Answers
To calculate the atoms of an element in a given molecule, we need to multiply stoichiometry by the number that is written on the foot of that element. Therefore, the balanced equation can be written as
H\(_2\)O\(_2\) + 2KI \(\rightarrow\) I\(_2\) + 2KOH
What is Balanced equation?Balanced equation is the one in which the total number of atoms of a species on reactant side is equal to the total number of atoms on product side. The mass of the overall reaction should be conserved. There are so many types of chemical reaction reaction like combination reaction, displacement reaction.
The other characteristic of balanced reaction is that physical state should be written with each compound or molecule on reactant and product side. Physical state should be written in brackets. s means solid, l means liquid, g means gas. The balanced equation for the given reaction can be given as
H\(_2\)O\(_2\) + 2KI \(\rightarrow\) I\(_2\) + 2KOH
Therefore, the balanced equation can be written as
H\(_2\)O\(_2\) + 2KI \(\rightarrow\) I\(_2\) + 2KOH
Learn more about the balanced equation, here:
https://brainly.com/question/7181548
#SPJ1
Related Questions
Question 25 (2 points) ✓ Saved
Consider these three compounds.
I
II
H
III
H
1
H-C-H
H-C-H
ннннн
! ! ! ! !
H-Ċ-Ċ-Ċ-Ċ-Ċ-H
II 1
Η Η Η Η Η
H H
1
1
H-C-C-C-H
HHH
H HH
1
H-C-C-C-C-H
I 1
Η Η Η Η
Which are isomers?

Answers
Answer:
May be its correct bro
Explanation:
take it easy
Why is fusion not a practical source of energy?
Answers
Answer:
Light i think
Explanation:
light is a source of energy also
write the conjugate bases of the following acids ? H₂S , HCOOH ,HSO₃¯ , HSO¯₄ , HS¯, HNO₂ ,HCN,?
Answers
Answer:
HS(-)
HCOO(-)
SO3(2-)
SO4(2-)
S(2-)
NO2(-)
CN(-)
Explanation:
The parenthesis are the charges of the compounds. When an acid is introduced in a base, it dissociates its Hydrogens giving protons to the base. In this case, if you remove one hydrogen of a compound, you will reduce the charge of a compound since basically you're removing a positive charge from a compound. If you need to, I can explain how the thing of the charges work.
In fact, the definition of a conjugate base is a compound created when the original (an acid) compound dissacioate an atom of hydrogen (a proton) in a base.
When all the soil pores are essentially water-filled, flow is termed _______________ .
Unsaturated
Saturated
Gravitional
Rapid
Answers
Nuclear fusion occurs when ___________________.
atomic nuclei are split
two or more atomic nuclei are joined together.
protons are destroyed, releasing large atoms of kinetic energy.
atoms are stripped of all their electrons and the mass of electrons turns to energy.
Answers
Explanation:
two or more atomic nuclei are joined together.
The nuclei 'fuse ' ....hence 'fusion'
How do you calculate the mass of an atom of silver? Need to have this answer in 7th grade terms,
Answers
Answer:
To calculate the mass of an atom of silver, we need to know the number of protons and neutrons in the nucleus of the atom. The atomic number of silver is 47, which means it has 47 protons in its nucleus. The mass number of silver is 108, which means that the total number of protons and neutrons in its nucleus is 108. Therefore, the mass of an atom of silver is approximately 108 times the mass of a single proton or neutron. This is because the mass of an electron is much smaller than the mass of a proton or neutron and can be neglected. The mass of a single proton or neutron is approximately 1 atomic mass unit (amu), so the mass of an atom of silver is approximately 108 amu.
Brandon's hands are cold from playing out in the snow. When he goes in the house, his mother hands him a mug of hot cocoa and his hands warm up. Why? A. Heat moves from the mug to his hands. B. Particles from the cocoa move to his hands. C. Cold from his hands is absorbed by the mug. D. Chemical energy from the cocoa changes to heat.
Answers
Answer:
A
Explanation:
One way that energy is transferred as heat is through direct contact between objects. is the process that moves energy from one object to another when they are touching. The heat energy moves from one object to another.
More than half the total present volume of ocean water resides in what ocean?
Answers
The Pacific Ocean is the largest ocean in the world based on water volume, totaling some 660 million cubic kilometers and is almost equally divided into the North and South Pacific waters.
The acid ionization constant, Ka, for propanoic acid, C2H5COOH, is 1.3x10-5.(a) Calculate the hydrogen ion concentration, [H+], in a 0.20-molar solution of propanoic acid.(b) Calculate the percentage of propanoic acid molecules that are ionized in the solution in (a).(c) What is the ratio of the concentration of propanoate ion, C2H5COO-, to that of propanoic acid in a buffer solution with a pH of 5.20?(d) In a 100.-milliliter sample of a different buffer solution, the propanoic acid concentration is0.35-molar and the sodium propanoate concentration is 0.50-molar. To this buffer solution,0.0040 mole of solid NaOH is added. Calculate the pH of the resulting solution
Answers
(a) The hydrogen ion concentration in the solution is [H+] = 1.14x10^-3 M. (b) 0.57%. (c) The ratio of the concentration of propanoate ion to that of propanoic acid in the buffer solution is 2.68.
(a) The balanced equation for the ionization of propanoic acid is:
C2H5COOH + H2O ⇌ C2H5COO- + H3O+
The equilibrium expression for this reaction is:
Ka = [C2H5COO-][H3O+] / [C2H5COOH]
At equilibrium, the concentration of propanoic acid that has ionized to form propanoate ion and hydronium ion is equal to the concentration of propanoic acid that has not ionized, so we can assume that [C2H5COO-] ≈ [H3O+]. Let x be the concentration of hydronium ion in the solution. Then the equilibrium expression becomes:
Ka = x^2 / (0.20 - x)
Solving for x, we get:
x = sqrt(Ka * (0.20 - x)) = sqrt(1.3x10^-5 * 0.20) = 1.14x10^-3 M
Therefore, the hydrogen ion concentration in the solution is [H+] = 1.14x10^-3 M.
(b) The percentage of propanoic acid molecules that are ionized in the solution is given by:
% ionization = [H3O+] / [C2H5COOH] x 100%
% ionization = (1.14x10^-3 / 0.20) x 100% = 0.57%
(c) The pH of a buffer solution can be calculated using the Henderson-Hasselbalch equation:
pH = pKa + log([C2H5COO-] / [C2H5COOH])
At pH 5.20, the hydronium ion concentration is 10^-5.20
= 6.31x10^-6 M.
Using the equilibrium expression for propanoic acid and the fact that [C2H5COO-] + [C2H5COOH] = total buffer concentration,
we can solve for the ratio of the concentrations of propanoate ion to propanoic acid:
Ka = [C2H5COO-][H3O+] / [C2H5COOH]
[C2H5COO-] = Ka[C2H5COOH] / [H3O+]
[C2H5COO-] = (1.3x10^-5)([C2H5COOH]) / (6.31x10^-6)
[C2H5COO-] / [C2H5COOH]
= 2.68
Therefore, the ratio of the concentration of propanoate ion to that of propanoic acid in the buffer solution is 2.68.
(d) When solid NaOH is added to the buffer solution, it reacts with the propanoic acid to form propanoate ion and water:
C2H5COOH + NaOH → C2H5COO- + H2O + Na+
The number of moles of propanoic acid that react with NaOH is equal to the number of moles of NaOH that were added. The new concentration of propanoic acid is:
0.35 M - (0.0040 mol / 0.100 L) = 0.346 M
The new concentration of propanoate ion is:
0.50 M + (0.0040 mol / 0.100 L) = 0.54 M
The new concentration of hydronium ion can be calculated using the equilibrium expression.
Learn more about equilibrium here:
https://brainly.com/question/3920294
#SPJ4
How mans, gram of HBr would exactly be
required to react with 2g of propyne ?
(Br=80,H=1, c=12) C3 H4+2HBr–> C3 H6 Br2
Answers
Answer:
The number of moles of HBr required to react with 2 g of propyne can be calculated by using the balanced chemical equation:
C3 H4 + 2HBr → C3 H6 Br2
The molar mass of propyne is (3 x 12 + 4 x 1) = 40 g/mol
So, 2 g of propyne is equal to 2/40 = 0.05 moles of propyne.
Since 2 moles of HBr are required to react with 1 mole of propyne, then 0.05 moles of propyne would require 0.05 x 2 = 0.1 moles of HBr.
Finally, to find the number of grams of HBr, we multiply the number of moles by its molar mass, which is (1 x 1 + 80 x 1) = 81 g/mol
So, 0.1 moles of HBr is equal to 0.1 x 81 = 8.1 g of HBr.
The spot on the fault where the pressure first releases is called the
Answers
Answer:
This movement releases energy and generates seismic waves that can be recorded by specialized instruments used by scientists. The point on a fault at which the first movement or break occurs during an earthquake is called the earthquake's hypocenter
Explanation:
Which of the following phrases does not describe a motor?
A. Changes electrical energy to mechanical energy
B. Uses the interaction between a spinning coil of wire and a magnet
C. Changes mechanical energy to electrical energy
D. Found in many appliances with moving parts
Answers
Answer:
Option C
Explanation:
A moter generally operates on electricity and mostly on Direct currentSo it converts electrical energy into mechanical energy to do the work .Hence option C is not correct
to an atom.
Neutrons add only
a. mass
b.ions
C. positive charge
d. negative charge
Please select the best answer from the choices provided
A
В
С
D
Pls help fast due in 10 mins!!! :(((((((( ughhh please!
Answers
Answer:
The answer is A, Neutron adds only mass
This is timed i need this fast
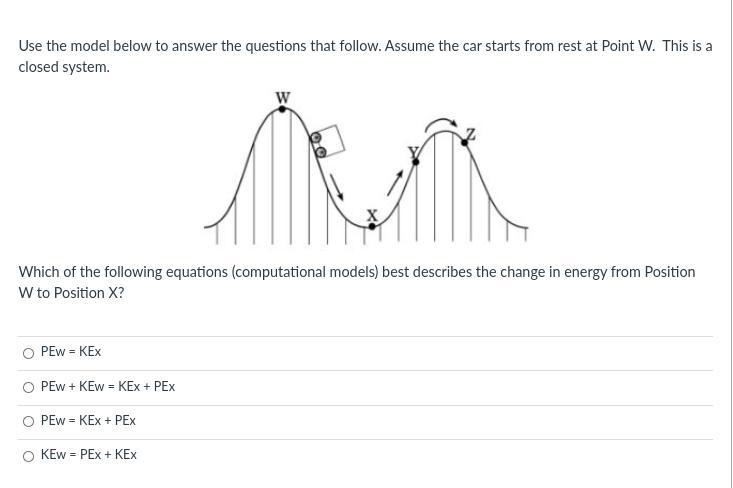
Answers
Answer:
1. or A
Explanation:
Explain how these results show that chlorine is more reactive than bromine and
lodine.
Answers
Chlorine is more reactive than bromine because it replaces both bromine and iodine.
How chlorine is more reactive than bromine?Fluorine is the most sensitive while on the other hand, the astatine is the least reactive as compared to other elements. The chlorine displaces both bromine and iodine, and bromine displaces iodine because of its high reactivity. The element that replaces other atom is considered as more reactive.
The order of reactivity is that the chlorine is more reactive than bromine, which indicates that chlorine is more reactive than iodine.
So we can conclude that chlorine is more reactive than bromine due to high reactivity.
Learn more about Chlorine here: https://brainly.com/question/24218286
#SPJ1
Is HCL a pure substance? Look at me already asking chemistry questions! //!roll eyes emoji/2#/
Answers
Answer:
yes it is as it has a chemical formula which cannot be changed
Helpppp meeeee!!! I beg you

Answers
Answer:
True
Explanation:
(30 pts) Please find the correct answer.

Answers
Answer:2
Explanation: Acids do indeed conduct electricity (Love the one peice pfp btw)
Nitrogen and hydrogen combine at a high temperature, in the presence of a catalyst, to produce ammonia.
N2(g)+3H2(g)⟶2NH3(g)
There are four molecules of nitrogen and nine molecules of hydrogen present in the diagram.
When the reaction is complete, how many molecules of NH3 are produced?
What is the limiting reactant?
How many molecules of each reactant are remain after the reaction is complete?
Answers
After the reaction is complete, no nitrogen and no hydrogen molecules remain, and 8.00 x 1014 molecules of NH3 are produced.
In the equation, nitrogen and hydrogen react at a high temperature, in the presence of a catalyst, to produce ammonia, according to the balanced chemical equation:N2(g)+3H2(g)⟶2NH3(g)The coefficients of each molecule suggest that one molecule of nitrogen reacts with three molecules of hydrogen to create two molecules of ammonia.
So, to determine how many molecules of ammonia are produced when four nitrogen and nine hydrogen molecules are present, we must first determine which of the two reactants is the limiting reactant.
To find the limiting reactant, the number of moles of each reactant present in the equation must be determined.
Calculations:
Nitrogen (N2) molecules = 4Hence, the number of moles of N2 = 4/6.02 x 1023 mol-1 = 6.64 x 10-24 mol
Hydrogen (H2) molecules = 9Hence, the number of moles of H2 = 9/6.02 x 1023 mol-1 = 1.50 x 10-23 mol
Now we have to calculate the number of moles of NH3 produced when the number of moles of nitrogen and hydrogen are known, i.e., mole ratio of N2 and H2 is 1:3.
The mole ratio of N2 to NH3 is 1:2; thus, for every 1 mole of N2 consumed, 2 moles of NH3 are produced.
The mole ratio of H2 to NH3 is 3:2; thus, for every 3 moles of H2 consumed, 2 moles of NH3 are produced.
From these mole ratios, it can be observed that the limiting reactant is nitrogen.
Calculation for NH3 production:
Nitrogen (N2) moles = 6.64 x 10-24 moles
The mole ratio of N2 to NH3 is 1:2; therefore, moles of NH3 produced is 2 × 6.64 × 10−24 = 1.33 × 10−23 moles.
Now, to determine how many molecules of NH3 are produced, we need to convert moles to molecules.
1 mole = 6.02 x 1023 molecules
Thus, 1.33 x 10-23 moles of NH3 = 8.00 x 1014 molecules of NH3 produced.
To find the amount of each reactant remaining after the reaction is complete, we must first determine how many moles of nitrogen are consumed, then how many moles of hydrogen are consumed, and then subtract these from the initial number of moles of each reactant.
The moles of nitrogen consumed = 4 moles × 1 mole/1 mole N2 × 2 mole NH3/1 mole N2 = 8 moles NH3
The moles of hydrogen consumed = 9 moles × 2 mole NH3/3 mole H2 × 2 mole NH3/1 mole N2 = 4 moles NH3
Thus, the moles of nitrogen remaining = 6.64 × 10−24 mol – 8 × 2/3 × 6.02 × 10^23 mol-1 = 5.06 × 10−24 mol
The moles of hydrogen remaining = 1.50 × 10−23 mol – 4 × 2/3 × 6.02 × 10^23 mol-1 = 8.77 × 10−24 mol
Finally, the number of molecules of each reactant remaining can be calculated as follows:
Number of N2 molecules remaining = 5.06 × 10−24 mol × 6.02 × 10^23 molecules/mol = 3.05 × 10−1 molecules ≈ 0 molecules
Number of H2 molecules remaining = 8.77 × 10−24 mol × 6.02 × 10^23 molecules/mol = 5.28 × 10−1 molecules ≈ 0 molecules.
For more such questions on molecules
https://brainly.com/question/24191825
#SPJ8
what is the name of the product when 3-methyl-2-butanol is oxidized?
Answers
Answer:
3-methyl-2-butanone.
Explanation:
Hello there!
In this case, for these types of oxidation of secondary alcohols, due to the 2-butanol, it is possible for us to remember that the product is a ketone exhibiting a carbonyl (C=O) group as the product of the oxidation. In such a way, the reaction would be:
CH3 - CH - CH - CH3 -------> CH3 - C - CH - CH3
| | || |
OH CH3 O CH3
Therefore, the product would be 3-methyl-2-butanone.
Best regards!
How does the generator work? Select the statement that describes that.
Answers
It turns electrical energy into KE.
This is the correct statement of how a generator works:
What is generator?A generator is described as a device that converts motive power or fuel-based power into electric power for use in an external circuit.
Generators contrary to general opinion do not create electricity instead it uses the mechanical energy supplied to it to force the movement of electric charges present in the wire of its windings through an external electric circuit. This flow of electrons makes up the output electric current supplied by the generator.
The present-day generators work on the principle of electromagnetic induction that was discovered by Michael Faraday.
Faraday realized that the above flow of current can be created by moving an electrical conductor in a magnetic field and this movement creates a voltage difference between the two ends of the conductor which causes the electric charges to flow, hence generating electric current.
Learn more about generators at: https://brainly.com/question/12950635
#SPJ1
Complete question:
How does the generator work? Select the statement that describes that.
______It turns electrical energy into KE.
_____ It turns sound energy into electrical energy.
_____ It turns electrical energy into sound energy.
_____ It turns electrical energy into thermal energy.
_____ It turns KE into electrical energy.
isoniazid is used in the treatment of tuberculosis and multiple sclerosis. identify each lone pair as either localized
Answers
The pairs has been identified as lone or localized, N is a lone pair and NH₂ is localized.
Isoniazid, also known as nydrazid, is a popular antibiotic used to treat and prevent tuberculosis and other illnesses. For specific forms of tremor in multiple sclerosis (MS), isoniazid is administered. Usually, substantial dosages of the antibiotic (600–1200 mg daily) are needed to treat MS tremors. To avoid contracting active TB disease, you can take medication. The medications Isoniazid and Rifapentine (INH-RPT) are combined to treat LTBI. They eliminate the dormant tuberculosis bacteria before you become unwell. The TB bacteria are powerful and can be killed by the medication over several months. Isoniazid is used to treat or prevent tuberculosis (TB) (reactivation). It can be used either on its own or in conjunction with other medications to cure TB or stop it from coming back (reactivation).
To learn more about isoniazid click here
brainly.in/question/18176701
#SPJ4
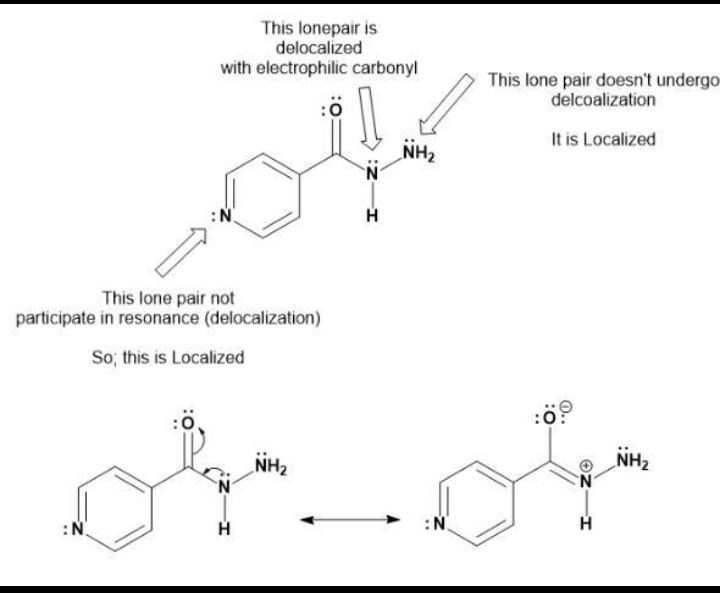
Consider an electrochemical cell formed from a Cu(s) electrode submerged in an aqueous Cu(NO_3)_2 solution and a Cd(s) electrode submerged in a Cd(NO_3)_2(aq) solution. The two electrodes are connected by a wire and the two solutions are connected by a salt bridge containing NaNO_3(aq). The following reaction takes place: Cu^2+(aq) + Cd(s) rightarrow Cu(s) + Cd^2+(aq) Which statement describes how the electrons or nitrate ions will flow? electrons will flow from Cu(s) to Cd(s) nitrate ions will flow from Cu compartment to Cd compartment nitrate ions will not flow between compartments nitrate ions will flow from Cd compartment to Cd compartment
Answers
Option B is the right answer, Nitrate ions will flow from Cu compartment to Cd compartment.
Define electrochemical cell.
An electrochemical cell is a device that uses electricity to induce a nonspontaneous process or to generate electricity from a spontaneous oxidation-reduction reaction.
Since in the given reaction Cu2+ is going to reduce to Cu and Cd is going to oxidize to Zn2+ . Electrons and cadmium ions will move towards cathode where reduction occurs.
And nitrate ions will move towards anode to maintain a neutral charge, where oxidation occurs.
So, nitrate will flow from Cu compartment to Cd compartment.
Therefore, option B is the right answer, Nitrate ions will flow from Cu compartment to Cd compartment.
To learn more about electrochemical cell from the given link.
https://brainly.com/question/10470515
#SPJ4
2.00M CO and 2.00M H2O are mixed in a sealed container and the system is left to reach equilibrium. Use the following information to calculate the equilibrium constant, Kc if at equilibrium the concentration of CO2 is 0.73M
CO(g) + H2O(g) ⇌ CO2(g) + H2(g
Answers
The equilibrium constant, Kc, for the response is 0. This approach that at equilibrium, the attention of merchandise is correctly 0 in comparison to the attention of reactants.
The equilibrium constant, Kc, may be calculated the use of the concentrations of the reactants and merchandise at equilibrium, consistent with the regulation of mass action. The balanced chemical equation for the response is:
CO(g) + H2O(g) ⇌ CO2(g) + H2(g)
At equilibrium, the attention of CO2 is given as 0.seventy three M. The preliminary attention of CO is 2.00 M, and the preliminary attention of H2O is likewise 2.00 M. Let x be the alternate in attention of CO and H2O, and y be the alternate in attention of CO2 and H2, then the equilibrium concentrations may be expressed as:
[CO] = 2.00 M - x
[H2O] = 2.00 M - x
[CO2] = 0.seventy three M + y
[H2] = y
The equilibrium expression for the response is:
Kc = ([CO2][H2])/([CO][H2O])
Substituting the equilibrium concentrations into the expression gives:
Kc = ([0.73 + y][y])/([(2.00 - x)][(2.00 - x)])
Assuming that x and y are small in comparison to the preliminary concentrations, we will approximate the equilibrium concentrations as:
[CO] ≈ 2.00 M
[H2O] ≈ 2.00 M
[CO2] ≈ 0.seventy three M
[H2] ≈ 0.00 M
Substituting those values into the equilibrium expression gives:
Kc = (0.730)/(2.002.00) = 0
This suggests that the response does now no longer continue to a enormous quantity withinside the ahead route and is in all likelihood to be an instance of a reversible response with a low equilibrium constant.
For such more questions on equilibrium
https://brainly.com/question/19340344
#SPJ11
Completely describe the electrolytic cell corresponding to the following equation. (Hint: you may need to combine 2 half reactions from Table 17-1 to make one of the half reactions for this cell)
Cr2O7^2– + I^– → Cr^3+ + IO3^–
With work please
Answers
The first half-reaction is the oxidation of Cr2O7^2– to Cr^3+ and the second half-reaction is the reduction of I^– to IO3^–. When combined, the overall reaction is Cr2O7^2– + I^– → Cr^3+ + IO3^–.
The electrolytic cell consists of two electrodes, one anode and one cathode, both of which are immersed in an electrolyte solution. At the anode, the Cr2O7^2– ions are oxidized to Cr^3+ ions, releasing electrons into the external circuit.
At the cathode, the I^– ions are reduced to IO3^– ions, and the electrons from the external circuit are used to drive the reaction. The electrolyte solution must contain both Cr2O7^2– and I^– ions in order to facilitate the transfer of electrons between the electrodes.
The overall reaction is driven by the potential difference between the anode and the cathode, which is created by the flow of electrons through the external circuit.
Learn more about electrolytic cell at:
https://brainly.com/question/4030224
#SPJ1
In the chemical equation CH4 + O2 -> CO2 + 2H2O for every one mole of carbon dioxide you produced, how many moles of water would you have?
Answers
Answer: 2 moles
Explanation:
In the balanced equation, it can be seen that everything reacts in a 1:1 mol ratio except water (H2O) as determined by the coefficient. It is simple stoichiometry, 1 mol CO2 * 2 mol H2O/1 mol CO2 = 2 mol CO2. After doing the math, the CO2 cancels out, leaving how many moles of H2O there are.
Find the number of representative particle in each of these substances.267 mol Y
Answers
The number of representative particles in 0.267 mole Y is 1.61 x 10²³ atoms.
What are particles?Particles can be simply defined as a measurable change in momentum involving a matter wave interaction with other substance or object.
1. 0.267 mole Y
1 mole of compound = 6.022 x 10²³
Than 0.267 mole of Y compound = 0.267 x 6.022 x 10²³
= 1.61 x 10²³
2. 0.200 mole NaI
0.200 mole of NaI compound = 0.200 x 6.022 x 10²³
= 1.2 x 10²³
3. 34.0 mole SO₃
34.0 mole of SO₃ compound = 34.0 x 6.022 x 10²³
= 204.75 x 10²³
4. 2.25 x 10⁻⁶ mole N₂
2.25 x 10⁻⁶ mole of N₂ compound = 2.25 x 10⁻⁶ x 6.022 x 10²³
= 13.55 x 10²³
Thus, the number of representative particles in 0.267 mole Y is 1.61 x 10²³ atoms.
To learn more about particles, refer to the link below:
https://brainly.com/question/2288334
#SPJ1
Your question is incomplete but most probably your full question was
Find the number of representative particles in each of these substances? In addition to the calculation, you MUST correctly identify the representative particle to get the points.
1 .267 mol Y
2 0.200 mol NaI
3 34.0 mol SO3
4 2.25 x 10 -6 mol N2
you are given experimental data below measured by a cole parmer rotary viscometer. although the fluid is unknown, you can classify the fluids as newtonian, shear thickening, and/or shear thinning. please classify each fluid.
Answers
Shear thickening fluid Fluid B, shear thinning fluid Fluid A, and Newtonian fluid Fluid C.
Describe what you mean by "fluid."Because there are more inter - particle gaps, fluids flow more fluidly and don't have a set structure. Fluids include both liquids and gases. Any fluid's molecules are always moving randomly and interacting with one another as well as any module's walls.
How many different kinds of fluid exist?Idea fluids, Real flow, Kinematic fluid, Non-Newtonian coolant, Viscoelastic secretions, and Number of initiatives Fluid are the various types of fluid. The thermodynamic characteristics of fluids are their temperatures, viscosity, pressure, as well as specific enthalpy. Physical characteristics: These characteristics, including as color and odor, aid in recognizing the fluid's physical state.
To know more about fluid visit:
https://brainly.com/question/13873557
#SPJ4
Complete question is:
You are given experimental data below measured by a Cole Parmer rotary viscometer. Although the fluid is unknown, you can classify the fluids as Newtonian, shear thickening, and/or shear thinning. Please classify each fluid. (LAB 1)
Fluid A = exponential decay
Fluid B = exponential growth
Fluid C = Linear horizontal line
Calculate the cell potential for the galvanic cell in which the given reaction occurs at 25 °C, given that [Sn2+]=0.0624 M, [Fe3+]=0.0437 M, [Sn4+]=0.00655 M, and [Fe2+]=0.01139 M. Standard reduction potentials can be found in this table.
Sn2+(aq)+2Fe3+(aq)↽−−⇀ Sn4+(aq)+2Fe2+(aq)
So far my incorrect answers have been:
0.28
0.798
0.178
0.142
0.881
0.61
and 0.812
Answers
Answer:
The cell potential for the given galvanic cell is 0.188 V.
Explanation:
To calculate the cell potential, we can use the Nernst equation:
Ecell = E°cell - (RT/nF)ln(Q)
where E°cell is the standard cell potential, R is the gas constant (8.314 J/mol·K), T is the temperature in Kelvin (25°C = 298 K), n is the number of moles of electrons transferred (in this case, n = 2), F is the Faraday constant (96,485 C/mol), and Q is the reaction quotient.
First, we need to write the half-reactions and their standard reduction potentials:
Sn4+(aq) + 2e- → Sn2+(aq) E°red = 0.15 V
Fe3+(aq) + e- → Fe2+(aq) E°red = 0.77 V
The overall reaction is the sum of the half-reactions:
Sn2+(aq) + 2Fe3+(aq) → Sn4+(aq) + 2Fe2+(aq)
The reaction quotient Q can be expressed as:
Q = [Sn4+][Fe2+]^2 / [Sn2+][Fe3+]^2
Substituting the given concentrations, we get:
Q = (0.00655)(0.01139)^2 / (0.0624)(0.0437)^2 = 0.209
Now we can calculate the cell potential:
Ecell = 0.15 V + 0.0592 V log([Fe2+]^2/[Fe3+]) + 0.0592 V log([Sn4+]/[Sn2+])
= 0.15 V + 0.0592 V log(0.01139^2/0.0437^2) + 0.0592 V log(0.00655/0.0624)
= 0.188 V
Therefore, the cell potential for the given galvanic cell is 0.188 V.
The cell potential for the given galvanic cell in which the given reaction occurs at 25 °C is 0.188 V.
How to the cell potential of galvanic cell?To find the cell potential, we take the Nernst equation:
Ecell = E°cell - (RT/nF)ln(Q)
In which R is the gas constant (8.314 J/mol·K) and E° cell is the standard cell potential.
T temperature in Kelvin (25°C = 298 K), and n is the number of moles of electrons transferred (n = 2), Q is the reaction quotient and F is the Faraday constant (96,485 C/mol).
Firstly, write the half-reactions and then their standard reduction potentials:
Sn⁴⁺(aq) + 2e⁻ → Sn²⁺(aq) E°red = 0.15 V
Fe³⁺(aq) + e⁻ → Fe²⁺(aq) E°red = 0.77 V
The overall reaction is the sum of the half-reactions:
Sn²⁺(aq) + 2Fe³⁺(aq) → Sn⁴⁺(aq) + 2Fe²⁺(aq)
The Q reaction quotient can be written as:
Q = [Sn⁴⁺][Fe²⁺]² ÷ [Sn²⁺][Fe²⁺]²
Substituting the given concentrations, we observe:
Q = (0.00655)(0.01139)² ÷ (0.0624)(0.0437)² = 0.209
Next, we can find the cell potential:
Ecell = 0.15 V + 0.0592 V log([Fe²⁺]²/[Fe³⁺]) + 0.0592 V log([Sn⁴⁺]/[Sn²⁺])
= 0.15 V + 0.0592 V log(0.01139²÷0.0437²) + 0.0592 V log(0.00655÷0.0624)
= 0.188 V
Thus, the cell potential for the given galvanic cell is 0.188 V.
Learn more about cell potential, here:
https://brainly.com/question/29719917
#SPJ2
Question 4 (2 points)
Which best describes Nuclear changes?
The substance stays the same, but the properties change.
Elements rearranging to become different substances.
The number of protons or neutrons changes, which may result in a different
atom.

Answers
Nuclear modifications are alterations that take place inside an atom's nucleus. The amount of protons and neutrons in the nucleus may change, and this is the most fundamental degree of change that can take place in a material.
The atom is considered to have experienced a nuclear transition and is now a distinct atom when the number of protons or neutrons changes. This is thus because the element is determined by the number of protons, and the element changes if the number of protons varies.
If an atom of uranium contains 92 protons, for instance, it is uranium; nevertheless, if it has 91 protons, it is protactinium. This nuclear shift produces a distinct atom with different properties.
Learn more about Nuclear reactions at:
https://brainly.com/question/16526663
#SPJ1