Determine the Molar mass of NI3
Answers
Answer:
The molar mass of \(NI_{3}\) is 394.719 g/mol.
Explanation:
This was on my periodic table.
Related Questions
how many liters of a 70 alcohol solution must be added to 50 liters of a 40 lcohol solution
Answers
we need approximately 28.57 liters of a 70% alcohol solution to add to 50 liters of a 40% alcohol solution.
To find out how many liters of a 70% alcohol solution must be added to 50 liters of a 40% alcohol solution, we can use the following formula:
$$\text{Volume of 70% alcohol solution} = \frac{\text{Amount of pure alcohol needed}}{\text{Concentration of pure alcohol}}$$Let V be the volume of the 70% alcohol solution needed.
Then, the amount of pure alcohol needed is 0.7V, since the concentration of the 70% alcohol solution is 70%.Similarly, the amount of pure alcohol in 50 liters of a 40% alcohol solution is 0.4(50) = 20 liters. Therefore, we can set up an equation:$$0.7V = 20$$Solving for V, we get:$$V = \frac{20}{0.7} \ approx 28.57$$Therefore, we need approximately 28.57 liters of a 70% alcohol solution to add to 50 liters of a 40% alcohol solution.
To learn more about alcohol visit
https://brainly.com/question/29268872
#SPJ11
Which value could be the y-coordinate for point Q?
215
42
2/13
8/2

Answers
Calculate the quantity of heat absorbed by 20 g of water that warms from 30
∘
C to 90
∘
C. Express your answer in calories.
Answers
The quantity of heat absorbed by 20 g of water as it warms from 30 °C to 90 °C is 1200 calories. To calculate the quantity of heat absorbed by the water, we can use the formula:
Q = m * c * ΔT
Where:
Q is the quantity of heat absorbed,
m is the mass of the water (in grams),
c is the specific heat capacity of water (which is approximately 1 calorie/gram·°C),
ΔT is the change in temperature (final temperature minus initial temperature).
In this case, we have:
m = 20 g (the mass of water)
c = 1 calorie/gram·°C (specific heat capacity of water)
ΔT = 90 °C - 30 °C = 60 °C (change in temperature)
Substituting the values into the formula, we get:
Q = 20 g * 1 calorie/gram·°C * 60 °C
Q = 1200 calories
Therefore, the quantity of heat absorbed by 20 g of water as it warms from 30 °C to 90 °C is 1200 calories.
To know more about mass of the water visit:
https://brainly.com/question/26789700
#SPJ11
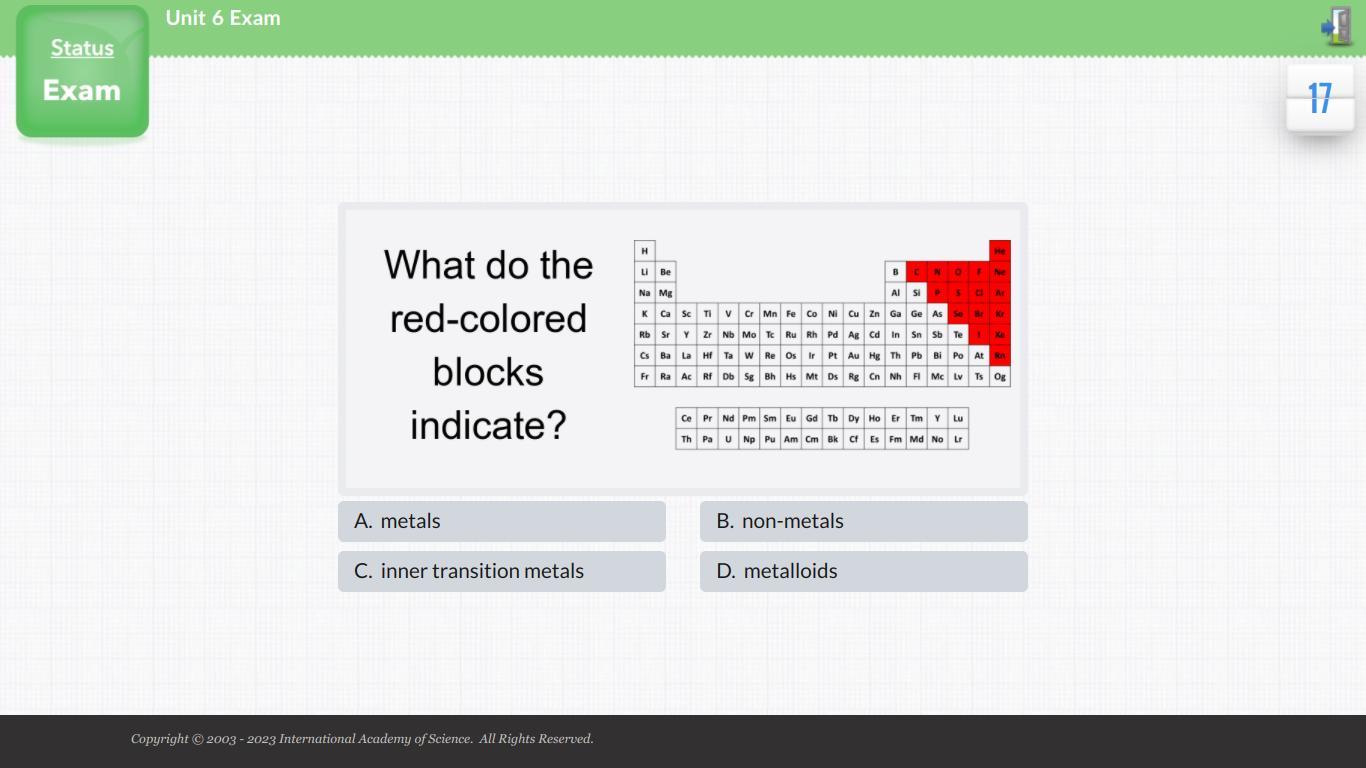
What do the red-colored blocks indicate?
Answers
The red-colored blocks indicate non metals in the periodic table. Nonmetals are located on the right side of the periodic table, in the p-block.
Non-metals are composed of a variety of elements, such as carbon, nitrogen, oxygen, sulfur, phosphorus, and selenium. These elements are generally non-reactive, but some of them can form compounds with other elements. They are also used in various industries, such as the production of plastics, fertilizers, and explosives. Non-metals are divided into two categories: metalloids and non-metalloids. Nonmetals are characterized by their lack of luster and their low thermal conductivity.
To learn more about non metals click here https://brainly.com/question/29404080
#SPJ1
Which of the following should you multiply by in order to solve the following: 43 m = ? ft
Answers
Answer:
3.28
Explanation:
At some point it is best to write out an equation to cancel out the units you don't want and to get to the units that you do want. So if you write this out in an equation, you can see how you get to the answer.
43 m 3.28 ft ? ft
-------- X ----------- = --------------
1 1 m 1
You can see now that the meters on the top will cancel out the meters on the bottom so all you are left with is the feet unit. When you write out the equation you put the unit on the bottom that you want to get rid of (meter). You put the unit you want to get to on the top which is feet. At this point you just have to know how many feet 1 meter is equal to and plug those number in. 1 meter is equal to 3.28 feet.
Use bonding theory to describe the following in terms of
electrons and orbitals: bonding electron, lone pair.
Pls help lols
Answers
Bonding electrons are involved in the formation of chemical bonds, whereas lone pair electrons are not involved in bond formation and occupy atomic orbitals.
What are bonding electrons and lone pair electrons?Bonding electrons are electrons that are involved in forming chemical bonds between atoms. In covalent bonds, atoms share electrons to achieve a full outer electron shell and attain stability. The number of bonding electrons is determined by the electron configuration of the atoms involved in the bond and their relative electronegativities.
Lone pair electrons, on the other hand, are non-bonding electrons that are not involved in forming chemical bonds. These electrons are found in the outermost electron shell of an atom and can be thought of as "unpaired" electrons. Lone pair electrons occupy atomic orbitals and do not participate in bond formation. Instead, they have a significant impact on molecular shape and reactivity due to their repulsive forces.
Learn more about electrons, here:
https://brainly.com/question/1255220
#SPJ9
9. Which is not a form of electromagnetic radiation?
O radio waves
O gravity
O gamma rays
O visible light
Answers
Answer:
Gravity
Explanation:
Electromagnetic radiation is self-sustaining energy with electric and magnetic field components.
Which of the following pieces of evidence suggests there was a chemical change?
Change in color
Change in shape
Change in size
Change in state
Answers
Answer:
Change in colour will be the answer.
at a given temperature, different liquids will have different equilibrium vapor pressures because
Answers
At a given temperature, different liquids will have different equilibrium vapor pressures because the vapor pressure of a liquid depends on its intermolecular forces, molecular weight, and temperature. The equilibrium vapor pressure is a measure of the tendency of a liquid to evaporate and become a gas at a specific temperature.
Intermolecular forces, such as hydrogen bonding or London dispersion forces, play a significant role in determining the strength of attractions between molecules in a liquid. Liquids with stronger intermolecular forces will have lower vapor pressures because it requires more energy to overcome these forces and transition into the gas phase.
Molecular weight also influences vapor pressure. Generally, liquids with larger and heavier molecules will have lower vapor pressures compared to liquids with smaller and lighter molecules. This is because larger molecules have stronger intermolecular forces and require more energy to transition into the gas phase.
Furthermore, temperature affects the vapor pressure of a liquid. As the temperature increases, the average kinetic energy of the liquid molecules increases, resulting in more molecules with sufficient energy to overcome intermolecular forces and transition into the gas phase. Therefore, at higher temperatures, the vapor pressure of a liquid increases.
In summary, the equilibrium vapor pressure of a liquid is determined by the interplay of intermolecular forces, molecular weight, and temperature. Different liquids with varying intermolecular forces and molecular weights will exhibit different equilibrium vapor pressures at the same temperature.
To know more about the vapor pressures refer here :
https://brainly.com/question/25715932#
#SPJ11
If beryllium has a mass number of 9 and an atomic number of 4
Calculate the number of protons, neutrons, and electrons
Answers
Answer:
mass number=9
atomic number=4
Explanation:
protons =4(protons is the same as atomic number)
neutrons=mass number-protons=3
electrons=4(it is the same as protons because it didn't gain or loose a charge)
Suppose you wanted to calculate the heat of reaction for the formation of ammonia gas and hydrochloric acid from solid ammonium chloride. Write a balanced equation for this reaction
Answers
Answer:
NH4Cl (s) → NH3 (g) + HCl (aq)
How are wavelength and frequency related to one another?
Answers
Which of these is true about pure substances? They can only contain one type of molecule. They may contain one type of atom or one type of molecule. They can only contain one type of atom. They can contain different types of atoms and molecules.
Answers
Answer:
its totally d
Explanation:
calculate the mass of iron (iii) carbonate that will be formed when 15.0 mL of 0.15 M iron (iii) chloride solution are reacted with 20.0 mL of 0.15 M sodium carbonate. Be sure to write the balanced metathesis reaction that is occuring.
Answers
Answer:
The molar mass of a substance is calculated as the mass of a given chemical compound or substance, which is then divided by the amount of the substance. The mass is calculated in grams and the amount of the substance is calculated in mol. Thus, the standard unit for molar mass is g/mol.
Explanation:
According to the given question, the reaction between iron chloride and sodium carbonate will be given as:
\(\text {3Na_{2}CO_{3}\text} \;\[+\] \;\text{ 2FeCl_{3} \text}\; \rightarrow\; \text {6NaCl}\; \[+\] \;\text { Fe_{2} (CO_3)_{3} \text}\)
Now, to calculate the mass of iron carbonate formed in the above chemical reaction, it will be given as:
\({ \text Moles\;of}\text \;\text {FeCl_{3} \text} \[=\dfrac{0.15 \;\text {moles} \times 0.015\; \text L}{1.0 { \;\text L}\text}\)
\(=\;0.00225\; \text {moles}\text\)
Similarly, for sodium carbonate
\(\text Moles\; of\; Na_{2}CO_{3}}\te \[=\]\dfrac{0.15 \;\text {moles} \times 0.020\; \text L}{1.0 { \;\text L}\text}\\\)
\(\[=\]\;0.0003\; {\text moles \text}\)
Limiting reactant is \(\text {FeCl_{3} \text}\)
Now, calculating iron chloride mass:
\(=\dfrac{0.00225 \times 1\;\text{moles}\;\text{Fe}_2 {(\text{CO}_3)}}{2\;\rm{moles\;of\;}Fecl_3}\)
\(=0.001125 \;\text {moles }\)
Now, molar mass of the \(\text{Fe}_{2}\text{CO}_{3}\) will be \(=291.71679\;\text{g/ mol}\)
\(\begin{aligned}\text{Mass}&=\frac{\text{Moles}}{\text{Molar mass}}\\\\&=\frac{0.001125\;\text{moles}}{291.71679\; \text{g/mol}} \\\\&=3.85648\times10^{-6} \end{aligned}\)
If 12.3 grams of CCl4 are produced from the reaction of 18.0 g of carbon disulphide with 22.0 grams of Cl2, what is the percent yield of CCl4???
Answers
The percent yield of CCl₄ from the reaction of 18.0 g of carbon disulphide with 22.0 grams of Cl₂ is 77.3%
Equation of reaction:
CS₂ + 3 Cl₂ ----> CCl₄ + S₂Cl₂
molar mass of CS₂ = 78 g/mol
molar mass of Cl₂ = 71 g/mol
molar mass of CCl₄ = 154 g/mol
1 mole of carbon disulphide reacts with 3 moles of chlorine gas78 g of CS₂ reacts with * 71 g of Cl₂
78 g of CS₂ reacts with 213 g of Cl₂
18 g of CS₂ reacts with 213 * 18/ 78 g of Cl₂
18 g of CS₂ requires 49.1 g of Cl₂
Therefore, Cl₂ is the limiting reactant3 moles of Cl₂ produces 1 mole of CCl₄
213 g of Cl₂ produces 154 g of CCl₄
22.0 g of Cl₂ will produce 154 * 22 / 213 g of CCl₄
22.0 g of Cl₂ will produce 15.9 g of CCl₄
Percent yield = (actual yield / expected yield) * 100%Actual yield = 12.3 g
Expected yield = 15.9 g
Percent yield = 12.3/15.9) * 100%
Percent yield = 77.3 %
The percent yield of CCl₄ from the reaction of 18.0 g of carbon disulphide with 22.0 grams of Cl₂ is 77.3%
Learn more about percent yield at: https://brainly.com/question/12890861
how much of the atmosphere is composed of nitrogen gas?
Answers
Approximately 78% of the atmosphere is composed of nitrogen gas.
The envelope of gases surrounding the Earth or another celestial body is referred to as the atmosphere. It is held in place by the planet's gravity. The atmosphere protects life on Earth by absorbing ultraviolet solar radiation, warming the surface through heat retention (greenhouse effect), and reducing temperature extremes between day and night (the diurnal temperature variation).
The atmosphere consists of various gases, including nitrogen, oxygen, and carbon dioxide. The percentage of each gas present in the atmosphere is referred to as its composition. The atmosphere of the Earth is made up of 78.08% nitrogen, 20.95% oxygen, 0.93% argon, and trace amounts of other gases such as carbon dioxide, neon, helium, and methane.
The following are the various layers of Earth's atmosphere:
Exosphere
Thermosphere
Mesosphere
Stratosphere
Troposphere
To learn more about Atmosphere refer: https://brainly.com/question/13092796
#SPJ11
A measure of the average kinetic energy of particles is
length
temperature
mass
volume
Answers
PLEASE HELP ASAP
There are 164 g H3PO3 formed during a
reaction. How many moles of H₂O are
required? (H3PO3: 82 g/mol)
P2O3 + 3H₂O → 2H3PO3
164 g H3PO3|
164 g H3PO3 → [?] mol H₂O

Answers
To find the number of moles of H₂O required, we need to determine the molar ratio between H3PO3 and H₂O in the balanced equation.
From the balanced equation:
P2O3 + 3H₂O → 2H3PO3
We can see that 2 moles of H3PO3 are produced from 3 moles of H₂O.
To calculate the number of moles of H₂O, we can set up a proportion using the molar ratios:
2 moles H3PO3 / 3 moles H₂O = 164 g H3PO3 / x moles H₂O
Cross-multiplying the proportion, we have:
2 moles H3PO3 * x moles H₂O = 3 moles H₂O * 164 g H3PO3
2x = 3 * 164
Simplifying:
2x = 492
x = 492 / 2
x = 246
Therefore, 246 moles of H₂O are required.
Learn more about balanced equation on:
https://brainly.com/question/31242898
#SPJ1
Answer:
3
Explanation:
Both plant and animal cells contain mitochondrion however they are not in equal amounts. Between the cheek and onion cells, which one do you think would contains the greater amount of mitochondrion and why?
Answers
Answer:
cells, like plants and animals, also have membrane-bound nuclei and organelles (e.g., mitochondria, cytoplasmic curriculum, lysosomes). Cheek cells, like other squamous cells in animals, appear scale-like under the microscope.
Hope this helps!! if it does...
Plants and animal cell also have membrane-bound nuclei and organelles (e.g., mitochondria, cytoplasmic curriculum, lysosomes). Cheek cells, like other squamous cells in animals, appear scale-like under the microscope.
What is cell?Cell is defined as the base of life as it is the structural as well as functional unit of life. Cell is made up of pre existing cells and the cell contain various cell organelles such as mitochondria, endoplasmic reticulum, ribosomes, golgi appratus, nucleus, and cytoplasm.
Basically, cell is of two type one in prokaryotic and another one is eukaryotic. The prokaryotic cell is known as pre mature cells as they do not contain cell organelles and eukaryotic cell are known as advanced and developed cells as they contain several cell organelles.
The unicellular animals are made up of single cell and all the functions carried out in a single cell like amoeba and multicellular organisms are made up of multiple cells and there is specific cell for specific functions.
Therefore, Plants and animal cell also have membrane-bound nuclei and organelles (e.g., mitochondria, cytoplasmic curriculum, lysosomes). Cheek cells, like other squamous cells in animals, appear scale-like under the microscope.
Learn more about cell here:
https://brainly.com/question/3142913
#SPJ2
Juan puts a pencil into a glass of water. The pencil appears broken at the point where it enters the water. What causes the pencil to look broken? A. The water in the glass absorbs incoming light waves so light cannot pass through the water. B. The light waves traveling through the glass are refracted as the waves pass from air to water. C. The water in the glass changes incoming light waves into a different form of energy. D. The light waves traveling through the glass are reflected by the pencil when it is underneath the water.
Answers
Answer:
d
Explanation:
What are the units of molar mass?
A. L/g
B. mol/g
C. g/L
D. g/mol
SUBST
Answers
Answer:
D - g/mol
step by step method
I think its the best answer
what characteristic of a solid is most responsible for their structure? select all that apply. the elements that make up the solid ability to withstand scratching bonding patterns between atoms amount of kinetic energy it can absorb before breaking
Answers
The elements that make up the solid bonding patterns between atoms is the characteristic of a solid is most responsible for their structure.
When the forces binding atoms or molecules together are greater than the energy separating them, solids are created. Atomic bonding patterns are one of the most crucial elements that determine the final physical makeup of a given solid item. The more solid an object is, the stronger the bond pattern. Solids have hard, rigidly defined shapes and volumes. Around fixed axes, particles oscillate. Strong intermolecular interactions, or strong interactions between molecules, result in the tight aggregation and fixed locations of solids' molecules. Solid particles are strongly attracted to one another.
To learn more about solids click here
https://brainly.com/question/17061172
#SPJ4
please help me quickly

Answers
Answer:
tell me the question i cant see the picture
Explanation:
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
ОА.
OB.
[Mg:0:
[Mg] [10:
[Mg]* 10:
[Mg*:07
Ос.
OD.
![Which diagram shows the correct way to represent an ionic compound of magnesium oxide?.OB.[Mg:0:[Mg]](https://i5t5.c14.e2-1.dev/h-images-qa/contents/attachments/wXKU4sszqhZVsGHV3kBmfVhr9ZMld5EF.png)
Answers
Answer:
the correct answer is D
Explanation:
D
The diagram which shows the correct way to represent the ionic compound, magnesium oxide is option D.
What is an electron dot structure?Electron dot structure is a representation of the valence electrons of an element, represented by the dots around the symbol of the element.
An ionic compound is formed by the combination of two or more ions. Note that the two ions that compose an ionic bond are held purely by electrostatic interaction of the oppositely charged ions.
The correct depiction of MgO is the arrangement in which the two ions, \(Mg^{2+}\) and \(O^{2-}\) are placed in different brackets and the oxygen ion has a complete octet as seen in option D.
Learn more about electron dot structure here:
https://brainly.com/question/2141967
#SPJ2
3.25 kcal is the same amount of energy as A. 3.25J. B. 0.7771. C. 777J. D. 13600 j.
Answers
Kilocalorie is a unit of measuring the amount of energy of a reaction, but this is not the only unit, we can also have Joules as a unit, and the conversion is:
1 Kcal = 4184 Joules
Therefore if we have 3.25 Kcal, we will have:
3.25 * 4184 = 13600 Joules of energy, therefore letter D
Make a suggestion on how you will deal with each problem.

Answers
Using the periodic table, identify the name and symbol of the three neutral atoms given their atomic numbers and masses. The neutral atom with an atomic number of 1 and a mass number of 1. bol. name: Hydrogen atomic symbol: H The neutral atom with an atomic number of 11 and a mass number of 23. name: (Sodium name: Sodium atomic symbol: | 22 Na dionie sympat yang The neutral atom with an atomic number of 7 and a mass number of 14. name: Nitrogen Nitrogen atomic symbol: 0 atomic symbol: N | N º
Answers
The neutral atom with an atomic number of 1 and a mass number of 1 is Hydrogen (H).
The neutral atom with an atomic number of 11 and a mass number of 23 is Sodium (Na).
The neutral atom with an atomic number of 7 and a mass number of 14 is Nitrogen (N).
The atomic number of an element corresponds to the number of protons in its nucleus, which determines its identity. The mass number represents the total number of protons and neutrons in an atom.
For the first atom, with an atomic number of 1 and a mass number of 1, there is only one proton and no neutrons, which corresponds to Hydrogen (H).
The second atom, with an atomic number of 11 and a mass number of 23, has 11 protons and 12 neutrons. This corresponds to the element Sodium (Na).
The third atom, with an atomic number of 7 and a mass number of 14, has 7 protons and 7 neutrons, which corresponds to Nitrogen (N).
To learn more about atomic numbers, here
https://brainly.com/question/16858932
#SPJ4
a material has volume 4.52m^3 and density 8.61kg/m^3. the mass of this material in scientific notation is 3.89 X 10^2 g
is it true or false?
Answers
You need to make an aqueous solution of 0.167 M iron(III) acetate for an experiment in lab, using a 125 mL volumetric flask. How much solid iron(III) acetate should you add?
How many milliliters of an aqueous solution of 0.137 M potassium fluoride is needed to obtain 16.8 grams of the salt
ion concentration
In the laboratory you dissolve 19.6 g of sodium carbonate in a volumetric flask and add water to a total volume of 375 mL.
What is the molarity of the solution?
M.
What is the concentration of the sodium cation? M.
What is the concentration of the carbonate anion? M.
In the laboratory you dissolve 16.1 g of nickel fluoride in a volumetric flask and add water to a total volume of 500 mL.
What is the molarity of the solution?
M.
What is the concentration of the nickel cation? M.
What is the concentration of the fluoride anion? M.
Answers
The amount of solid iron (III) acetate required to make an aqueous solution of 0.167 M of iron (III) acetate using 125 ml volumetric flask is 13.5g.
To address this problem, molarity, number of moles, ion molarity
To determine the molarity of a solution, divide the amount of solute in moles by the volume of the solution in liters. Calculate the number of moles by dividing the substance's mass by its molar mass. To calculate the ion concentration, multiply the molarity of the ionic compound by the mole ratio.
The formula for calculating the molarity of a solution is
M = n/V
Here, n is the number of moles of solute and V is the volume of solution in liters.
The number of moles of a substance, use the formula
n = m/MM
where MM represents the molar mass of the substance and m stands for the mass of the substance.
The molarity iron (III) acetate is 0.167 M
Volume of a solution is 0.125 mL
So V = 125ml(1L/1000ml)
= 0.125L
The molarity of a solution is calculated as:
M = n/V
Rearrange the above equation for .
n=MxV
Substitute 0.167 M for M and 0.125 L for V
n=0.167 Mx0.125 L =0.0208 (mel 1) = 0.0208 mol
The molar mass of iron (III) acetate, Fe(C,H,02), is 650.9 g/mol .
The number of moles of a substance is calculated as:
n = m/MM
Rearrange the above equation for .
m=nx MM
Substitute 0.0208 mol for n and 650.9 g/mol for MM in the above equation.
m=0.0208 mol x 650.9 g/mol = 13.5 g
Hence the mass of solid iron (III) acetate is 13.5g
Learn more about molarity here brainly.com/question/8732513
#SPJ4
what is the solubility in moles/liter for copper(ii) oxalate at 25 oc given a ksp value of 2.9 x 10-8. write using scientific notation and use 1 or 2 decimal places (even though this is strictly incorrect!)
Answers
The solubility product constant expression for copper(II) oxalate is:
CuC₂O₄(s) ⇌ Cu²⁺(aq) + C₂O₄²⁻(aq)
The Ksp value given is 2.9 x 10⁻⁸ at 25°C.
Let's assume that "x" is the molar solubility of C₂O₄²⁻ in water, then the equilibrium concentrations of Cu²⁺ and C₂O₄²⁻ ions are also "x" because 1 mol of C₂O₄²⁻ produces 1 mol of Cu²⁺ and 1 mol of C₂O₄²⁻
So, it can be written as:
Ksp = [Cu²⁺ ][C₂O₄²⁻ ] = x²
Substituting the given Ksp value, we get:
2.9 x 10⁻⁸ = x²
Taking the square root of both sides gives:
x = 1.7 x 10⁻⁴ M
Therefore, the solubility of copper(II) oxalate at 25°C is 1.7 x 10⁻⁴ moles/litre, rounded to two decimal places.
Learn more about copper(II) oxalate, here:
https://brainly.com/question/15225438
#SPJ4