Determine the electron-group arrangement, molecular shape, and ideal bond angle(s) for each of the following:
(b) NO²⁻
Answers
The electron group arrangement of NO²⁻is trigonal planar. The molecular shape is bent, and the bond angle is 120°.
What is the molecular shape of a compound?The molecular geometry of the compound shows the position of nuclei and the electron of the compound. It shows how the joining of electrons and nuclei makes the shape of the compound.
Like here, the shape of nitrite is bent with lone pair which is shown by Lewis's structure The bond angle will be the distance between the nuclei of the neighbor atoms.
Thus, the electron geometry arrangement of nitrite is trigonal planer with a bent shape and the bond angle will be 120°.
To learn more about molecular geometry, refer to the link:
https://brainly.com/question/7558603
#SPJ4
Related Questions
I WILL GIVE BRAINLEST IF YOU ANSWER THIS QUESTION QUICKLY!!!
what is an orbital?
Answers
Answer:
Electric Patterns and has many different variations.
Explanation:
A population of a wild animal is a frequent target for hunters. Hunters tend to hunt the larger animals with big horns. How might this affect the populations in the wild?
A.
Animals with average horns will increase in number.
B.
Smaller animals with a trait for short horns will become endangered.
C.
Traits for larger animals with big horns will be selected.
D.
Traits for smaller animals with short horns will be selected.
Answers
Traits for larger animals with big horns will be selected.
what is the standard form of 12,341 us?
Answers
Answer:
12341 in standard form = 1.2341 × 10⁴
Explanation:
When you split the number 12341 into a coefficient and a power of 10 you do get 12341 in exponential form, but there is an indefinite number of possibilities.
As you probably want 12341 in normalized scientific notation, the coefficient or significand of 12341 in scientific notation must be in the interval [1,10[.
As there are many ways to express 12341 in scientific notation, in this post we mean 12341 in normalized scientific notation, unless stated otherwise. Therefore:
12341 in scientific notation = 1.2341 × 10⁴.
This can also be expressed as 1.2341 × 10⁴, using the caret symbol, or as 1.2341e+4, which is called 12341 in e-notation.
Decide whether each proposed multiplication or division is possible from the image below and restore the results if they are I don’t need an explanation just the answer please

Answers
The question presents a table with many multiplications using different units (mass and velocity) and requires us to decide whether the operations presented are possible.
To solve this question, we should keep in mind this general rule: for units reffering to the same measurement (for example: mass, volume, distance etc.), we are able to multiply the units as long as they are the same. For example: g x g, L x L, m x m. If units are about the same measurement but still different, we should convert one of them in order to make them the same.
On the other hand, when we are talking about division, if we have the same unit on the operator and denominator position, we will have a result as "1" - then we need to analyze the entire value.
Now, considering the operations presented in the question:
On the first one, we have kg multiplied by kg. Although it isn't usual to see square kg (kg^2), we are able to make this operation:
\(9.0\text{ kg }\times5.0\text{ kg}=45kg^2\)On the second line, we have square g multiplied by kg. In this case, since the first unit is square, we can assume they are not about the same measurement. Thus, we can't complete this operation.
On the third line, there is a division and we should proceed as a normal operation (although the unit km/s isn't usual):
\(\frac{5.6\text{ km}}{7.0\text{ s}}=0.8\text{ km/s}\)In summary, the answers should be:
1) Is it possible? YES / Result = 45 kg^2
2) Is it possible? NO
3) Is it possible? YES / Result = 0.8 km/s
ordering question click and drag on elements in order rank the following 0.1 m salt solutions in order of increasing ph (lowest ph at the top of the list).
Answers
NaHSO4 <NH4NO3<NaHCO3<Na2CO3 salt solutions in order of increasing ph (lowest ph at the top of the list).
The pH scale determines how basic or acidic aqueous or other liquid solutions are. The expression converts the hydrogen ion concentration, which typically ranges between 0 and 14, into values between 1 and 1014 gram-equivalents per litre. It is widely employed in the fields of biology, agronomy, and chemistry. Because it has a pH of 7, or 107 gram-equivalents of hydrogen ions per litre, pure water is neutral (neither acidic nor alkaline).
In an aqueous solution, the amount of hydrogen ions expressed in equivalents per litre is equal to log(H+). Since it indicates which crops will thrive there, a soil's pH is probably the most significant factor affecting its moisture content in agriculture.
To know more about pH visit : https://brainly.com/question/17603212
#SPJ4
Prediction for Scandium (II) and Cl
Answers
Answer:
(a) Eka-aluminum and gallium are two names of the same element as Eka-Aluminium has almost exactly the same properties as the actual properties of the gallium element. The properties: atomic mass, density, melting point, formula of chloride and formula of oxide are almost the same.
Explanation:
Scandium - Eka boron.
(ii) Gallium - Eka aluminium.
(iii) Germanium - Eka silicon.
I am the 1st element in the periodic table
Your answel
Answers
Answer:
Its Hydrogen
Explanation:
Which one will fill up first?? and explain how?,
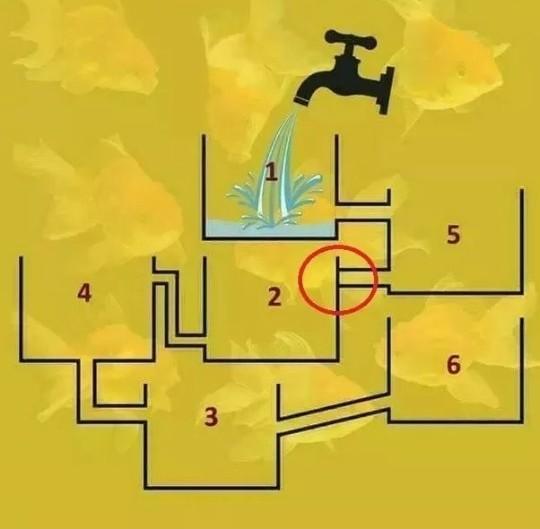
Answers
Answer:
5
Explanation:
because 2 box is close mark brainlist plss
Answer:
5th fill first....
hope it's help you
What part of the plant takes up water and nutrients from the soil?
Answers
Answer:
the stem
Explanation:
yjsbsksvznzbfb
What is the chemical formula for 8.6 mol of sulfur and 3.42 mol of phosphorus
Answers
The chemical formula for the compound containing 8.6 mol of sulfur and 3.42 mol of phosphorus is P₂S₅
How do I determine the formula of the compound?From the question given above, the following data were obatined:
Sulphur (S) = 8.6 molesPhosphorus (P) = 3.42 moleChemical formula =?The chemical formula of the compound can be obtained as follow:
Divide by their molar mass
S = 8.6 / 32 = 0.26875
P = 3.42 / 31 = 0.11032
Divide by the smallest
S = 0.26875 / 0.11032 = 2.44
P = 0.11032 / 0.11032 = 1
Multiply by 2 to express in whole number
S = 2.44 × 2 = 5
P = 1 × 2 = 2
Thus, the chemical formula is P₂S₅
Learn more about empirical formula:
https://brainly.com/question/9459553
#SPJ1
The three major minerals involved in bone maintenance are
A. calcium, potassium, and phosphorus.
B. calcium, magnesium, and phosphorus.
C. calcium, magnesium, and potassium.
D. magnesium, phosphorus, and potassium.
E. calcium, sulfur, and potassium.
Answers
The correct answer is B. calcium, magnesium, and phosphorus. These three minerals are essential for bone health and maintenance. Calcium is the primary mineral that provides strength and structure to bones.
Magnesium is important for the activation of enzymes involved in bone metabolism and is also required for the proper utilization of calcium. Phosphorus is another crucial mineral that makes up a significant portion of the mineralized matrix of bones. Together, these three minerals play a vital role in maintaining healthy bones.
To know more about calcium refer here
https://brainly.com/question/30954368#
#SPJ11
What is the formula for Cobalt (2) Phosphide?
Answers
How many grams of H20 will be formed when 32.0 g H2 reacts with 16.0 g
O2? *
2H2 + O2 + 2H2O
9.00 g
16.0 g
18.0 g
32.0g
Answers
Answer:
18.0 g
Explanation:
M(H2) = 2.0 g/mol
32.0g * 1 mol/2 g= 16 mol H2
M(O2) = 32 g/mol
16.0 g * 1mol/32 g= 0.5 mol O2
2H2 + O2 ---> 2H2O
from reaction 2 mol 1 mol
given 16 mol 0.5 mol
We see that O2 is a limiting reactant.
2H2 + O2 +-------> 2H2O
from reaction 1 mol 2 mol
given 0.5 mol x mol
x =(0.5*2)/1= 1 mol H2O
M(H2O)= 18 g/mol
18 g/mol* 1 mol = 18 g H2O
(A) The mass of zinc sulfide that is dissolved in 150 mL of a saturated solution is _____ grams.
(B) The mass of manganese(II) hydroxide that is dissolved in 175 mL of a saturated solution is____ grams.
Answers
After considering the given data we conclude that the answers for the given sub questions are
a) the mass of zinc sulfide that is dissolved in 150 mL of a saturated solution is \(2.0*10^{-12} grams\).
b) the mass of manganese(II) hydroxide that is dissolved in 175 mL of a saturated solution is \(1.9*10^{-4} grams\).
(a) To calculate the mass of zinc sulfide that is dissolved in 150 mL of a saturated solution, we can use the Ksp value for zinc sulfide, which is \(2.0*10^{-25}\). The molar solubility of zinc sulfide can be calculated using the Ksp value as follows:
\(Ksp = [Zn^{2+} ][S^{2-} ] = (x)(x) = x^2\)
\(x = \sqrt(Ksp) = \sqrt(2.0*10^{-25} ) = 1.4*10^{-13} M\)
The number of moles of zinc sulfide dissolved in 150 mL of the saturated solution is:
\(n = M *V = (1.4*10^{-13} mol/L) *0.150 L = 2.1*10^{-14} mol\)
The mass of zinc sulfide dissolved in 150 mL of the saturated solution is:
\(mass = n * molar mass = (2.1*10^{-14} mol) * (97.47 g/mol) = 2.0*10^{-12} g\)
The mass of zinc sulfide dissolved in 150 mL of the saturated solution is:
\(mass = n * molar mass = (2.1*10^{-14} mol) *(97.47 g/mol) = 2.0*10^{-12} g\)
Therefore, the mass of zinc sulfide that is dissolved in 150 mL of a saturated solution is \(2.0*10^{-12} grams.\)
(b) To calculate the mass of manganese(II) hydroxide that is dissolved in 175 mL of a saturated solution, we can use the Ksp value for manganese(II) hydroxide, which is \(4.5*10^{-14}\). The molar solubility of manganese(II) hydroxide can be calculated using the Ksp value as follows:
\(Ksp = [Mn^{2+} ][OH^{-} ]^2 = (x)(2x)^2 = 4x^3\)
\(x = (Ksp/4)^{(1/3)} = (4.5*10^{-14} /4} )^{(1/3)} = 1.2*10^{-5} M\)
The number of moles of manganese(II) hydroxide dissolved in 175 mL of the saturated solution is:
\(n = M * V = (1.2*10^{-5} mol/L) * 0.175 L = 2.1*10^{-6} mol\)
The mass of manganese(II) hydroxide dissolved in 175 mL of the saturated solution is:
\(mass = n *molar mass = (2.1*10^{-6} mol) * (88.94 g/mol) = 1.9*10^{-4} g\)
Therefore, the mass of manganese(II) hydroxide that is dissolved in 175 mL of a saturated solution is \(1.9*10^{-4} grams.\)
To learn more about molar solubility
https://brainly.com/question/30298910
#SPJ4
The complete question is
(a) The mass of zinc sulfide that is dissolved in 150 mL of a saturated solution is _____ grams.
(b) The mass of manganese(II) hydroxide that is dissolved in 175 mL of a saturated solution is____ grams.
ksp for zinc sulfide is 2.0x10-25
ksp for manganese hydroxide is 4.5x10-14
How might the structure of an atom be modeled
Answers
Answer:
you can explore the structure of atoms as you add protons, neutrons, or electrons to a model and the name of the element you have created
Explanation:
A The birthrate is higher than the death rate.
B The fertility rate is decreasing quickly.
C The immigration rate is higher than the emigration rate.
D The carrying capacity has been surpassed.

Answers
Answer:
A. The birthrate is higher than the death rate
Explanation:
How is baking bread a chemical reaction
Answers
Answer:
In the process of baking bread, the chemical reaction happens because by consuming the sugar, the yeast has created new substances—carbon dioxide and ethanol.
Explanation:
Brainliest please! I am so close to getting my next ranking! I would really appreciate it, and it would make my day! Thank you so much, and have a wonderful rest of your day!
Which type of plant used light, feathered seeds?
What would a zebra be?
consumer
decomposer
producer
Answers
Answer:
Zebra- consumer
Tiger- consumer
Worm- decomposer
Tree-Producer
Molded bread- decomposer
Plant- producer
Answer:
Type of plant - Dandelion .Zebra is a Consumer .Explanation:
Dandelion plant produces light and feathery seeds .
Zebra consumes / eats grass so it is a consumer .
What is the best description of radiation
Answers
Radiation is the emission and propagation of energy in the form of waves, rays, or particles. There are three main types of radiation Non-ionizing radiation , Ionizing radiation, and Neutrons.
Non-ionizing radiation is the release of energy from the lower-energy region of the electromagnetic spectrum. Ionizing radiation is radiation with sufficient energy to remove an electron from an atomic orbital, forming an ion. Neutrons are particles found in the atomic nucleus.
Radiation includes the emanation of any portion of the electromagnetic spectrum, plus it includes the release of particles. An example is a burning candle that emits radiation in the form of heat and light. Radiation is the release of energy, whether it takes the form of waves or particles. Radioactivity refers to the decay or splitting of an atomic nucleus. A radioactive material releases radiation when it decays.
learn more about radiation at -
brainly.com/question/2496523
how many atoms are in 4(NH3)2NO2?
Answers
There are a total of 38 atoms in the compound 4(NH3)2NO2.
How to find the number of atomsTo determine the number of atoms in the given compound, we first need to break down the formula and identify the number of atoms in each element.
The formula is 4(NH3)2NO2, which can be written as:
4 x 2(NH3) + 1 x 2(NO2)
Each molecule of NH3 contains
one atom of nitrogen and three atoms of hydrogenEach molecule of NO2 contains
one atom of nitrogen and two atoms of oxygen.Therefore, the total number of atoms in the given compound is:
4 x 2 x (1 nitrogen atom + 3 hydrogen atoms) + 1 x 2 x (1 nitrogen atom + 2 oxygen atoms)
= 4 x 2 x 4 + 1 x 2 x 3
= 32 + 6
= 38
Learn more about atoms at
https://brainly.com/question/6258301
#SPJ1
Chemists can identify the composition of some unknown salts by conducting a flame test. When potassium salts are heated in a flame, a purple color is observed.
This is due to the movement of electrons between energy levels. What is the electron configuration of a potassium atom at ground state?
answer choices
1s2; 2s2; 2p6; 3s2; 3p6; 4d1
1s2; 2s2; 2p6; 3s2;3p6; 3d1
1s2; 2s2; 2d6; 3s2; 3d6; 4s1
1s2; 2s2; 2p6; 3s2; 3p6; 4s1
Answers
The electron configuration of a potassium atom at ground state is 1s²2s²2p⁶3s²3p⁶4s¹. Therefore, option D is correct.
What is an electronic configuration?The electron configuration of an element can be explained as electrons being occupied in different energy levels of an atom of a specific element. In the electron configuration, the electrons are usually written as a superscript of atomic subshells. For example, the electron configuration of Helium can be represented as 1s²2s².
The sequence of completely filled subshells similar to neighboring the electronic configuration of a noble gas is represented by square brackets. The principal quantum number (n) will be used to denote the maximum number of electrons in an electron shell.
The total number of electrons occupied in the given electronic configuration 1s²2s²2p⁶3s²3p⁶4s¹ is 19. The atomic number of potassium is 19 therefore it is the configuration of potassium.
Learn more about electronic configuration, here:
brainly.com/question/5624100
#SPJ4
difference between soap and detergent
Answers
Soap is potassium or sodium salts of a carboxylic acid attached to a long aliphatic chain. Detergent is the potassium or sodium salts of a long alkyl chain ending with a sulfonate group.
students mixed two liquids in a beaker and listed their observations
> Liquid 1 was colorless
> Liquid 2 was colorless
> The mixture of liquids 1 and 2 formed a colorless solution
> small, solid particles formed and fell to the bottom of the beaker
based on these observations, which statement contains the best evidence that a chemical reaction occurred?
A. There is a change in shape
B. there is a change in volume
C. the two liquid mix into a solution
D. the two liquids made a new substance
Answers
The two liquids made a new substance.
What is a physical and chemical change?A chemical change results from a chemical reaction, while a physical change is when matter changes forms but not chemical identity.
A chemical change occurs when some substances are transformed into different substances, with different nature and properties. In this case when both liquids are mixed a solid is formed. This is an example of a change of state of the liquids reactants to the solid product, which has properties and characteristics different from that of the initial liquids of the mixture which is called a chemical change.
Hence, option D is correct.
Learn more about physical and chemical change.
https://brainly.com/question/13161870
#SPJ1
why isn't lactic acid fermentation a reasonable long term solution to lack of oxygen
Answers
Lactic acid fermentation is not a reasonable long-term solution to a lack of oxygen because it is an inefficient process in terms of energy production and can lead to the accumulation of lactic acid.
During lactic acid fermentation, glucose is converted into lactic acid in the absence of oxygen. This process allows for the regeneration of NAD+ to sustain glycolysis, which is the primary pathway for ATP production in the absence of oxygen. However, lactic acid fermentation produces only a limited amount of ATP compared to aerobic respiration, which is the process that occurs in the presence of oxygen.
Additionally, lactic acid buildup can lead to a decrease in intracellular pH, causing acidification of the cells. This acidic environment can negatively impact cellular processes and potentially lead to cell damage. The accumulation of lactic acid also limits the ability of cells to perform essential functions, including muscle contraction.
In contrast, aerobic respiration, which occurs in the presence of oxygen, is a much more efficient process for ATP production. It generates a significantly higher amount of ATP per glucose molecule compared to lactic acid fermentation. Therefore, in the long term, relying solely on lactic acid fermentation would result in reduced energy production and potential harm to cellular function.
To learn more about fermentation , click here: brainly.com/question/29760642
#SPJ11
How is Mass converted to the energy released in the fire
Answers
Answer:
The heat and light released by fire comes from the breaking of chemical bonds. ... The mass of each molecule, before burning, exceeds the total mass of its atoms by a tiny amount that is equivalent (through E=mc²) to the total energy of all its atomic bonds.
Explanation:
I hope this helps (:
a small cylinder of oxygen contains 300.0mL of gas at 15 atm. What will the volume of this gas be when released into the atmosphere at 0.900atm?
Answers
Under pressure, oxygen is contained in oxygen cylinders, and as the cylinder is used down, the pressure gauge steadily decreases.
What is the capacity of small oxygen cylinder?A small cylinder called the D size, which can contain 340 litres of oxygen at 13 700 kPa, is frequently fastened to the side of an ambulance stretcher. With an integrated valve and 460 litres of capacity at 23 000 kPa, the CD size is a more recent variation of the D.The small, transportable E cylinder, which has a volume of 0.68 m3 and weighs only 7 kg, is one of the sizes available for industrial oxygen cylinders.Three sizes of medical oxygen cylinders are offered. Small (.4 m3) is referred to as A size, Medium (1.6 m3) as D size, and Large (2.5 m3) as E size.The patient's oxygen saturation rises by 3–4% for every liter/minute of oxygen. There are 21% oxygen molecules in room air. In this case, a patient receiving 4 L/min of oxygen.To learn more about oxygen refer to:
https://brainly.com/question/29016884
#SPJ1
The picture above is a diagram of what earth-sun-moon phenomena?
A.Solar System
B.Solar Eclipse
C.Lunar Eclipse
D.Earth-Sun-Moon
If you don't know plz don't answer

Answers
a student obtained a wet burette from the cart but failed to rinse it with a small amount of the base before starting a titration. will more or less titrant (base) be required to neutralize the acid?
Answers
The student failed to rinse a wet burette with a small amount of base before starting a titration. The student would need more titrant (base) to neutralize the acid than if they had rinsed the burette before starting the titration.
The reason for this is that when a wet burette is not rinsed with the titrant, the remaining water in the burette can dilute the titrant, thereby decreasing its concentration. If the titrant is diluted, more of it would be required to neutralize the same amount of acid. This would result in a titration that requires more titrant (base) to neutralize the acid than if the burette had been properly rinsed with the base.
In other words, not rinsing the burette with a small amount of base can affect the accuracy of the titration results. It is, therefore, important to properly rinse the burette with the titrant before starting a titration to avoid diluting the titrant and ensure accurate titration results.
In conclusion, more titrant (base) would be required to neutralize the acid if a wet burette is not rinsed with a small amount of base before starting a titration. It is essential to rinse the burette before starting a titration to avoid dilution of the titrant and ensure accurate titration results.
for more such question on titration
https://brainly.com/question/186765
#SPJ11
Helium only has two valence electrons and is a stable atom. How is this possible when atoms
need 8 valence electron to become stable?
Answers
Answer:
Explanation:
Helium (He) is similar in that it, too, only has room for two electrons in its only valence shell. Hydrogen and helium have only one electron shell. The first shell has only one s orbital and no p orbital, so it holds only two electrons. Therefore, these elements are most stable when they have two electrons.
Thermal energy always moves from _ _ _ _ to _ _ _ energy.
Fill in the blanks.
Answers
Thermal energy always moves from higher energy area to lower energy area over a temperature gradient.
What is thermal energy?Thermal energy is a form of energy by virtue of temperature of a body. As the temperature of a body increase its thermal energy increases. Thermal energy or heat energy transfers though medium and vacuum from higher energy area to lower energy area.
Heat transfer can be through conduction in solids, convection in fluids and by radiation through space. In conduction, the thermal energy easily transfers through a chain of close packed molecules.
In all these mode of heat transfer heat energy is flowing from a hotter region to a colder region.
Find more on thermal energy:
https://brainly.com/question/18989562
#SPJ1