determine the molar mass (in g/mol) of a gas that travels with an effusion time that is 1.55 times longer than that of co2(g).
Answers
The molar mass (in g/mol) of a gas that travels with an effusion time that is 1.55 times longer than that of CO₂ is 54.77 g/mol
given that :
Rate of effusion of CO₂= R1
rate of effusion of gas = R2
molar mass of CO₂ , M1 = 44 g/mol
molar mass of gas M2 = ?
the rate of effusion is given as :
R1 / R2 = √M2 / M1
R2 = 1.55 R1
1 / 1.55 = √ 36.45 / M2
0.803 = 44 / M2
M2 = 54.77 g/mol
Thus, the molar mass of the gas is 54.77 g/mol
To learn more about effusion here
https://brainly.com/question/29805920
#SPJ4
Related Questions
In Three to five sentences, explain the two scientific claims regarding the depletion of ozone over Antarctica
Answers
When sunlight returns to Antarctica each year in September and October, this activation of chlorine and bromine causes ozone to rapidly lose its protective properties, creating the Antarctic ozone hole.
What is causing ozone depletion over Antarctica?Around the world, winds are constantly stirring the Earth's atmosphere. Because of this, wherever they are released, ozone-depleting gases mix with other airborne particles, including those from Antarctica. These gases are more effective at removing ozone from the atmosphere in Antarctica than they are anyplace else due to the region's unique meteorological conditions.The Northern Hemisphere has seen the majority of human emissions of bromine- and chlorofluorocarbon-containing gases like halons. In the latitudes corresponding to Europe, Russia, Japan, and North America, almost 90% of the satellites have been launched. Within a year or two, gases that are largely unreactive and insoluble in water, including CFCs and halons, are intermingled throughout the lower atmosphere.Mostly at tropical latitudes, the CFCs and halons in this well-mixed air rise from the lower atmosphere into the stratosphere. Winds then carry this air poleward, both north and south, from the tropics, ensuring that chlorine and bromine are present in almost equal levels throughout the whole global stratosphere.The South Pole is situated in the Southern Hemisphere within Antarctica, a very large land mass that is entirely encircled by water. This symmetry is represented in the weather patterns that permit the establishment of a particularly cold zone in the stratosphere over the Antarctic continent in winter. This region is isolated by a ring of strong winds that circulates around its perimeter. Polar stratospheric clouds, which occur as a result of the extremely low stratospheric temperatures, are responsible for chemical changes that encourage the synthesis of chlorine and bromine, two elements with chemical activity. When sunlight returns to Antarctica each year in September and October, this activation of chlorine and bromine causes ozone to rapidly lose its protective properties, creating the Antarctic ozone hole.To Learn more About sunlight returns Refer To:
https://brainly.com/question/14053556
#SPJ1

Are two atoms of the same element identical?
Please answer this and I will mark the best answer as the brainiest answer.
Answers
Answer:
No
Explanation:
Are two atoms of the same element identical?
No they are not, for multiple reasons,
1) Heisenberg uncertainty principle: Electrons don't have a definite position, and direction of motion, at the same time. So we can only know of the two. Therefore the atoms are not identical in terms of the location or direction of motion at any fixed point in time.
2) Isotopes, while the number of protons in the nucleus determine the element, atoms of the same element can have different numbers of neutrons in their nuclei.
3) Electrons again, atoms can exist in energized states, therefore we may have a different number of electrons
The diagram below represents the bone
arrangements in the front limbs of three different
species of mammals.
The similarities and differences in these limbs
suggest that all three species developed from the
same ancestor, but
Answers
Answer:
these species share a common ancestor
Explanation:
How many significant figures are in 0.57
Answers
Answer:
0.57
Sig Figs
2
Decimals
2
Scientific Notation
5.7 × 10-1
Words
zero point five seven
Explanation:
Answer:
2
Explanation:
What quantities will you need to measure to determine the specific heat of one of these liquids?
Answers
The specific heat capacity of water is 4.2 joules per gram per degree Celsius or 1 calorie per gram per degree Celsius.
The work of anthropologists shirley lindenbaum, david simmons, and paul farmer serve as examples of applying anthropology to successfully address health-care problems around the world. Identify the techniques that these three anthropologists used in their work that helped them assist communities overcome debilitating health issues.
Answers
The techniques that helped these three anthropologists their work that helped them assist communities overcome debilitating health issues are:
language skillscommunity involvementWho are anthropologists?An anthropologist is described as a person engaged in the practice of anthropology.
Anthropology is said to be the study of aspects of humans within past and present societies.
The work of anthropologists like Shirley lindenbaum, david simmons, and paul farmer serve as examples of applying anthropology to successfully address health-care problems around the world. These three anthropologists had techniques used in their work their work that helped them assist communities overcome debilitating health issues. Such techniques include:
language skillscommunity involvementLearn more about anthropology at: https://brainly.com/question/1799013
#SPJ1
The data below were collected for the following reaction: 2NO2(g)+F2(g)?2NO2F(g)
[NO2](mol L?1) [F2](mol L?1) Initial Rate (mol L?1 s?1)
0.100 0.100 0.026
0.200 0.100 0.051
0.400 0.400 0.411
A) Write an expression for the reaction rate law.
Write an expression for the reaction rate law.
Rate=k[NO2][F2]
Rate=k[NO2]
Rate=k[F2]
Rate=k[NO2]2[F2]
B) calculate the value for the rate constant, k
C) What is the overall order of the reaction?
Answers
A) The expression for the reaction rate law can be written as: Rate=k[NO2][F2]
B) To calculate the value for the rate constant, we need to choose any set of concentrations and corresponding rates from the data provided and substitute them in the rate law expression. Let's use the first set of concentrations and rates:
Rate = k[NO2][F2]
0.026 mol L^-1 s^-1 = k(0.1 mol L^-1)(0.1 mol L^-1)
k = 0.026 mol L^-1 s^-1 / (0.1 mol L^-1)(0.1 mol L^-1)
k = 2.6 L^2 mol^-2 s^-1
C) The overall order of the reaction is the sum of the orders with respect to each reactant. In this case, the reaction rate law is first order with respect to both [NO2] and [F2]. Therefore, the overall order of the reaction is 1 + 1 = 2.
In summary, the expression for the reaction rate law is Rate=k[NO2][F2], the rate constant (k) was calculated to be 2.6 L^2 mol^-2 s^-1 using the initial rate data, and the overall order of the reaction is 2 since it is the sum of the orders with respect to each reactant.
To learn more about reaction click here: brainly.com/question/30464598
#SPJ11
Name these compounds according to IUPAC. (ASAPP)

Answers
The IUPAC name of the compound is propanal and acetone.
What is propanal and acetones?Propanal is also called propanaldehyde, and it is an aldehyde which has one double bond and oxygen.
Acetone is a ketone. Ketones have functional group of R2CO.
Thus, the IUPAC name of the compound is propanal and acetone.
Learn more about propanal and acetones
https://brainly.com/question/25702257
#SPJ1
what is the correct name for cr2o3
Answers
The correct name for the chemical compound \(Cr_2O_3\)is Chromium (III) Oxide.
Chromium (III) oxide is a chemical compound composed of the elements chromium and oxygen. It is a blue-green powder, and it has the chemical formula \(Cr_2O_3\). The substance is known as chromia or chrome oxide in the art world.Chromium (III) oxide is a powder that is used as a pigment and metal polish, among other things.
It is also utilized as a catalyst in a variety of chemical reactions, as well as in the production of pigments and coatings. In the field of optics, it is also utilized as a polishing agent for glass, metals, and other materials.
Chromium (III) oxide is insoluble in water and does not dissolve in any acid other than hot concentrated sulfuric acid. However, it is soluble in molten alkalis and acids. Chromium (III) oxide is used in the production of aluminum oxide, which is used as an abrasive and a refining agent in the refining of steel.
For mor esuch questions on Chromium visit:
https://brainly.com/question/30163849
#SPJ8
A gas heated and it absorbs 100 kJ of heat. It expand doing 25 kJ of work. What is the internal energy change of the gas?
125 kJ
-125 kJ
75 kJ
-75 kJ
Answers
The internal energy change of the gas is 125 kJ. So, the correct option is A.
The first law of thermodynamics can be used to calculate the internal energy change of a gas:
ΔU = Q - W
where
Q is the heat that the gas absorbs,
W is the work that the gas does, and
U is the change in internal energy.
Q = 100 kJ (stated heat absorbed by the gas).
W = -25 kJ (work done by the gas, which is negative because the system does work on itself).
Inserting Values:
ΔU = 100 kJ - (-25 kJ)
ΔU = 100 kJ + 25 kJ
ΔU = 125 kJ
As a result, the internal energy change of the gas is 125 kJ. So, the correct option is A.
Learn more about internal energy change, here:
https://brainly.com/question/3453679
#SPJ4
Among the groups of elements listed below, which have the same number of electrons in their outermost energy levels? C, K, Ca, Rb, Sr
Na,Mg,AI,S
Li,B,C,F
F,CI,Br,I
Answers
Answer:
i believe its d
Explanation:
Answer:
I checked and I too think its D
What is the electron configuration for magnesium?

Answers
Answer:
D.1s2 2s2 2p6 3s2
Explanation:
The electron configuration of magnesium is [Ne] 3s2, where [Ne] represents the electron configuration of a neutral neon atom. This means that magnesium has two electrons in its 3s orbital, with the other ten electrons being in the 1s and 2s orbitals. This electron configuration represents the arrangement of electrons in the shells around the magnesium atom's nucleus. The electron configuration is used to predict the chemical and physical properties of an element and its behavior in chemical reactions.
Allen
What gas is used in freezers and fridges?
Answers
Answer:
Chloro-Flouro-Carbon
Explanation:
Chloro-Flouro-Carbon (CFC Gas) is used as a refrigerant in fridges and freezers.
Which term describes a chemical equation in which all highly soluble compounds are written as dissociated ions
Answers
Answer: IONIC EQUATION.
Explanation:
A chemical equation is defined as the form by which a chemical reaction is represented mathematically. These are written in the form of symbols and chemical formulas of reactants and products which are taking part in the chemical reaction. A chemical equation can be written in two forms, these include:
--> MOLECULAR EQUATION: in this type of equations, the compounds are written and represented in a molecular form. This is sometimes referred to as a balanced equation.
--> IONIC EQUATION: This is a type of chemical equation in which the electrolytes in aqueous solution are expressed as dissociated ions. A typical illustrated example is seen in the reaction between AgNO3(aq) and NaCl(aq) :
Ag+(aq) + NO3-(aq) + Na+(aq) + Cl-(aq) → AgCl(s) + Na+(aq) + NO3-(aq)
The (aq) written in the above equation signifies they are in aqueous solution.
How many grams of sulfur trioxide are required to produce 2.00 mol of sulfuric acid in an
excess of water?
Answers
Describe how you would prepare a pure dry sample of lead(II) sulfate crystals starting from solutions of lead(II) nitrate and sodium sulfate.
Include a series of key steps in your answer.
Answers
Answer:
Method: Measure out 25 cm3 of 0.5 mol dm3 lead(II)nitrate solution and add it to a small beaker. Measure out 25 cm3 of 0.5 mol dm3 of potassium sulfate add it to the beaker and mix together using a stirring rod.
true/false. it is observed that when dull, grey magnesium is placed in acid, bubbles stream from the metal and the temperature rises.
Answers
The statement it is observed that when dull, grey magnesium is placed in acid, bubbles stream from the metal and the temperature rises is true.
Magnesium metal, which is drab and grey, reacts chemically with acid to produce hydrogen gas and magnesium ions in solution. Exothermic means that heat is emitted, which raises the temperature as a result of the reaction. The hydrogen gas that is being created as a result of the reaction is what is visible as bubbles.
A chemical reaction happens when magnesium (Mg) metal is dissolved in an acidic liquid like hydrochloric acid (HCl). The hydrogen ions (H+) from the acid react with the magnesium atoms on the metal's surface to create magnesium ions (Mg2+) and hydrogen gas (H2). In this particular single replacement or displacement process, magnesium replaces the acid's hydrogen:
MgCl2(aq) + H2(g) = Mg(s) + 2HCl(aq)
The fact that heat is emitted as a byproduct of this process indicates that it is exothermic. The temperature of the solution may rise as a result of the heat produced during the reaction. Because hydrogen gas is created during the reaction, which is less dense than the liquid and rises to the surface, bubbles are visible during the process.
To know more about the magnesium refer here :
https://brainly.com/question/1533548#
#SPJ11
___________ was the first major antimicrobial chemical used with toxic and irritating side effects.
Answers
This was the first major antimicrobial chemical used with toxic and irritating side effects.
One of the main jobs of _________ is to support the leaves
The flowers
the seeds
the roots
the stem
Answers
Answer:
Stem
Explanation:
Answer:
option-(d) stem
Explanation:
Stems have four main functions which are: Support for and the elevation of leaves, flowers and fruits. The stems keep the leaves in the light and provide a place for the plant to keep its flowers and fruits. Transport of fluids between the roots and the shoots in the xylem and phloem...
I hope it help you
A 2 kg bowling pin is at rest. A 6 kg bowling ball is rolling towards it at 10 m/s. If the ball
transfers all of it's momentum to the pin, what is the velocity of the pin after the collision?
Answers
P1 = P2 and since momentum is conserved, 21 = (6.0)(2.77) + (0.70). (vpin). 4.38 = 0.70 vpin, vpin = 6.3 m/s to the right, 21 = 16.62 + 0.70 vpin,
How do you determine a bowling ball's force?The bowling ball is solely affected by friction, hence Ff is used in place of F and equals mass times acceleration. The normal force (mass times gravity) multiplied by the friction coefficient equals the friction force, which also equals mass times acceleration.
How is ball velocity determined?The falling ball travels a distance of d = 12 9.8 (m/s2) t2, with a speed of v = 9.8 (m/s2) t as a function of time. The ball travels 4.9 m in a second. The falling ball's velocity is v = -9.8 (m/s2) t j, and its position is r = (4.9 m - 12 9.8 (m/s2) t2) j as a function of time.
to know more about velocity here:
brainly.com/question/18084516?
#SPJ1
how would doubling the concentration of t-bucl affect the rate of the reaction? why?
Answers
Answer:
Explanation: It will not
Which choices is a biotic factor of an ecosystem?
• sun
• ants
• air
• soil
Answers
Answer:
Aboitic factor would be soil
Biotic factor would be ants.
Explanation:
Which orbital has the highest energy in a multielectron atom? The quantum numbers are given as (n, l, ml).
Select one:
a. (3, 0, 0)
b. (4, 3, -1)
c. (4, 4, 0)
d. (4, 1, 1)
e. (3, 2, 0)
Answers
The orbital with the highest energy in a multielectron atom is represented by the quantum numbers (4, 4, 0), which is option c.
The principal quantum number (n) represents the energy level or shell in which an electron resides. As the value of n increases, the energy of the electron also increases.
Among the given options, we can see that (4, 3, -1), (4, 4, 0), and (4, 1, 1) have the same principal quantum number (n = 4). Therefore, we need to compare their values of the azimuthal quantum number (l) to determine which orbital has the highest energy.
The azimuthal quantum number (l) defines the shape of the orbital. As the value of l increases, the energy of the orbital increases. In this case, (4, 4, 0) has the highest value of l, indicating a higher energy level compared to (4, 3, -1) and (4, 1, 1).
Hence, the orbital with the highest energy in a multielectron atom is represented by the quantum numbers (4, 4, 0), which is option c.
To learn more about orbital click here: brainly.com/question/32355752
#SPJ11
PLEASE HELP FIRST GETS BRAINLIEST
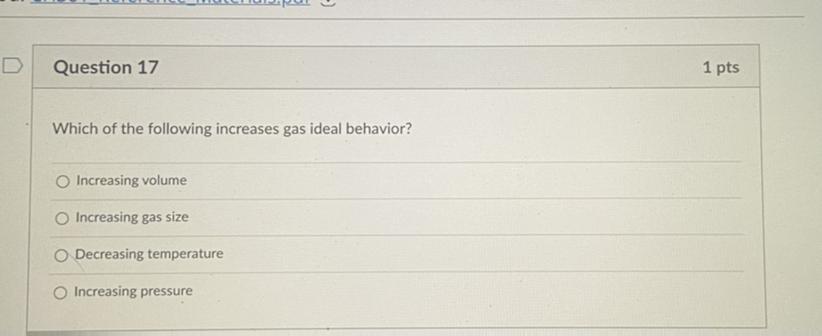
Answers
Answer:
c i guess, decrease temperature
Compared to compounds with network structures, compounds arranged in molecules (covalent bonds) have ____________ melting points.
Answers
Explanation:
.... have lower melting points.
Perform the following mathematical operation, and report the answer to the correct number of significant figures. 0.97584 ÷ 5.68 = [?]
Answers
Answer: 0.172
Explanation:
In the early 1900's many scientists thought that an atom consisted of a positive substance with negative charges scattered throughout the substance. Then Ernest Rutherford completed an experiment that changed the concept of an atom. His discovery led to the understanding that an atom. His discovery led to the understanding that an atom consists of mostly empty space with
Answers
The question is incomplete; the complete question is;
In the early 1900s many scientists thought that an atom consisted of a positive substance with negative charges scattered throughout the substance. Then Ernest Rutherford completed an experiment that changed the concept of an atom. His discovery led to the understanding that an atom consists mostly of empty with space with-
Protons orbiting a dense nucleus made of electrons and neutrons
Electrons orbiting a dense nucleus made of protons and neutrons
Neutrons and protons orbiting a cloud of electrons
Electrons and protons orbiting a cloud of neutrons
Answer:
Electrons orbiting a dense nucleus made of protons and neutrons
Explanation:
Rutherford established the nuclear theory of the atom by his famous experiment. In his experiment, alpha particles were used to bombard a thin gold foil and the movement of the particles was observed on a moveable zinc sulphide screen.
It was discovered from the experiment that the atom was mostly made up of empty space. The electrons orbit a dense nucleus comprising of protons and neutrons.
i need help Which of the following shows the correct order in which organs take part in the process of digestion
Answers
Answer:
Mouth
Esophagus
Stomach
The small intestine
Colon (large intestine)
Rectum
The solubility of KCl at 40 degrees Celsius is 39 g in 100 g of water. Suppose 82 g of KCl is added to 200 g of water at 40 degrees Celsius. Is it true that at equilibrium, most, but not all, of the KCl is dissolved and the solution is saturated? Explain.
Answers
It's not true, no. The resultant solution will be unsaturated because it contains less KCl than the amount that will solubilize in 100 g of water at 40°C, which is 39 g, when 82 g of KCl is added to just 200 g of water.
The greatest quantity of solute that may dissolve in a given amount of solvent at a certain temperature and pressure to create a saturated solution is known as solubility. The solubility of KCl in this situation at 40°C is 39 g in 100 g of water. The solution will be unsaturated if 200 g of water are combined with 82 g of KCl at 40 °C. Because more KCl has been supplied than can dissolve in 200 g of water but less than can dissolve in 300 g of water, this has occurred (100 g for each 100 g of water).
learn more about solubility here:
https://brainly.com/question/22185953
#SPJ4
(a) calculate k1 and k2. (for h3aso4, ka1 = 2.5x10-4. ka2 = 5.6x10-8, ka3 = 3.0x10-13)
Answers
Using the equations for the ionization of each proton (H+) :
k1 = 2.5x10^-4 and k2 = 2.5x10^-7. (for H₃AsO₄, ka1 = 2.5x10-4. ka2 = 5.6x10-8, ka3 = 3.0x10-13)
k1 and k2 for H₃AsO₄, we need to use the equations for the ionization of each proton (H+):
H₃AsO₄ + H₂O ⇌ H₃O+ + H₂AsO₄⁻ (Ka1)
H₂AsO₄⁻ + H₂O ⇌ H₃O+ + HAsO₄²⁻ (Ka2)
Ka1 = [H₃O+][H₂AsO₄⁻]/[H₃AsO₄]
2.5x10^-4 = [H₃O+][H₂AsO₄⁻]/[H₃AsO₄]
Ka2 = [H₃O+][HAsO₄²⁻]/[H₂AsO₄⁻]
5.6x10^-8 = [H₃O+][HAsO₄²⁻]/[H₂AsO₄⁻]
Since we are given the concentrations of H₃AsO₄, H₂AsO₄⁻, and HAsO₄²⁻ are initially negligible, we can assume that the concentrations of H₃O+ and H₂AsO₄⁻ are equal to x at equilibrium. Then, the concentration of HAsO₄²⁻ at equilibrium is (x^2)/[H₃AsO₄].
Using these assumptions and solving the equations for x, we get:
Ka1 = x^2/[H₃AsO₄] = x^2/(0.1 M) = 2.5x10^-4
x^2 = 2.5x10^-5
x = 5.0x10^-3 M
Ka2 = x^2/[H₂AsO₄⁻] = (5.0x10^-3 M)^2/(0.1 M) = 2.5x10^-7
Therefore, k1 = 2.5x10^-4 and k2 = 2.5x10^-7.
To learn more about ionization refer here:
https://brainly.com/question/1602374#
#SPJ11