Rb: [Kr]5s¹Cs: [Xe]6s¹Fr: [Rn]7s¹We can see that hydrogen has 1 shell and Cs has 6 shells.
E. They have the same number of inner core electrons.
This is false. As we said at point D, the number of shells increases from top to bottom in a group, so the number of inner core electrons also increases in this order. For example, in the alkaline earth metals group, the electron configuration of the elements is:
Be: [He]2s²Mg: [Ne]3s²Ca: [Ar]4s² Sr: [Kr]5s²Ba: [Xe]6s²Ra: [Rn]7s²As we can see, the number of inner shells increases from Be ([He]) to Ra ([Rn]).
F. They have the same outer shell electron configuration.
This is true. As we said at point A, the elements in the same group will have the same electron configuration of the outer shell (valence electron configuration). At points D and E, we can see that the valence electron configuration is the same for all the elements in the groups.
Therefore, statements A and F are true.
You can find more about the periodic table here: https://brainly.com/question/4287157?referrer=searchResults
I hope it helps you!
What is the uneven distribution of charge on a water molecule called ?
Answers
Answer:
polar molecule
Explanation:
Answer:
Polar molecule
Explanation:
It is called a polar molecule which results in the uneven distribution of charge.
Water is polar because the electronegativities of oxygen and hydrogen are different.
Electronegativity is the ability of an atom to attract a bonding pair of electrons in a covalent bond. As the number of protons in the nucleus increases, the electronegativity or attraction increases. Oxygen has 8 protons, while hydrogen has 1 proton, hence oxygen is more electronegative than hydrogen.
This gives oxygen a slightly negative charge, which pulls the hydrogen electrons towards the oxygen. Hydrogen has a slightly positive charge as it has a lower electronegativity value, so is less able to attract the electrons from oxygen.
This uneven distribution of charges brings about polarity in water.
A large difference in electronegativity makes a compound polar, which you can also tell from using a Pauling scale (a measure of electronegativity).
For more on polar molecules:
https://brainly.com/question/20337982?referrer=searchResults
Find the amount of negative charge in one mole of oxygen gas, O2.
Answers
Since there are 6.022 1023 molecules in 1 mole of oxygen (O2), it has a mass of 32 grams.
One mole of oxygen gas is equal to how many molecules of oxygen gas?One mole of oxygen gas, with the formula O2, weighs 32 g and includes 6.02 x 1023 molecules of oxygen, but also contains 12.04 x 1023 (2 x 6.02 x 1023) atoms of oxygen, since each molecule of oxygen contains two oxygen atoms.Since there are 6.022 1023 molecules in 1 mole of oxygen (O2), it has a mass of 32 grams.One mole of oxygen weighs 15.998 grams, whereas one mole of hydrogen weighs 1.008 grams.Since there are 6.022 1023 molecules in 1 mole of oxygen (O2), it has a mass of 32 grams.To learn more about oxygen refer to:
https://brainly.com/question/26073928
#SPJ4
The amount of negative charge in one mole of oxygen gas, \(O_{2}\) is 9.647 x \(10^{14}\) coulomb.
One mole of oxygen gas is equal to how many molecules of oxygen gas?One mole of oxygen gas, with the formula O2, weighs 32 g and includes 6.02 x \(10^{23}\) molecules of oxygen, but also contains 12.04 x \(10^{23}\) (2 x 6.02 x \(10^{23}\)) atoms of oxygen, since each molecule of oxygen contains two oxygen atoms.
Since there are 6.022 1023 molecules in 1 mole of oxygen (\(O_{2}\)), it has a mass of 32 grams. One mole of oxygen weighs 15.998 grams, whereas one mole of hydrogen weighs 1.008 grams. Since there are 6.022 1023 molecules in 1 mole of oxygen (\(O_{2}\)), it has a mass of 32 grams.
Determination of negative charge:Mass of 1 mole of electrons:
9.10 x \(10^{13}\) × 6.023× \(10^{23}\) 5.486× \(10^{-7}\) kg
Charge of 1 mole of electrons:
1.602× \(10^{-19}\) × 6.023× \(10^{23}\) = 9.647× \(10^{4}\) coulomb
To learn more about oxygen refer to:
brainly.com/question/26073928
#SPJ4
Hi i need help please. I have to list the following forms of lights from lowest energy to highest energy:
red lights, violet lights, gamma rays, microwaves, infrared light, and radio waves. Thank you so much!
Answers
Answer:
radio waves, microwaves, infrared light, red lights, violet light, gamma rays
Given this equation: N2 + 3 H2 → 2 NH3, how many moles of NH3 can be produced from 3.1 moles of H2?
Answers
First, we write down our reaction:
N2 + 3H2 → 2NH3
Don't forget to balance it.
We only use moles as units.
Procedure:
3 x 1 mole H2 ------------ 2 x 1 mole NH3
3.1 moles H2 ------------- x
x = 2.1 moles NH3 are produced
Answer: 2.1 moles NH3
an unstable nucleus exhibits radioactivity; i.e., it spontaneously disintegrates or by emitting particles and/or electromagnetic .
Answers
An unstable nucleus undergoes radioactivity, which refers to the spontaneous disintegration of the nucleus or the emission of particles and/or electromagnetic radiation. Radioactivity is a natural process that occurs in certain atomic nuclei that are inherently unstable. These nuclei strive to attain stability by undergoing radioactive decay, which can involve the emission of alpha particles, beta particles, gamma rays, or a combination thereof.
Unstable nuclei contain an imbalance in the number of protons and neutrons, making them energetically unstable. In order to reach a more stable configuration, these nuclei undergo radioactive decay. This process involves the spontaneous transformation of the nucleus, where it disintegrates or emits particles and/or electromagnetic radiation.
There are several types of radioactive decay. One type is alpha decay, where an alpha particle consisting of two protons and two neutrons is emitted from the nucleus. Another type is beta decay, which can occur in two forms: beta-minus (β-) decay and beta-plus (β+) decay. In beta-minus decay, a neutron within the nucleus is converted into a proton, and an electron (beta particle) is emitted. In beta-plus decay, a proton in the nucleus is converted into a neutron, and a positron (positive beta particle) is emitted.
Additionally, radioactive decay can also involve the emission of gamma rays. Gamma rays are high-energy photons that are released during the transition of the nucleus to a lower energy state. These rays are a form of electromagnetic radiation.
Overall, the radioactivity of an unstable nucleus manifests through the spontaneous disintegration of the nucleus or the emission of particles (alpha and beta particles) and/or electromagnetic radiation (gamma rays). The specific type of radioactive decay that occurs depends on the characteristics and composition of the unstable nucleus.
To learn more about electromagnetic radiation visit: brainly.com/question/29646884
#SPJ11
Which property do transition metals have in common?
Answers
Explanation: google (:
transition metals are good conductors of heat and electricity and it can hammered into shape easily and have high melting points.
what is transition metal?Transition metal are the chemical elements which have valence electron and it can participate in chemical bonds formation process in two shells.
The position of the transition metals in periodic table is the middle portions of the long periods between the groups on the left-hand side and the groups on the right.
Most of the transition metals are hard, strong, and lustrous and it has high melting and boiling points, good conductors of heat and electricity.
Some of the elements are important technologically are titanium, iron, nickel, and copper as they are used in electrical technology.
Other transition metals are used in useful alloys, with one another and with other metallic elements, dissolve in mineral acids, platinum, silver, and gold are some Nobel transition metal.
Learn more about transition metals, here:
https://brainly.com/question/12843347
#SPJ2
Matter can be altered in many different ways for example what are all the ways you can change a piece of binder paper
Answers
There are several ways through which a piece of binder paper can be altered. Some of these ways include crumpling, tearing, burning, cutting, and soaking reaction in water.
Crumpling: Folding, twisting, and crumpling a piece of binder paper will alter its appearance. By crumpling a piece of paper, the paper fibers will align differently, and the paper will become denser and more rigid.Tearing: When paper is torn, it is physically damaged, which alters its appearance. The paper will develop a rougher edge or a jagged edge, depending on the direction of the tear.
Burning: Burning paper alters the chemical composition of the paper. When paper is burned, the cellulose fibers that make up the paper break down and release gases such as water vapor and carbon dioxide.Cutting: Cutting paper alters its shape, but the fibers in the paper remain mostly intact. The paper will have a clean, straight edge where it was cut.Soaking in water: When paper is soaked in water, the fibers in the paper absorb the water and swell. The paper will become soft and mushy and can easily be torn or destroyed.
To know more about reaction visit:
https://brainly.com/question/30464598
#SPJ11
Physical and chemical properties
Answers
Answer:
Chemical properties
-It is not reverseable
-Change in energy is seen
-It is permanent change
-The change take place in both physical and chemical properties of matter
Physical properties
-The change takes place only in physical property of matter
-It can be reversed back
-It is temporary change
-Change in energy is not seen
Vinegar, which contains acetic acid, is used in foods and has few safety concerns. Hydrochloric acid is used in chemistry labs and requires the use of safety goggles and gloves. Why do the safety concerns for these two acids differ? 2 ... Acetic acid is a weak acid, and hydrochloric acid is a strong acid.
Answers
Answer:
acid
Explanation:
pls answer question will mark brainliset tyty

Answers
In these given options, flooding is most likely to reduced human population.
Humans are affected by floods in a variety of ways, including: flooding can cause injury or death to individuals. Sewage is frequently present in floodwater, which can compromise clean drinking water and cause disease. Power supply may become unstable.Environmental stress is a term that refers to a variety of circumstances, such as food shortages, predator activity, and other density-dependent variables, that cause population growth to slow down.Therefore, the infrastructure in our environment and the ecosystem itself may be harmed by severe flooding. They can first demolish houses and render them inhabitable. Additionally, they can clear farms of sand, which makes it harder to cultivate crops. In addition to the aforementioned, flooding taints clean water, resulting in illnesses.
Limiting variables prevent populations from growing either through increased death rates or emigration, decreased birthrates or immigration, or a combination of these factors.
To know more about flooding
https://brainly.com/question/5402454
#SPJ1
What measuring tool is used to find the volume of a shoe box?
A.water displacement
B.scale
C.beaker
D.ruler
Answers
Answer:
D. Ruler
Explanation:
Use the formula to find the volume of a cuboid by measuring the length, width, and height and multiplying all of them by each other.
How could you find the number of particles in 4 moles of a substance ?
Answers
Answer:
below
Explanation:
Calculating the number of particles
1. The number of particles in a substance can be calculated using:
2. Number of particles = Avogadro constant × the amount of substance in mol.
3. Calculate the number of water molecules in 0.5 mol of water.
4. Number of water molecules = Avogadro constant x amount of substance in mol.
Watch the movie to learn about the three types of heat transfer. As you watch, record any observations that help you answer the Guiding Question
Answers
Heat transfer is the process of thermal energy moving from one object to another due to a temperature difference. There are three types of heat transfer: conduction, convection, and radiation.
Conduction is the transfer of heat through direct contact between two objects, such as a pot on a stove or a person touching a hot surface. A common application of conduction is in cooking, where heat is transferred from a stove or oven to the food being cooked.
Convection is the transfer of heat through the movement of fluids, such as air or water. A common application of convection is in heating and cooling systems, where warm or cool air is circulated through a building to regulate its temperature.
Radiation is the transfer of heat through electromagnetic waves, such as heat radiating from a fire or the sun. A common application of radiation is in solar panels, which convert sunlight into usable energy.
For such more question Heat transfer
https://brainly.com/question/16055406
#SPJ4
Note: The correct question would be as bellow,
What is heat transfer and what are the 3 types of heat transfer Support your answer with applications for each type?
Which of the following is true? Question 1 options:
(A) A person's weight on the moon is less than on Earth, due to the force of gravity.
(B) A person's weight on the moon is the more than on Earth, due to the force of gravity.
(C) A person's weight on the moon is the same as on Earth, due to the force of gravity.
(D) A person's mass on the moon is more than on Earth, due to the force of gravity.
Answers
Among the given options, the option A which is A person's weight on the moon is the less than on the Earth, due to the force of gravity, is true.
The weight of a person is usually one-sixth less than their Earth's weight. Only the value of weight fluctuates from Earth to the moon, whereas the mass stays the same on both earth and moon.
This happens due to the gravitational force of the moon. Moon is smaller than the Earth, therefore it has low gravitational forces. Moon's gravitational force leads to the difference in the weights on the Earth and the moon.
The moon of Earth has comparitively less amount of mass than Earth. It is not only small in size than but it also is not as dense as earth. It is only 60 % dense which leads to lower gravitational pull, hence leading to difference in the weight.
To learn more about earth and moon,
brainly.com/question/1415075
#SPJ1
acetic acid has a boiling point of 118oc. what is the boiling point of acetic acid in k?
Answers
acetic acid has a boiling point of 118oc. 391 K (118+273 = 391) is the boiling point of acetic acid in k.
Acetic acid, which has the molecular formula \(CH_{3} COOH\), is also referred to as ethanoic acid, ethylic acid, vinegar acid, and methane carboxylic acid. As a byproduct of fermentation, acetic acid lends vinegar its distinctive smell. About 4-6% of the acetic acid in vinegar is water. In laboratories, more concentrated concentrations are often used, and glacial acetic acid is pure acetic acid with very little water present.
The 33rd most frequently manufactured chemical in the US is acetic acid. Acetic anhydride, cellulose acetate, vinyl acetate monomer, acetic esters, chloracetic acid, plastics, dyes, pesticides, photographic chemicals, and rubber are all products made with acetic acid. Other industrial applications include the production of organic chemicals, vitamins, antibiotics, hormones, and dietary additives. Acetic acid typically has amounts of 700 to 1,200 milligrams per kilogram (mg/kg) in wines, up to 860 mg/kg in aged cheeses, and 2.8 mg/kg in fresh orange juice when it is present naturally in food.
To know more about acetic acid:
https://brainly.com/question/15202177
#SPJ4
Please show how you solved :)
What is oxygen solubility at 10m depth below sea level, 25 deg
C, 30 g/L salinity?
Answers
The solubility of oxygen at 10m depth below sea level, 25 degrees Celsius, and 30 g/L salinity is approximately 6.59 mg/L.
To calculate the solubility of oxygen at a specific depth below sea level, temperature, and salinity, we can refer to the oxygen solubility tables. The solubility of oxygen can vary depending on these factors.
1. Begin by identifying the given parameters:
- Depth: 10m below sea level
- Temperature: 25 degrees Celsius
- Salinity: 30 g/L
2. Use the given parameters to locate the corresponding values in the oxygen solubility table.
3. The solubility of oxygen at a depth of 10m below sea level, 25 degrees Celsius, and 30 g/L salinity is typically around 6.59 mg/L.
Therefore, the solubility of oxygen at 10m depth below sea level, 25 degrees Celsius, and 30 g/L salinity is approximately 6.59 mg/L.
Learn more about solubility from this link:
https://brainly.com/question/9098308
#SPJ11
The oxygen solubility at 10m depth below sea level, 25°C, and 30 g/L salinity is approximately 1538 mol/L.
To calculate the oxygen solubility at a specific depth below sea level, temperature, and salinity, we can use the solubility formula.
The solubility of a gas decreases with increasing temperature and salinity, and increases with increasing pressure.
Here's how you can calculate the oxygen solubility at 10m depth below sea level, 25°C, and 30 g/L salinity:
1. Determine the pressure at 10m depth below sea level: -
The pressure at sea level is approximately 1 atmosphere (atm).
The pressure increases by approximately 1 atm for every 10 meters of depth.
Therefore, at 10m depth, the pressure is approximately 2 atm.
2. Convert the temperature to Kelvin: -
To convert from Celsius to Kelvin, add 273 to the temperature.
25°C + 273 = 298 K.
3. Use the solubility formula:
The solubility of oxygen in water can be calculated using Henry's law:
S = k * P * C.
S is the solubility of oxygen in moles per liter (mol/L).
k is the Henry's law constant for oxygen in water at a specific temperature and salinity.
P is the partial pressure of oxygen in atmospheres (atm).
C is the concentration of oxygen in moles per liter (mol/L).
4. Look up the Henry's law constant for oxygen at 25°C and 30 g/L salinity:
The Henry's law constant for oxygen at 25°C and 30 g/L salinity is approximately 769 L*atm/mol.
5. Calculate the solubility:
S = (769 L*atm/mol) * (2 atm) * (1 mol/L). - S ≈ 1538 mol/L.
Therefore, the oxygen solubility at 10m depth below sea level, 25°C, and 30 g/L salinity is approximately 1538 mol/L.
Learn more about solubility from this link:
brainly.com/question/9098308
#SPJ11
Which flask has the highest
pressure, assuming they all contain
1 mole He at 298K?
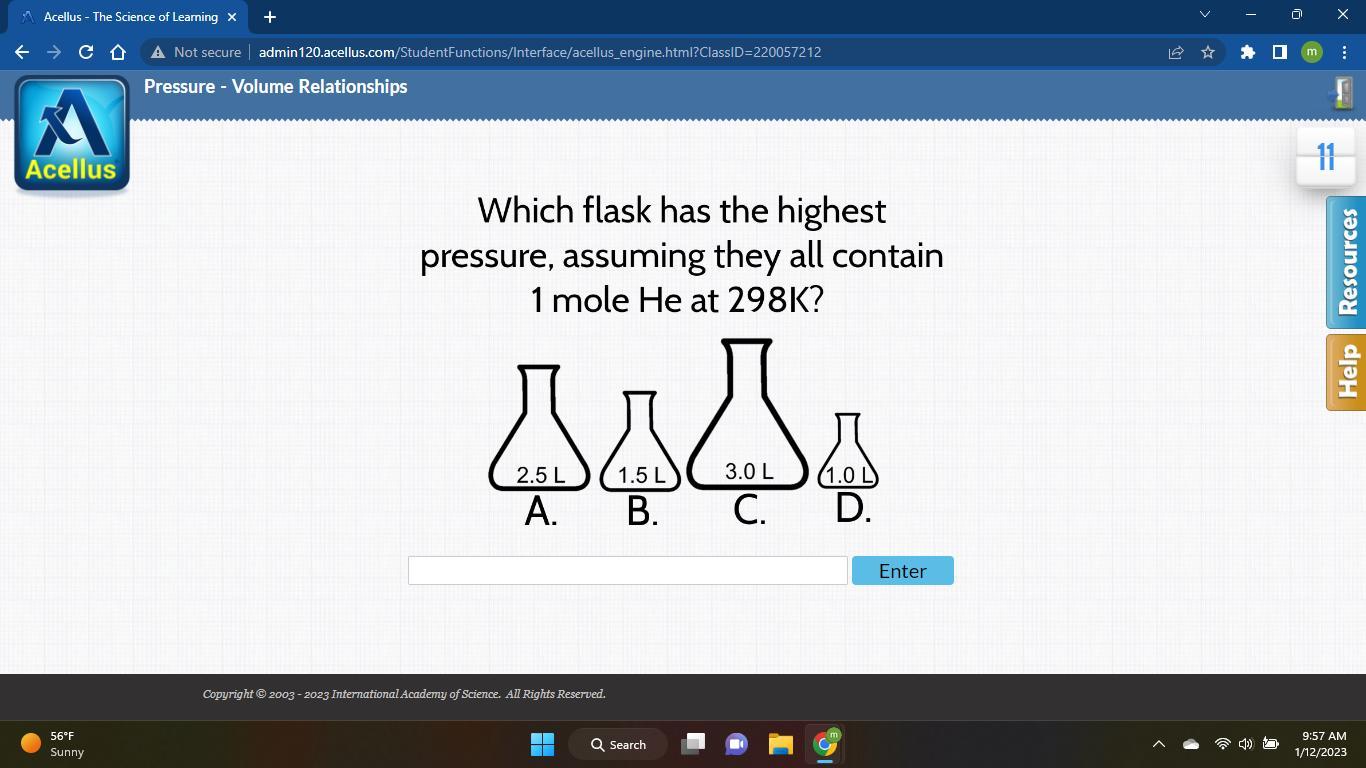
Answers
They all have the same amount of helium inside, so the flask with the smallest size has the most pressure.
Answer: d 1.0l
Explanation:
is 3NaOH + H3PO4--->Na3PO4 + 3H2O balanced?
Answers
Answer:
Yes
Explanation:
3NaOH + H3PO4
3Na + 3O + 3H + 3H + P + 4O
3Na + 7O + 6H + P
Na3PO4 + 3H2O
3Na + P + 4O + 6H + 3O
3Na + 7O + 6H + P
Computer architecture. Can you
please answer all the question. True or false, Thank you
1. Indicate whether the following statements are true of false. ( ) In the same OSM, all pages must have the same size. ( ) In the same OSM, all segments must have the same size. ) Internal fragmentat
Answers
a. False. In the same OSM (Operating System Memory), it is not necessary for all pages to have the same size. Different pages can have varying sizes depending on the memory allocation requirements of the system.
b. False. In the same OSM, all segments do not need to have the same size.
Segments can have different sizes based on the specific needs of the programs or processes running in the system.
Segmentation allows for flexible memory allocation by dividing the memory into logical segments.
c. True. Internal fragmentation occurs when memory is divided into smaller areas to accommodate smaller data blocks, but the remaining memory within each block is too small to be utilized for another block.
As a result, these small unused portions of memory remain empty, leading to inefficient memory utilization.
This is a disadvantage of the paging architecture employed in a computer's memory management system. It can result in wasted memory space and lower overall system performance.
Read more about Operating System Memory
https://brainly.com/question/30593994
#SPJ11
A circuit is set up with two parallel resistors, each of a resistance of 250Ω.
b. If another resistor of resistance 300Ω is added in series with these two parallel resistors, what is the total
resistance?
c. If a voltage of 120V is put across the circuit in b, what will the current be in the circuit?
Answers
Answer:
425 and 0.28A
Explanation:
Resistance for resistors in parallel
1/ R = 1/250 +1/250
=0.008
R = 1/ 0.008 = 125
Total resistance
R= 125+ 300
=425
...
V= IR
I= V/R
I = 120/425
= 0.28 A
Use your knowledge of atoms, bonding, and the periodic table to complete the chemical equation .”CH4 + O2
and predict the products of the chemical reaction on your paper. Label this equation “Initial Prediction.
Answers
Answer:
CH4 + O2 = CO2 + H2O.
Answer:
CH4 + O2 = CO2 + H2O.
Explanation:
n alloy used in an artificial hip contains 17 g of Ni, 23 g of Cr, and 40 g of O. Calculate the mole fractions and mass fractions of each element in the alloy. Also, calculate the average molecular weight of the a
Answers
The mole fractions and mass fractions of each element in the alloy are: Mole fractions: Ni = 0.0896, Cr = 0.1366, O = 0.7738 Mass fractions: Ni = 21.25%, Cr = 28.75%, O = 50% Average molecular weight: 20.8 g/mol.
To calculate the mole fractions and mass fractions of each element in the alloy, we need to first determine the total number of moles of each element:
moles of Ni = 17 g / 58.69 g/mol = 0.290 mol
moles of Cr = 23 g / 51.99 g/mol = 0.442 mol
moles of O = 40 g / 15.99 g/mol = 2.501 mol
The total number of moles in the alloy is then:
total moles = 0.290 mol + 0.442 mol + 2.501 mol = 3.233 mol
The mole fractions of each element are then:
mole fraction of Ni = 0.290 mol / 3.233 mol = 0.0896
mole fraction of Cr = 0.442 mol / 3.233 mol = 0.1366
mole fraction of O = 2.501 mol / 3.233 mol = 0.7738
The mass fractions of each element can be calculated as follows:
mass fraction of Ni = (17 g / 80 g) x 100% = 21.25%
mass fraction of Cr = (23 g / 80 g) x 100% = 28.75%
mass fraction of O = (40 g / 80 g) x 100% = 50%
The average molecular weight of the alloy can be calculated using the formula:
average molecular weight = (mass of Ni + mass of Cr + mass of O) / total moles
The mass of each element can be calculated as follows:
mass of Ni = 0.290 mol x 58.69 g/mol = 17.0 g
mass of Cr = 0.442 mol x 51.99 g/mol = 23.0 g
mass of O = 2.501 mol x 15.99 g/mol = 40.0 g
Substituting these values into the formula, we get:
average molecular weight = (17.0 g + 23.0 g + 40.0 g) / 3.233 mol
average molecular weight = 20.8 g/mol.
Learn more about mole fraction here:
https://brainly.com/question/14498215
#SPJ11
What reaction is used to remove one phosphate group from ATP?A. hydrolysis reactionB. redox reactionC. combustion reactionD. neutralization reaction

Answers
Answer:
A. hydrolysis reaction.
Explanation:
Chemical Reactions.
First, let's review each concept of the group of answer choices:
- hydrolysis: is a reaction in which the net reaction is an organic compound reacting with water to give either two molar equivalents of a single product or more than one product.
- redox: is a type of chemical reaction that involves a transfer of electrons between two species.
- combustion: is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat.
- neutralization: is a reaction that occurs when an acid and a base react to form water and a salt and involves the combination of H+ ions and OH- ions to generate water.
The problem is asking for the reaction that removes a phosphate group from ATP, so let's see the structure of ATP with one phosphate group:
What is enclosed in the red box is the phosphate group.
The reaction that removes this phosphate group represents a rupture of the structure and based on the logic of the definitions of the given concepts, the answer would be that the reaction to remove one phosphate group from ATP is A. hydrolysis reaction. This reaction looks like this:
ATP + water (H2O) -> ADP + Pi,
where ADP is the same molecule of ATP but it has two phosphate groups and Pi is the phosphate group removed.
