Determine the number of moles of c2o4 in a sample with 0.48 moles of mno4 at endpoint
Answers
There are 2.4 moles of C2O4^2- in the given sample
To determine the number of moles of C2O4 in the given sample, we need to use the balanced chemical equation of the reaction between MnO4 and C2O4. The equation is:
MnO4- + 5C2O4^2- + 8H+ → Mn2+ + 10CO2 + 4H2O
From the equation, we can see that 1 mole of MnO4- reacts with 5 moles of C2O4^2-. Therefore, if we have 0.48 moles of MnO4- at the endpoint, we can calculate the number of moles of C2O4^2- as follows:
0.48 moles MnO4- x (5 moles C2O4^2-/1 mole MnO4-) = 2.4 moles C2O4^2-
Therefore, there are 2.4 moles of C2O4^2- in the given sample.
It is important to note that moles are a unit of measurement used in chemistry to represent the amount of a substance, and it is equal to the mass of a substance in grams divided by its molar mass. In this case, we were able to determine the number of moles of C2O4^2- in the sample by using the stoichiometry of the balanced chemical equation.
To know more about moles, visit:
https://brainly.com/question/31597231#
#SPJ11
Related Questions
How many particles are in 0.75 moles of AgNO3?
Answers
Explanation:
Assume if its asking about molecule particle:
1 mol of AgNO3 = 6.022 x 10^23 molecules
0.75 mol of AgNO3 = 0.75 x 6.022 x 10^23
= 4.5165 x 10^23 molecules
Assume if its asking about atom particle:
AgNO3 has 5 elements
0.75 mol of AgNO3 = 0.75( 5 x 6.022 x 10^23)
= 2.2583 x 10^24 atoms
Lithium has an atomic number of 3 and an average atomic mass of 6.941. how many protons does lithium have in the nucleus? 3 3 4 4 6 6 10
Answers
Lithium has 3 protons in the nucleus.
Atomic number is the number of protons or the number of electrons in the nucleus of a neutral atom.Mass number is the sum of the protons as well as the neutrons in the nucleus of an atom. ( sum of neutrons and protons = nucleons)Atomic number =number of protons =number of electrons
According to the question,
we have been given atomic number= 3
So, number of protons will be 3 and number of electrons will also be 3.
We can additionally calculate number of neutrons as follows-
number of neutrons= mass number - number of protons
= 6.941 - 3
≈ 4
Hence, the number of protons in lithium will be 3.
To learn more about atomic number refer-https://brainly.com/question/1805828
#SPJ4
The nucleus of Lithium has three protons.
The number of protons or electrons in the nucleus of a neutral atom is known as its Atomic number.
The total mass of an atom's protons and neutrons is known as its mass number. (neutrons and protons added together Equals nucleons)
Atomic mass= Mass number + Neutrons
Atomic number equals the sum of the protons and electrons
Since, atomic number given is 3
Therefore, protons in the nucleus will be 3.
Additionally, we may determine the neutron number using the formula below.
mass number = number of neutrons + number of protons
⇒ 6.941 = number of neutrons + 3
⇒ number of neutrons will be approximately 4.
Thus, lithium will contain three protons.
To learn more about atomic number refer- https://brainly.com/question/17684922
#SPJ4
find the formal charge (fc) of the atoms in nitrobenzene (shown below). which atom in this structure is likely to be attracted to an anion? which atom is likely to be attracted to a cation?
Answers
Therefore, the formal charge for each atom in nitrobenzene is: C: 0, N: 0, O: -1
The structure of nitrobenzene is:
NO2
|
____C____
| |
H C6H5
|
H
To determine the formal charge (FC) of each atom, we need to compare the number of valence electrons in the neutral atom to the number of electrons assigned to the atom in the molecule.
The FC of an atom is calculated using the formula:
FC = valence electrons - lone pair electrons - 1/2(bonding electrons)
Valence electrons for carbon (C) = 4
Valence electrons for nitrogen (N) = 5
Valence electrons for oxygen (O) = 6
FC for C = 4 - 0 - 1/2(8) = 0
FC for N = 5 - 2 - 1/2(6) = 0
FC for O = 6 - 4 - 1/2(4) = -1
An atom that is likely to be attracted to an anion is one that has a positive formal charge or is electron deficient. In nitrobenzene, the carbon atom is least electronegative and has a formal charge of 0, so it is more likely to be attracted to an anion.
An atom that is likely to be attracted to a cation is one that has a negative formal charge or is electron rich. In nitrobenzene, the oxygen atom has a formal charge of -1 and is therefore more likely to be attracted to a cation.
For more question on formal charge click on
https://brainly.com/question/11723212
#SPJ11
Please help me. No links or false answers!! Correct the following expressions into the correct scientific notation.
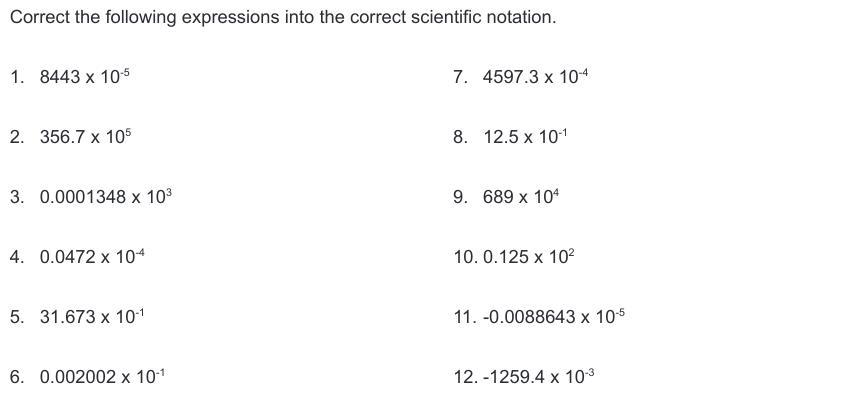
Answers
The aim of the scientific notation is to be able to reduce the bulk of the figures that we have to deal with.
What is the scientific notation?The aim of the scientific notation is to be able to reduce the bulk of the figures that we have to deal with. Thus, when we apply the scientific notation, we reduce the digits that we are dealing with to manageable number and this makes it easy for us to convey scientific information.
Let us now write the correct expression of the given numbers in scientific notation:
1) 8.443 * 10^-1
2) 3.567 * 10^8
3) 1.348 * 10^-1
4) 4.72 * 10^-6
5) 3.1673 * 10^0
6) 2.002 * 10^-4
7) 4.5973 * 10^-1
8) 1.25 * 10^0
9) 6.89 * 10^6
10) 1.25 * 10^1
11) -8.8643 * 10^-8
12) - 1.2594 * 10^0
Learn more scientific notation:https://brainly.com/question/18073768
#SPJ1
PLEASE ANSWER!!! NEED HELP!!!
In the reaction MgC12 + 2KOH Mg(OH)2 + 2KCl, if 1 mole MgC12 is added to
3 moles KOH, what is the limiting reagent?
KCI
Mg(OH)2
KOH
MgCl2
Answers
Answer:
For 1 mole of MgCl2, it would require 2 moles of KOH. ( 1 : 2 mole ratio)
Since you have 3 moles of KOH, it is in excess, and MgCl2 is the limiting reactant.
Explain why a halogen bulb has a longer bulb life than a traditional incandescent bulb filled with argon.
Answers
Answer:
The halogen bulb lasts longer because the filament lasts longer. It can have twice the life of an incandescent and use the same amount or slightly less (10%) electricity.
Explanation:
How many milliliters of a 5.0 M H2SO4 stock solution would you need to prepare 100.0 mL of 0.25 M H2SO4?
Answers
Answer:
5 milliliters
Explanation:
Use the formula M1V1 = M2V2 where M is molarity and V is volume
Plug in numbers
(.25 M)(100 mL) = (5 M)V2
V2 = 5 mL
The volume of stock solution of 5M H₂SO₄ needed to prepare 100mL of 0.25M H₂SO₄ is 5mL.
How do we calculate the volume?Volume of stock solution to prepare any dilute solution will be calculated by using the following formula:
M₁V₁ = M₂V₂, where
M₁ & V₁ is the molarity and volume of stock solution, and
M₂ & V₂ is the molarity and volume of final prepared solution.
On putting values from the question to the above formula and calculate for the value of V₁ as:
V₁ = (0.25)(100) / (5)
V₁ = 5 mL
Hence required volume is 5mL.
To know more about volume & molarity, visit the below link:
https://brainly.com/question/24881505
According to the kinetic theory of gases and Gay Lussac's Law, which of the following statements is incorrect?
A. The pressure of a gas results from collisions between the gas particles and the walls of the
container.
B. The average kinetic energy of the particles in a gas is inversely proportional to the
temperature of the gas.
C. Collisions between gas particles in the container are inelastic, but particle collisions with the
walls of the container are elastic.
D. During perfectly elastic collisions, no energy is lost due to friction.
E. As heat is added to a gas, the number of particle collisions increases and the pressure decreases.
Answers
Answer:B C E
Explanation:
Answer:
BCE
Explanation:
I got it right
Hope it helps
Which of the following diagrams has correctly shaded the metals in blue?

Answers
1) Groups of elements in the periodic table.
Metals are located in the middle and left side of the periodic table.
The second picture has correctly shaded the metals.
.

Match the landform to its description.
caldera
made of pieces of lava
volcanic soil
material fills in valleys
cinder cone
bowl-shaped depression
shield volcano
wide summit, gentle slope
lava plateau
rich in nutrients
Answers
Answer:
cinder cone: made of pieces of lava
shield volcano: wide summit, gentle slope
volcanic soil: rich in nutrients
caldera: bowl shaped depression
lava plateau: material fills in valleys
Explanation:
it’s right
Answer:
Caldera, Bowl-Shaped depression
Shield volcano, Wide summit, Gentle slope
Lava plateau, Material fills in valleys
Volcanic soil, Rich nutrients
Cinder Cone, Made of pieces of lava
Explanation:
Got it right on edge
Which of the following correctly pairs the strongest type of force present in each of the given compounds?dispersion forces,HBr PH3,hydrogen bonding Cl2,dipole-dipole forcesCH3OCH3,dipole-dipole force
Answers
CH3OCH3 dipole-dipole forces.
Which of the aforementioned forces is the most powerful?The four forces are the electromagnetic force, gravity, a weak nuclear force, and the strong nuclear force, in that order.
How powerful is the powerful force?Scientists have now measured the intensity of the strong force upto at 1.5 trillion electronvolts, that is approximately the average energy of each and every particle inside the universe soon after the Big Bang, after turning on the LHC, doubling their energy reach.
To know more about hydrogen bonding visit:
https://brainly.com/question/21054466
#SPJ4
the forces that moves against movement
Answers
Answer:
Friction
Explanation:
What characterizes a radioactive atom?
A. Its nucleus is unstable.
O B. Its nucleus has too few quarks.
O C. Its electrons gain energy.
O D. Its protons repel the neutrons.
Answers
. Its nucleus is unstable.
Its nucleus is unstable. This characterizes a radioactive atom. Therefore, the correct option is option A.
What is radioactive atom?The smallest parts of common stuff that can be separated without releasing electrically charged particles are called atoms. The atoms are divided into two sections. an electron cloud and an atomic nucleus. Atoms with an unstable nucleus and the potential for radioactive decay are referred to as radioactive atoms.
As only nuclei often experience decay and changes with electron configuration only come from nucleus configuration changes, the term "radioactive atom" is misleading. This isn't a rule, it must be said. As a parent nucleus needs capture any of its orbital electrons, the electron cloud also plays a significant role in the case of electron capture. Its nucleus is unstable. This characterizes a radioactive atom.
Therefore, the correct option is option A.
To know more about radioactive atom, here:
https://brainly.com/question/13673451
#SPJ7
What is the identity of the element that has 19 protons and 22 neutrons?
Answers
Answer:
A titanium ion
I answered all of the previous questions, but I'm not sure I understand questions 4 and 5, so I'm hoping you can assist me.

Answers
For 2. Look up what bonds the elements make when combined.
For 3. Look up the detention of the bond.
I hope this helps!
how many milliseconds are there exactly in 30 days
Answers
2.592e+9. I looked it up on
Answer:
2592000000 Milliseconds
Explanation:
To calculate 30 Days to the corresponding value in Milliseconds, multiply the quantity in Days by 86400000 (conversion factor). In this case we should multiply 30 Days by 86400000 to get the equivalent result in Milliseconds:
30 Days x 86400000 = 2592000000 Milliseconds
I took it from https://whatisconvert.com/30-days-in-milliseconds
The weathering of a tall mountain down into a low-lying hill is an example of a landform being changed through a _______ process. The buildup of sand dunes by the deposition of sediment is an example of landforms being created through a _______ process. A. Destructive; destructiveB. Constructive; destructiveC. Constructive; constructiveD. Destructive; constructive
Answers
The solution for this question is A. Destructive; constructive
The weathering of a tall mountain down into a low-lying hill involves the breakdown and erosion of the mountain over time, which is a destructive process. This process typically occurs due to various factors such as wind, water, and ice erosion, which gradually wear away the mountain's structure.
On the other hand, the buildup of sand dunes through the deposition of sediment is a constructive process. This occurs when wind or water carries and deposits sand or sediment in a specific location, gradually forming dunes over time.
Therefore, the weathering of a tall mountain represents a landform being changed through a destructive process, while the creation of sand dunes through the deposition of sediment represents a landform being created through a constructive process.
To know more about Weathering related question visit:
https://brainly.com/question/23449272
#SPJ11
reaction stoichiometry is based on chemical equations and
Answers
Reaction stoichiometry is based on chemical equations and the law of conservation of mass.
What is law of conservation of mass? The law of conservation of mass states that matter cannot be created or destroyed in a chemical reaction. This means that the mass of the products of a reaction must equal the mass of the reactants. This law is one of the most fundamental laws of chemistry and has been known since the 18th century. The law is based on the principle that matter is neither created nor destroyed in a chemical reaction, only converted from one state to another.Conservation of mass is an important concept in chemistry and is used to explain the behavior of many chemical reactions. It is often used to determine the amount of a substance that is produced or consumed in a reaction. It also helps scientists understand the relationships between reactants and products in a reaction. The law is also used to calculate the mass of products formed when a given amount of a reactant is used. Conservation of mass is also an important part of thermodynamics, allowing scientists to calculate the energy changes that occur in a reaction.To learn more about reaction stoichiometry refer to:
https://brainly.com/question/30186191
#SPJ4
A sample of krypton gas in a container of volume 1.90 L exerts a pressure of 0.553 atm at 21°C. How many moles of gas are present?
Answers
Answer:
0.064 moles
Explanation:
First, we need to convert the temperature in Kelvin, which can be done by adding 273 to the temperature in Celsius. So the temperature in Kelvin is 294 K. We can now use the Ideal Gas Law, PV = nRT, to solve for the number of moles, n. Rearranging the equation gives us n = PV/RT. Plugging in our values gives us n = (0.553 atm)(1.90 L)/[(0.0821 L·atm/K·mol)(294 K)] = 0.064 mol. Therefore, there are 0.064 moles of krypton gas present in the container.
How many kinds of chemically non-equivalent hydrogens are there in each of the following compounds? a the number of chemically non-equivalent hydrogens is. B the number of chemically non-equivalent hydrogens is.
Answers
Answer:
he number of chemically non-equivalent hydrogens is 6.
can someone answer the 4 questions? (4 is optional) it would really help me out thanks

Answers
Answer:
1)Energy converter is the transformation of one form of energy into another form .It is more specifically that the term energy conversions refers to the process through which energy is changed into forms that are useful to humans .For 100 of years humans have used an array of devices and systems for this purpose.
2) The three energy converters that you or your family use regularly are
Electric Iron Electric BulbElectric generator3)The three kinds of power plants that produce electricity in Ontario are
Nuclear Power PlantsHydroelectric Power PlantsGeothermal Power Plants4)A power plant is an industrial facility that generates electricity from primary energy most of the power plants use one more generators that convert mechanical energy into electrical energy in order to supply power to the electrical grid for society's electrical needs.
may be this was ur answer for ur question u wanted
3. How many grams are in 9.015 x 1035 atoms of Cobalt?
Answers
Answer:
8.822 × 10¹³ g Co
General Formulas and Concepts:
Chemistry - Atomic Structure
Reading a Periodic TableUsing Dimensional AnalysisAvogadro's Number - 6.022 × 10²³ atoms, molecules, formula units, etc.Explanation:
Step 1: Define
9.015 × 10³⁵ atoms Co
Step 2: Identify Conversions
Avogadro's Number
Molar Mass of Co - 58.93 g/mol
Step 3: Convert
\(9.015 \cdot 10^{35} \ atoms \ Co(\frac{1 \ mol \ Co}{6.022 \cdot 10^{23} \ atoms \ Co} )(\frac{58.93 \ g \ Co}{1 \ mol \ Co} )\) = 8.82189 × 10¹³ g Co
Step 4: Check
We are given 4 sig figs. Follow sig fig rules and round.
8.82189 × 10¹³ g Co ≈ 8.822 × 10¹³ g Co
I NEED HELP PLEASE, THANKS!
Electrochemistry is important in many aspects of daily life.
i. Define voltaic cell.
ii. Fill in the blanks for the drawing of a voltaic cell that’s made with copper/copper (II) nitrate (E° = 0.34 V) and zinc/zinc (II) nitrate (E° = –0.76 V). Briefly explain the role of the salt bridge.
iii. Using the equation E°cell = E°cathode – E°anode, calculate the overall cell potential for the cell.
iiii.
a. _____________
b. _____________
c. _____________
d. _____________
e. _____________
f. _____________
g. _____________
h. _____________

Answers
Answer:
Here's what I get
Explanation:
(i) Voltaic cell
A voltaic cell is a device that uses a chemical reaction to produce electrical energy.
(ii) Overall Cell Potential
The standard reduction potentials for the half-reactions are
ℰ°/V
Cu²⁺ + 2e⁻ ⇌ Cu 0.34
Zn²⁺ + 2e⁻ ⇌ Zn -0.76
The half-reaction with the more positive potential is the reduction half-reaction. It is the reaction that occurs at the cathode.
The half-reaction with the more negative potential is the oxidation half-reaction. It is the reaction that occurs at the anode.
We reverse that half-reaction and subtract the voltages to get the cell reaction.
ℰ°/V
Cathode: Cu²⁺ + 2e⁻ ⇌ Cu 0.34
Anode: Zn ⇌ Zn²⁺ + 2e⁻ -0.76
Cell: Zn + Cu²⁺ ⇌ Zn²⁺ + Cu 1.10
\(\mathcal{E}_{\text{cell}}^{\circ} = \mathcal{E}_{\text{cat}}^{\circ} - \mathcal{E}_{\text{an}}^{\circ} = \text{0.34 V} - \text{(-0.76 V)} = \text{0.34 V} + \text{0.76 V} = \textbf{1.10 V}\)
(iii) Diagram
The specific labels will depend on your textbook.
They are often as follows.
a. Electron flow
b. Voltmeter or lightbulb
c. Electron flow
d. Cathode or Cu
e. Cu²⁺(aq) and NO₃⁻(aq)
f. Salt bridge
g. Zn²⁺(aq) and NO₃⁻(aq)
h. Anode or Zn
The salt bridge enables ions to flow in the internal circuit and to maintain electrical neutrality in the two compartments.
It often consists of a saturated solution of KCl.
As Zn²⁺ ions form in the anode compartment, Cl⁻ ions move in to provide partners for them.
As Cu²⁺ ions are removed from the cathode compartment, K⁺ ions move in to replace them.
Answer:a. Electron flow
b. Voltmeter or lightbulb
c. Electron flow
d. Cathode or Cu
e. Cu²⁺(aq) and NO₃⁻(aq)
f. Salt bridge
g. Zn²⁺(aq) and NO₃⁻(aq)
h. Anode or Zn
Explanation:
Scientists estimate that a single chlorine molecule in the CFC structure can destroy as many as ___________ ozone molecules.
100,000
10,000
1,000
100
Answers
Scientists estimate that a single chlorine molecule in the CFC structure can destroy as many as 100,000 ozone molecules. So The correct answer is 100,000.
CFCs are fully halogenated paraffin hydrocarbons that contain only carbon, chlorine, and fluorine atoms. These organic compounds were discovered by scientists in 1928 and were initially used as a refrigerant, solvents, and aerosol propellants.
CFCs are known to be the primary cause of the depletion of the ozone layer. When these chemicals are exposed to ultraviolet light, they break down and release chlorine atoms. The chlorine atoms then react with ozone molecules, resulting in the destruction of the ozone layer.
Ozone is critical to the Earth's atmosphere because it helps protect it from the sun's harmful ultraviolet radiation. Ozone depletion exposes the planet to harmful UV radiation, which has been linked to skin cancer, cataracts, and other health problems.
To know more about chlorine molecule please refer to:
https://brainly.com/question/20485611
#SPJ11
Chemists can identify the composition of some unknown salts by conducting a flame test. When potassium salts are heated in a flame, a purple color is observed.
This is due to the movement of electrons between energy levels. What is the electron configuration of a potassium atom at ground state?
answer choices
1s2; 2s2; 2p6; 3s2; 3p6; 4d1
1s2; 2s2; 2p6; 3s2;3p6; 3d1
1s2; 2s2; 2d6; 3s2; 3d6; 4s1
1s2; 2s2; 2p6; 3s2; 3p6; 4s1
Answers
The electron configuration of a potassium atom at ground state is 1s²2s²2p⁶3s²3p⁶4s¹. Therefore, option D is correct.
What is an electronic configuration?The electron configuration of an element can be explained as electrons being occupied in different energy levels of an atom of a specific element. In the electron configuration, the electrons are usually written as a superscript of atomic subshells. For example, the electron configuration of Helium can be represented as 1s²2s².
The sequence of completely filled subshells similar to neighboring the electronic configuration of a noble gas is represented by square brackets. The principal quantum number (n) will be used to denote the maximum number of electrons in an electron shell.
The total number of electrons occupied in the given electronic configuration 1s²2s²2p⁶3s²3p⁶4s¹ is 19. The atomic number of potassium is 19 therefore it is the configuration of potassium.
Learn more about electronic configuration, here:
brainly.com/question/5624100
#SPJ4
what is [oh − ] (in m) in a solution of 1.24 m hoc2h4nh2 and 0.80 m hoc2h4nh3no3? (assume kw = 1.01 ✕ 10−14.)
Answers
The concentration of hydroxide ions ([OH-]) in the given solution is 1.24 M.
Describe the dissociation of a weak base ?
The dissociation of a weak base in an aqueous solution involves the breaking of chemical bonds within the base molecule to release hydroxide ions (OH-) into the solution. Unlike strong bases, which completely dissociate in water, weak bases only partially dissociate, resulting in a dynamic equilibrium between the undissociated base and the hydroxide ions.
To determine the concentration of hydroxide ions ([OH-]) in the given solution, we can first write the chemical equation for the dissociation of the weak base, \(HOC_2H_4NH_2\):
\(HOC_2H_4NH_2 + H_2O\) ⇌ \(OC_2H_4NH_2- + H_3O+\)
Here for every molecule of \(HOC_2H_4NH_2\) that dissociates, one hydroxide ion (OH-) is formed. Therefore, the concentration of hydroxide ions ([OH-]) will be equal to the concentration of the conjugate base (\(OC_2H_4NH_2-\)).
Given:
[\(HOC_2H_4NH_2\)] = 1.24 M
[\(HOC_2H_4NH_3NO_3\)] = 0.80 M
\(K_w\) = \(1.01*10^{-14}\)
Since,\(HOC_2H_4NH_2\) is a weak base, we can assume that the concentration of the conjugate acid (\(HOC_2H_4NH_3+\)) is remove compared to the concentration of\(HOC_2H_4NH_2\).
To find [\(OC_2H_4NH_2-\)], we have to find the concentration of \(HOC_2H_4NH_2\) that has dissociated. This can be done using the equation for the dissociation of a weak base and the equilibrium constant (\(K_b\)):
\(K_b\) = [\(OC_2H_4NH_2-\)] * [\(H_3O+\)] / [\(HOC_2H_4NH_2\)]
Given that \(K_b\) is not provided, we can assume that the value of \(K_b\) is insignificant compared to \(K_w\), as\(HOC_2H_4NH_2\) is a weak base. Therefore, we can omit the contribution of\(H_3O+\) and let it is equal to 0.
\(K_b\) = [\(OC_2H_4NH_2-\)] × 0 / [\(HOC_2H_4NH_2\)]
Since [\(H_3O+\) is insignificant , the concentration of [\(OC_2H_4NH_2-\)] will be same to the concentration of \(HOC_2H_4NH_2\) that has dissociated.
[\(OC_2H_4NH_2-\)] = [\(HOC_2H_4NH_2\)] = 1.24 M
Thus, the concentration of hydroxide ions ([OH-]) in the given solution is 1.24 M.
To learn more about the dissociation of a weak base from the given link
brainly.com/question/3484994
#SPJ4
the number of moles of charge contributed to a solution by one mole of dissolved ions is called a(n) .
Answers
The number of moles of charge contributed to a solution by one mole of dissolved ions is called the Faraday constant (F). It represents the charge of one mole of electrons and is essential in electrochemical calculations involving charge transfer and electrolysis.
In a solution, when one mole of ions dissolves, each ion can carry a certain charge. For example, if a particular ion carries a charge of +1, then one mole of that ion would contribute +1 Faraday of charge to the solution. The Faraday constant is crucial in various electrochemical calculations, such as determining the amount of charge transferred during an electrochemical reaction or calculating the amount of substance produced or consumed in an electrolysis process.
Learn more about "moles" here:
brainly.com/question/29367909
#SPJ11
Help me pleaseeeeeee!!!!
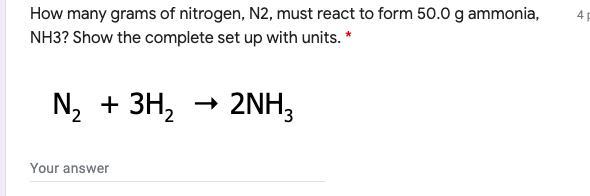
Answers
true or false: the molar enthalpy of sublimation of a given substance can be determined if its enthalpies of fusion and vaporization are known.
Answers
The given statement "The molar enthalpy of sublimation of a given substance can be determined if its enthalpies of fusion and vaporization are known" is true.
The molar enthalpy of sublimation of a substance can be determined if its enthalpies of fusion (melting) and vaporization (boiling) are known. The enthalpy of sublimation refers to the energy required to change a substance from the solid phase directly to the gaseous phase, bypassing the liquid phase.
The enthalpy change during sublimation can be calculated by considering the enthalpies of fusion and vaporization. When a substance undergoes sublimation, it first requires energy to melt from the solid phase to the liquid phase (enthalpy of fusion) and then additional energy to vaporize from the liquid phase to the gaseous phase (enthalpy of vaporization). The sum of these two enthalpies represents the overall energy change during sublimation.
Therefore, by adding the enthalpy of fusion and the enthalpy of vaporization, one can determine the molar enthalpy of sublimation for a given substance.
Learn more about molar enthalpy from the link given below.
https://brainly.com/question/32136429
#SPJ4
Which of these often forms sedimentary
rock?
A. mud and sand
B. plant and animal remains
C. chemicals on the ocean floor
D. all of the above
Answers
D. all of the above often forms sedimentary rock.
All of the options listed often contribute to the formation of sedimentary rocks. Sedimentary rocks are formed from the accumulation and consolidation of sediments, which are particles or fragments of pre-existing rocks or organic material.
A. Mud and sand: Sedimentary rocks such as shale, mudstone, and sandstone are primarily composed of compacted and cemented mud and sand particles, respectively. Over time, these particles settle and undergo compaction and lithification processes to form sedimentary rocks.
B. Plant and animal remains: Organic matter, including plant and animal remains, can accumulate in certain environments such as swamps, lakes, and marine environments. Over time, these organic materials may undergo partial decomposition and become incorporated into sediment layers. Under specific conditions, this organic material can be preserved and eventually transformed into coal, oil, or natural gas. These substances can be found in sedimentary rocks such as coal beds or oil shale.
C. Chemicals on the ocean floor: Sedimentary rocks can also form from the precipitation of dissolved chemicals in bodies of water, including the ocean. As water evaporates or becomes oversaturated with certain minerals, these minerals can precipitate and settle on the ocean floor. Over time, these accumulated minerals can compact and form sedimentary rocks such as limestone, gypsum, or rock salt.
Therefore, all of the options mentioned (mud and sand, plant and animal remains, and chemicals on the ocean floor) contribute to the formation of sedimentary rocks through different geological processes.
for more such questions on rock
https://brainly.com/question/1158219
#SPJ11