Do electrons attract hydrogen?
Answers
Answer:
Explanation:
When two hydrogen atoms come close enough to each other, their electrons are attracted to the proton of the other atom.
Related Questions
2. what do u mean by
Decomposition chemical Reaction
Answers
A decomposition reaction is a reaction in which a compound breaks down into two or more simpler substances.
which probing question lies within the scope of physics
Answers
A probing question that lies within the scope of physics could be: "What is the nature of dark matter and dark energy, and how do they influence the expansion of the universe?
Dark matter and dark energy are hypothetical forms of matter and energy that are believed to exist based on their observed gravitational effects on galaxies and the expansion of the universe.
Dark matter is thought to be a type of matter that does not interact with light or other electromagnetic radiation, while dark energy is an unknown form of energy that is causing the universe to expand at an accelerated rate.
Exploring the nature of dark matter and dark energy falls under the purview of physics because it involves understanding the fundamental forces and particles that govern the behavior of the universe.
Physicists study these phenomena by analyzing the gravitational effects they have on visible matter, conducting experiments to detect dark matter particles, and developing theoretical models to explain their properties and interactions.
Addressing this probing question requires the application of various branches of physics, such as cosmology, astrophysics, particle physics, and quantum mechanics.
Researchers employ observational data, theoretical frameworks, and advanced technologies to investigate the nature and origin of dark matter and dark energy, contributing to our understanding of the fundamental nature of the universe and its evolution.
For more such questions on probing question visit:
https://brainly.com/question/10753271
#SPJ8
How are Transpiration and Photosynthesis connected to each other?
Answers
Answer:
A leaf needs carbon dioxide and water for photosynthesis. ... For carbon dioxide to enter, the stomata on the surface of the leaf must be open. As you have seen, transpiration draws water from the roots into the leaf mesophyll.
I hope this helps you :)
2. The vertical columns in the periodic
table are called
O families
O periods
O rows
Answers
Answer:
2. Periods
Explanation:
The elements are arranged in seven horizontal rows, called periods or series, and 18 vertical columns, called groups. ... Elements in the periodic table are organized according to their properties.
Has two naturally occurring isotopes, and , with masses of 68.9257 u and 70.9249 u, respectively. calculate the percent abundances of these isotopes of gallium.
Answers
The percentage abundance of isotopes of gallium is 61.56% is Ga-70.92 & 38.44% is Ga-68.93.
What do you mean by gallium?Gallium (31Ga) is composed of two stable isotopes: gallium-69 and gallium-71. Gallium-67 and gallium-68 are the most commercially important radioisotopes.
Gallium-67 (half-life: 3.3 days) is a gamma-emitting isotope (the gamma ray emitted immediately after electron capture) that is used in standard nuclear medical imaging procedures known as gallium scans. It is most commonly used as the free ion, Ga3+. It is the gallium radioisotope with the longest half-life.
Are gallium isotopes naturally occurring?Gallium has two naturally occurring isotopes: Ga-69 (mass 68.9256 amu, natural abundance 60.11%), and Ga-71 (mass 70.9247 amu, natural abundance 39.89%).
Calculate the percent abundances of these isotopes of gallium.Gallium's average atomic mass =\(\frac{68.9257 +70.9249}{2}\)
=69.9253
Let x equal the percentage abundance of the first isotope.
by utilizing
AAM=\(\frac{W_{1}X_{1} * W_{2} X_{2} }{100}\)
\(69.70 = \frac{70.925 *X +68.926 * (1-X)}{100}\)
\(69.70 = 70.925X + 68.926 - 68.926 X\)
x = 61.56%
61.56% is Ga-70.92. (100-61.56)
& 38.44% is Ga-68.93.
The percentage abundance of isotopes of gallium is 61.56% is Ga-70.92 & 38.44% is Ga-68.93.
Learn more about isotopes here:-
https://brainly.com/question/2026214
#SPJ4
Which of the following statements best summarizes a consequence of the second law of thermodynamics? O Each chemical reaction in an organism must decrease the total entropy of the universe. O If the entropy of a system decreases, there must be a corresponding decrease in the entropy of the universe. O If the entropy of a system increases, there must be a corresponding decrease in the entropy of the universe. If entropy of a system decreases, there must be a corresponding increase in the entropy of the universe.
Answers
The statement that best summarizes a consequence of the second law of thermodynamics is (c) "If the entropy of a system decreases, there must be a corresponding increase in the entropy of the universe."
The second law of thermodynamics states that the total entropy of an isolated system can only increase over time. Entropy is a measure of the amount of disorder or randomness in a system. In any energy conversion or chemical reaction, some of the energy becomes unusable or is lost as heat, which increases the entropy of the surroundings.
When the entropy of a system decreases, it means that the system becomes more ordered. However, this cannot happen without an increase in the entropy of the surroundings, such as the release of heat into the environment. This ensures that the total entropy of the universe increases, as dictated by the second law of thermodynamics.
In summary, if the entropy of a system decreases, there must be a corresponding increase in the entropy of the universe, maintaining the overall increase in entropy. This principle governs energy conversions and chemical reactions in various systems, including those in living organisms.
To know more about entropy, refer here:
https://brainly.com/question/13135498#
#SPJ11
7. The different colors of the visible light spectrum coalesce to make white
light. What is the best synonym (similar meaning) for "coalesce?"
O Divide
O Separate
O Combine
O End up
Answers
a comparative study of coagulation, granular- and powdered-activated carbon for the removal of perfluorooctane sulfonate and perfluorooctanoate in drinking water treatment
Answers
Comparative study: Coagulation, GAC, and PAC for PFOS/PFOA removal in drinking water treatment. GAC/PAC demonstrated higher efficiency than coagulation.
Title: Comparative Study of Coagulation, Granular-Activated Carbon, and Powdered-Activated Carbon for the Removal of Perfluorooctane Sulfonate and Perfluorooctanoate in Drinking Water TreatmentAbstract:Perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) are persistent organic pollutants that have been detected in drinking water sources worldwide. These compounds pose potential risks to human health due to their persistence, bioaccumulative nature, and adverse effects on various organ systems. To mitigate the presence of PFOS and PFOA in drinking water, various treatment methods have been explored. This study aims to compare the efficiency of coagulation, granular-activated carbon (GAC), and powdered-activated carbon (PAC) in removing PFOS and PFOA during drinking water treatment.
Introduction:PFOS and PFOA are part of a larger group of per- and polyfluoroalkyl substances (PFAS) that have gained significant attention in recent years due to their widespread occurrence and potential health implications. These compounds are resistant to environmental degradation and have been used in various industrial and consumer applications, including firefighting foams, surface coatings, and water repellents.
Methods:In this study, water samples containing PFOS and PFOA were subjected to three treatment methods: coagulation, GAC adsorption, and PAC adsorption. Coagulation involved the addition of a coagulant (e.g., aluminum or iron salts) followed by flocculation and sedimentation. GAC and PAC adsorption involved the contact of water with a bed of respective carbon media to facilitate adsorption of PFOS and PFOA. The initial concentrations of PFOS and PFOA, contact time, pH, and carbon dosages were systematically varied to evaluate their effects on removal efficiency.
Results:The comparative study revealed that all three treatment methods exhibited the ability to remove PFOS and PFOA from drinking water. However, significant differences were observed in their removal efficiencies. Coagulation showed moderate removal efficiency for both PFOS and PFOA, with removal rates ranging from 40% to 60%. GAC and PAC exhibited higher removal efficiencies, with removal rates exceeding 90% for both compounds. However, the effectiveness of GAC and PAC was influenced by factors such as contact time, pH, and carbon dosage. Optimal conditions were determined for each treatment method to achieve maximum removal efficiency.
Discussion:The results indicate that GAC and PAC adsorption are more effective in removing PFOS and PFOA compared to coagulation. The adsorptive capacity of activated carbon provides a higher surface area for PFOS and PFOA adsorption, leading to superior removal efficiencies. Additionally, the extended contact time achieved through GAC and PAC beds allows for increased adsorption. However, it is important to note that the selection of the optimal treatment method should consider factors such as cost, ease of operation, and the presence of other contaminants in the water.
Conclusion:This comparative study highlights the superior performance of GAC and PAC adsorption over coagulation for the removal of PFOS and PFOA during drinking water treatment. Both GAC and PAC demonstrated high removal efficiencies, emphasizing their potential as viable treatment options for PFOS and PFOA-contaminated water sources. Further research and pilot-scale studies are warranted to evaluate the long-term performance, cost-effectiveness, and operational considerations associated with these treatment methods in real-world scenarios.
Learn more about Removal
brainly.com/question/27125411
#SPJ11
12. The smallest unit of an element that has all of the properties of the element is a/an
A. molecule.
B. cell.
C. atom.
D. neutron.
Answers
n atom is a particle of matter that uniquely defines achemical element. An atom consists of a central nucleus that is usually surrounded by one or more electrons. Each electron is negatively charged. The nucleus is positively charged, and contains one or more relatively heavy particles known as protons and neutrons.
Answer:
Explanation:
You have given the exact definition of what an atom is. It is the smallest entity that has all the same properties as a bunch of atoms put together.
Classify the following examples as exothermic or endothermic.
N₂+O2+ energy-
2NO
perspiring
a burning matchstick
toasting bread
H₂+12-2H1+
energy
melting wax
exploding dynamite
a glowing light stick
1. endothermic
2. exothermic
Answers
Answer:
I classify the following examples as Endothermic
What is the structural formula of 4-methyl pentan-2-ol
Answers
The 4-methyl pentane-2-ol (\(C_6H_{14}O\)) is an alcohol compound with a methyl group attached to the fourth carbon atom and a hydroxyl group attached to the second carbon atom in a five-carbon chain.
The structural formula of 4-methyl pentane-2-ol is \(C_6H_{14}O\). This is an alcohol compound with six carbon atoms, fourteen hydrogen atoms, and one oxygen atom. The first part of the name, 4-methyl, indicates that there is a methyl group (\(CH_3\)) attached to the fourth carbon atom in the chain. Pentan-2-ol tells us that there are five carbon atoms in the chain and that the hydroxyl group (OH) is attached to the second carbon atom. Therefore, the structural formula of 4-methyl pentane-2-ol can be written as \(CH_3CH(CH_3)CH(CH_2OH)CH_2CH_3\). This can be further simplified as \(CH_3CH(CH_3)CH(CH_2OH)CH_2CH_3\)which represents the complete structural formula of 4-methyl pentan-2-ol.4-methyl pentane-2-oil is an organic compound with a wide range of applications, including as a solvent, in the manufacture of cosmetics and perfumes, and as a flavoring agent in food and beverages. Its unique structure and properties make it a valuable component in various chemical and industrial processes.For more questions on methyl group
https://brainly.com/question/31238796
#SPJ8
Identify the missing reagent needed to carry out the following reaction. NaF Benzene Br ? A. 12-crown-5
B. 12-crown-4
C. 15-crown-5
D. 18-crown-6
E. None of these.
Answers
The correct answer is (D) 18-crown-6 depending on the specific conditions of the reaction.
The reaction shown is a nucleophilic substitution reaction where benzene is treated with a halide ion in the presence of a crown ether to increase the solubility of the salt formed. Crown ethers are cyclic polyethers that can encapsulate cations, making them more soluble in organic solvents.
The missing reagent needed to carry out the reaction is the crown ether that will help solubilize the NaBr salt formed. The most commonly used crown ether for this purpose is 18-crown-6, which can encapsulate Na+ cation.
Click the below link, to learn more about Nucleophilic substitution:
https://brainly.com/question/28325919
#SPJ11
which gas would most likely be found in the greatest amount in the bubbles? a) oxygen. b) ozone. c) nitrogen. d) carbon dioxide
Answers
The gas that would most likely be found in the greatest amount in bubbles is nitrogen, option c.
When a liquid undergoes a rapid change in pressure or temperature, bubbles are usually formed due to the presence of dissolved gases. Nitrogen is the most abundant gas available in natural environments such as water, due to its high solubility. Nitrogen is the primary component in Earth's atmosphere, with nearly 78% of it dissolved in water bodies.
Nitrogen is readily drawn out of the solution when you reduce pressure or the water temperature rises, leading to bubbles.
Learn more about Nitrogen Bubbles and Decompression sickness:
https://brainly.com/question/30286878
The gas that would most likely be found in the greatest amount in the bubbles is d) carbon dioxide.
During various natural processes and chemical reactions, gases can be released in the form of bubbles. When considering the options given, the gas that is commonly produced and released in significant amounts is carbon dioxide (CO2). Carbon dioxide is a byproduct of respiration, combustion, and other metabolic activities in living organisms. It is also released during the process of fermentation, photosynthesis, and decomposition of organic matter.
Oxygen (O2) is an essential gas for respiration, but it is typically consumed rather than produced in significant quantities during most natural processes. Ozone (O3) is a less common gas and is typically found in the Earth's ozone layer. Nitrogen (N2) is a major component of the Earth's atmosphere, but it is relatively inert and does not readily form bubbles.
Carbon dioxide, on the other hand, is frequently produced and released in various natural and chemical processes, making it the gas most likely to be found in the greatest amount in bubbles.
To learn more about carbon dioxide click here: brainly.com/question/15153008
#SPJ11
All of the following properties of alcohols are affected by hydrogen bonding except
A) molecular weight.
B) boiling point.
C) miscibility with water.
D) ability to dissolve polar substances.
E) none of the above
Answers
The property of alcohols that is not affected by hydrogen bonding is molecular weight. Hydrogen bonding is a type of intermolecular force that occurs between hydrogen atoms and highly electronegative atoms such as oxygen, nitrogen, or fluorine. Alcohols have an -OH group, which makes them capable of hydrogen bonding. When alcohols undergo hydrogen bonding, the intermolecular attraction between the molecules increases, which affects various properties of alcohols.
Boiling point and miscibility with water are two properties of alcohols that are affected by hydrogen bonding. Due to the stronger intermolecular forces caused by hydrogen bonding, alcohols have higher boiling points and are more soluble in water.
The ability of alcohols to dissolve polar substances is also affected by hydrogen bonding. Alcohols are able to dissolve polar substances such as sugars, aldehydes, and ketones because of the polar -OH group and the ability to form hydrogen bonds with other polar molecules.
However, molecular weight is not affected by hydrogen bonding. The molecular weight of an alcohol is determined by the sum of the atomic weights of its constituent atoms. It is not influenced by the presence or absence of hydrogen bonding. Therefore, the correct answer is option E, none of the above.
In summary, hydrogen bonding affects the boiling point, miscibility with water, and ability to dissolve polar substances of alcohols, but it does not affect their molecular weight.
learn more about Hydrogen bonding
https://brainly.com/question/1426421
#SPJ11
The graph represents the change in that occurs when food is cooked over a charcoal grill. Which statement correctly explains the graph?A. The reactants are unlit charcoal that has already released its energy, and the products are charcoal that has already burned.B. The reactants are charcoal that has already burned and released its energy, and the products are unlit charcoal.C. The reactants are unlit charcoal, and the products are charcoal that has already burned and released its energy.D. The reactants are charcoal that has already burned, and the products are unlit charcoal that has already released its energy.

Answers
The answer is C.
The reactants are charcoal that is unlit + oxygen and the products are the burnt charcoal + energy.
\(C_xH_y+O_{2\text{ }}\rightarrow CO_2+H_2O\text{ + heat}\)So for every combustion reaction like this one, CxHy is the wood. So before you light the wood, it is actally a reactant together with oxygen, because without oxygen the wood will not burn. So under the influence of heat, wood produces substances like carbon dioxide and heat, the moment you see wood burning it it already producing products, CO2 and heat (which is the fire). This is a combustion reaction.
Combustion reaction is exothermic because it releases energy.
How many grams of aluminum oxide (Al2O3) will be formed from 10.0 grams of aluminum (Al)? 4 AL + 3 02 --> 2 AL203
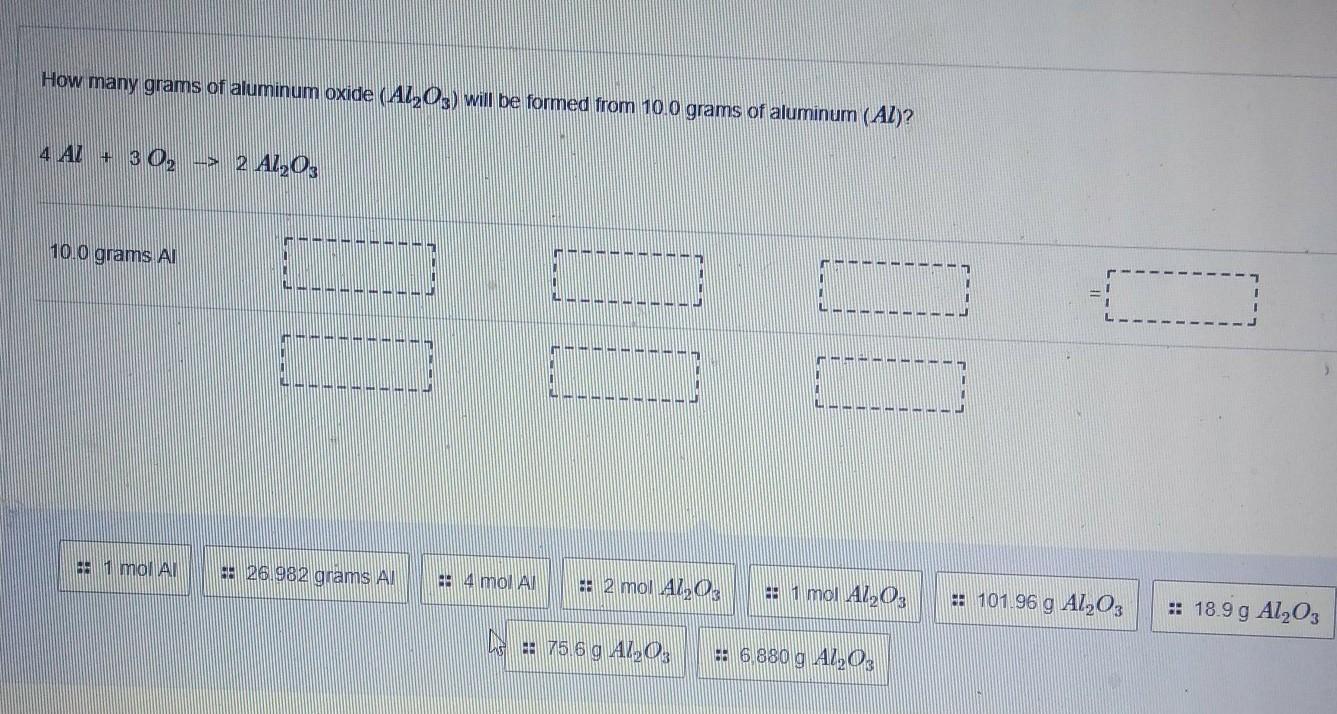
Answers
Answer:
Mass = 18.9 g
Explanation:
Given data:
Mass of Al₂O₃ formed = ?
Mass of Al = 10.0 g
Solution:
Chemical equation:
4Al + 3O₂ → 2Al₂O₃
Number of moles of Al:
Number of moles = mass/molar mass
Number of moles = 10.0 g/ 27 g/mol
Number of moles = 0.37 mol
Now we will compare the moles of Al and Al₂O₃.
Al : Al₂O₃
4 : 2
0.37 : 2/4×0.37 = 0.185 mol
Mass of Al₂O₃:
Mass = number of moles × molar mass
Mass = 0.185 mol × 101.9 g/mol
Mass = 18.9 g
aspartame is the methyl ester of a dipeptide made of phenylalanine and aspartic acid. what are the expected products of the hydrolysis of aspartame?
Answers
Aspartame is an artificial sweetener that is 200 times sweeter than sucrose. The expected products from the hydrolysis of aspartame are;
MethanolAmino acids (Phenylanaline)AspartateAspartame is a methyl ester that is gotten from aspartic acid and phenylalanine dipeptide. Aspartame is used by many food companies as a replacement for sugar. It is deemed suitable for consumption.
The hydrolysis of aspartame takes place in the small intestine. There, it is immediately transformed to methanol, phenylalanine, and aspartate.
Further products that are obtained after hydrolysis are; formaldehyde and formic acid.
Learn more here:
https://brainly.com/question/25225559
A bag of potato chips is packaged at sea level (1.00 atm) at a temperature of
300k and has a volume of 615 ml. if this bag of chips is transported to denver
(0.775 atm), and is at a temperature of 290k what will the new volume of the bag
be?
Answers
the new volume of the bag of chips in Denver will be approximately 633 ml. The new volume of the bag of chips in Denver will be 633 ml.
According to Boyle's Law, the product of pressure and volume is constant, assuming constant temperature. So, we can use the equation P₁V₁ = P₂V₂ to solve for the new volume (V₂). The initial pressure (P₁) is 1.00 atm, the initial volume (V₁) is 615 ml, the new pressure (P₂) is 0.775 atm, and the new temperature (T₂) is 290 K. Rearranging the equation, we get V₂ = (P₁ * V₁ * T₂) / (P₂ * T₁). Plugging in the values, we have V₂ = (1.00 atm * 615 ml * 290 K) / (0.775 atm * 300 K), which gives us V₂ ≈ 633 ml. Therefore, the new volume of the bag of chips in Denver will be approximately 633 ml.
learn more about volume here:
https://brainly.com/question/28058531
#SPJ11
Calcium is element 20 in the Periodic Table, has a mass of 40 amu and forms a 2+ ionic species. The calcium ion therefore has a. 18 protons, 18 neutrons and 22 electrons b. 22 protons, 18 neutrons and 18 electrons c. 20 protons, 20 neutrons and 18 electrons d. 18 protons, 20 neutrons and 20 electrons e. 20 protons, 18 neutrons and 20 electrons 1. In the following expression a∼1/b, what is the relationship between the components a and b ? a. Direct proportion b. None of the above c. Exact equation d. Inverse proportion e. Proportionality constant
Answers
The calcium ion has 18 protons, 20 neutrons, and 20 electrons.
The relationship between the components a and b is Inverse proportion.
The calcium ion (Ca2+) has a 2+ charge, indicating that it has lost 2 electrons from its neutral state. To determine the number of protons, neutrons, and electrons in the calcium ion, we need to consider its atomic number and mass.
The atomic number of calcium is 20, which indicates that it has 20 protons. Since the calcium ion has a 2+ charge, it means it has lost 2 electrons. Therefore, the number of electrons in the calcium ion is 20 - 2 = 18.
The mass number of calcium is 40 amu, which represents the total number of protons and neutrons. Since the calcium ion has 20 protons, the number of neutrons can be calculated as 40 - 20 = 20.
So, the correct option is: d. 18 protons, 20 neutrons, and 20 electrons
In the expression a∼1/b, the relationship between the components a and b is an inverse proportion. This means that as the value of a increases, the value of b decreases, and vice versa. The symbol ∼ represents the proportional relationship between a and 1/b, indicating that they are inversely related. Therefore, the correct answer is: Inverse proportion
To know more about calcium , click here, https://brainly.com/question/32135261
#SPJ11
QUICK PLEASE ANSWER THIS QUICK 70 POINTS RIGHT ANSWERS ONLY!! :)

Answers
Explanation:
To find the freezing point of the solution using the freezing point depression (ATf) and the freezing point of water, we can use the equation:
FPsolution = FPwater - ATf
where FPwater is the freezing point of pure water (0.00 °C). We know that ATf for this solution is 5.58 °C, as found in the previous step. Therefore:
FPsolution = 0.00 °C - 5.58 °C
FPsolution = -5.58 °C
However, a freezing point below zero degrees Celsius is not physically possible, since water freezes at 0.00 °C. Therefore, the solution would not actually freeze at this temperature, and we need to round the answer to zero °C:
FPsolution ≈ 0.00 °C
Therefore, the freezing point of the solution is around 0.00 °C, or the solution will not freeze at this temperature.
I NEED THE ANSWER ASAP
Chemicals that have leached into the soil can be made harmless by _______________________. (binary fission, bioremediation)
Answers
Chemicals that have leached into the soil can be made harmless by bio remediation .
What is bio remediation?Bioremediation is a biotechnology process that reduces or eliminates pollution. It is a type of waste management technology that uses organisms to remove or recycle pollutants from polluted areas. There are several remedial measures to clean up contaminated water and solids through chemical treatment, incineration and landfill. There are other types of waste management technologies such as solid waste management, nuclear waste management, etc.Bioremediation is different because it does not use toxic chemicals.How does it work?Bioremediation relies on stimulating the growth of specific microorganisms that use contaminants such as oils, solvents and pesticides as food and energy sources.These microbes convert pollutants into small amounts of water and harmless gases such as carbon dioxide.Can learn more about bio remediation from
https://brainly.com/question/14353375?
#SPJ4
When electrons lost a blank ion is formed
When electrons are gained a blank ion is formed

Answers
Answer:
I love it
Explanation:
☁️ Answer ☁️
annyeonghaseyo!
Your answer is:
When electrons are lost, a positively charged ion is formed. When electrons are gained, a negatively charged ion is formed. (I like the car)
Hope it helps.
Have a nice day noona/hyung!~  ̄▽ ̄❤️



How many joules are required to eat 5.25 g of titanium from 85.5 celsius to 132.5 celsius
Answers
The amount of energy required to eat 5.25 g of Titanium from 85.5 celsius to 132.5 celsius is 129 joules.
How do we calculate required heat?Absorbed or released amount of heat will be calculated by using the below formula as:
Q = mcΔT, where
m = mass of titanium = 5.25g
c = specific heat of titanium = 0.523 J/gram degree celsius
ΔT = change in temperature = 132.5 - 85.5 = 47 degree celsius
On putting these values on the above equation we get,
Q = (5.25)(0.523)(47) = 129 joules
Hence required heat is 129 joules.
To know more about absorbed heat, visit the below link:
https://brainly.com/question/8828503
7. C2H4O2 is what? vinegar
Answers
Answer:
Yes its vinegar
Explanation:
Actually \( \bold{\red {C_2H_4O_2}} \) is the formula for acetic acid which is found in vinegar.
Attractive forces of liquids allow for _____.
Answers
Answer:
They allow particles to stay close together.
The attractive forces (bonds) in a liquid are strong enough to keep the particles close together, but weak enough to let them move around each other. For example, Liquids are useful in car brake systems because they flow and cannot be compressed.
Explanation:
Hope this helps :)
Answer:
intermolecular attractive forces hold the molecules in contact, although they still have sufficient KE to move past each other. Intermolecular attractive forces, collectively referred to as van der Waals forces, are responsible for the behavior of liquids and solids and are electrostatic in nature.
Explanation:
I hope this helps!!!!!
a mixture of 141.3 g of p and 146.0 g of o2 reacts completely to form p4o6 and p4o10. find the masses of p4o6 and p4o10 that are formed by the reaction.
Answers
By using the molar masses of the products obtained in the reaction, we can calculate that the mass P₄O₆ formed is 125.1 g, and the mass of P₄O₁₀ formed is 162.2 g.
If the phosphorus (P) and oxygen (O₂) react completely, then the total mass of the reactants will be equal to the total mass of the products. So, if we label the mass of P₄O₆ as X, and the mass of P₄O₁₀ as Y, we know that:
X + Y = m(P) + m(O₂)
X + Y = 141.3 g + 146.0 g
X + Y = 287.3 g
X = 287.3 g - Y
Now, all the phosphorus has been used in the oxidation reaction. So, if we wanted to calculate the mass of phosphorus in P₄O₆, we would use its molar mass (M = 220 g/mol):
4 * 31 g : 220 g = mass of P in P₄O₆ : X
mass of P in P₄O₆ = 124 * X / 220
mass of P in P₄O₆ = 0.5636 * X
We can do the same for the mass of phosphorus in P₄O₁₀ (M = 284 g/mol):
4 * 31 g : 284 g = mass of P in P₄O₁₀ : Y
mass of P in P₄O₁₀ = 124 * Y / 284
mass of P in P₄O₁₀ = 0.4366 * Y
We know that the total mass of P used is 141.3 g, so:
mass of P in P₄O₆ + mass of P in P₄O₁₀ = 141.3 g
0.5636 * X + 0.4366 * Y = 141.3 g
If we substitute X from the first set of unknowns, we get:
0.5636 * (287.3 g - Y) + 0.4366 * Y = 141.3 g
161.9 g - 0.5636 * Y + 0.4366 * Y = 141.3 g
20.6 g = 0.127 * Y
Y = 20.6 g / 0.127
Y = 162.2 g
X = 287.3 g - 162.2 g
X = 125.1 g
You can learn more about the molar mass here:
brainly.com/question/12127540
#SPJ4
Why is paper towel absorbing water a chemical change
Answers
Answer:
it is not a chemical change
Explanation:
it is a physical change as a paper towel can be dried in the sun to turn into a regular paper towel thus proving that a paper towel absorbing water is a physical change not a chemical change
A train station in Tokyo uses tiles that convert people’s footsteps into electricity.
A.Restore
B. Rethink
C. Reduce
D. reuse
Answers
Answer:
reuse
Explanation:
Why might increasing the temperature alter the rate of a chemical reaction?
Answers
Answer:
Because after increasing of temperature kinetic energy of molecules increases and reaction speed increases in forward or reverse direction.
Explanation:
Which phrase provides the best definition of mass movement?
the movement of material caused by erosion
the slow flow of a glacier as it moves through the valley of a mountain
the movement of large amounts of soil and rock debris down a slope
the fast movement of a boulder rolling down a slope
Answers
Answer:
the movement of large amounts of soil and rock debris down a slope
Explanation:
yeetyeetyeetyeetyeetyeetyeetyeetyeetyeetyeetyeetyeetyeetyeetyeetyeetyeetyeetyeetyeetyeetyeetyeetyeetyeetyeetyeetyeetyeetyeetyeetyeetyeetyeet