describe the difference between corrosion and rust
Answers
The main difference between corrosion and rust is that corrosion occurs as a result of the chemical influence and it affects a lot of materials whereas rusting is only accelerated by certain chemicals and usually affects iron substances
Hopes this helps :)
Related Questions
Express the dosage using the ratio format you prefer. (Use mg for milligrams and mL for an injectable solution that contains 250mg in each 0.6 mL 3. [-/3 Points] CURRENMEDMATH11 12.3.002. EP. Consider the following. A 40mg in 2.5 mL solution will be used to prepare a 26mg dosage. Calculate the dosage using ratio and proportion. Express your final answer in mL to the
40mg
mL
=
X mL
26mg
40x
X
=
=
mL
[-/1 Points] CURRENMEDMATH11 12.3.004. Calculate the dosage (in milliliters). Express your answer to the nearest tenth. Assess y A 36mg per 2 mL strength solution is used to prepare 22mg. mL
Answers
The dosage of 26mg can be prepared using approximately 1.625 mL of the 40mg in 2.5 mL solution.
The dosage of 22mg can be prepared using approximately 1.222 mL of the 36mg per 2 mL strength solution.
To calculate the dosage using ratio and proportion, we can set up a proportion based on the strength of the solution.
40mg in 2.5 mL solution will be used to prepare a 26mg dosage.
Let X represent the mL of the solution needed to prepare the 26mg dosage.
We can set up the proportion as follows:
40mg/2.5mL = 26mg/X mL
Cross-multiplying and solving for X, we have:
40mg * X mL = 2.5mL * 26mg
40X = 65
X = 65/40
X ≈ 1.625 mL
For the second question:
36mg per 2 mL strength solution is used to prepare 22mg.
Let Y represent the mL of the solution needed to prepare the 22mg dosage.
We can set up the proportion as follows:
36mg/2mL = 22mg/Y mL
Cross-multiplying and solving for Y, we have:
36mg * Y mL = 2mL * 22mg
36Y = 44
Y = 44/36
Y ≈ 1.222 mL
To know more about solution
https://brainly.com/question/1616939
#SPJ11
Using the data below and Coulomb's law, calculate the energy change for this reaction (per formula unit of CsBr).
Cs(g) + Br(g)
CsBr(g)
Ionization Energy
Atom I1 (aJ)
Na 0.824
K 0.696
Cs 0.624
Electron Affinity
Atom E A1 (aJ)
F -0.545
Cl -0.580
Br -0.540
I -0.490
Ionic Radius
Cation Radius (pm)
Na+ 102
K+ 138
Cs+ 167
Ionic Radius
Anion Radius (pm)
F- 133
Cl- 181
Br- 196
I- 220
Answers
The energy change for the reaction (per formula unit of CsBr) is approximately -6.22 x 10^14 kJ.
Ionization Energy (I1) of Cs: 0.624 aJ
Electron Affinity (EA1) of Br: -0.540 aJ
Cation (Cs+) Ionic Radius: 167 pm
Anion (Br-) Ionic Radius: 196 pm
1. Calculate the lattice energy using Coulomb's law:
Lattice energy = (k * |Q1 * Q2|) / r
Where k is the electrostatic constant (8.99 x 10^9 N·m^2/C^2), Q1 and Q2 are the charges of the ions, and r is the distance between the ions.
Q1 = +1 (charge of Cs+)
Q2 = -1 (charge of Br-)
r = sum of the ionic radii = 167 pm + 196 pm = 363 pm = 3.63 x 10^-10 m
Lattice energy = (8.99 x 10^9 N·m^2/C^2) * |(1.602 x 10^-19 C * 1) * (1.602 x 10^-19 C * -1)| / (3.63 x 10^-10 m)
Lattice energy = (8.99 x 10^9 N·m^2/C^2) * (2.571 x 10^-38 C^2) / (3.63 x 10^-10 m)
Lattice energy ≈ 6.34 x 10^-19 J
2. Convert the energy change to kilojoules:
Energy change = (0.624 aJ + (-0.540 aJ) - 6.34 x 10^-19 J) * (1 x 10^-3 kJ / 1 J)
Energy change ≈ (0.624 - 0.540 - 6.34 x 10^-19) x 10^-3 kJ
≈ -6.22 x 10^14 kJ.
Learn more about Ionization Energy at https://brainly.com/question/30831422
#SPJ11
The arrangement of particles is most ordered in a sample of
1.
NaCl(aq)
2.
NaCl(l)
3.
NaCl(g)
4.
NaCl(s)
PLEASE HELP
Answers
We have that the arrangement of particles of NaCl is most ordered in a sample of
NaCl(s)
i.e solid NaCl
From the question we are told
The arrangement of particles is most ordered in a sample of
1. NaCl(aq)
2. NaCl(l)
3. NaCl(g)
4. NaCl(s)
NaCl Generally known as sodium chloride or salt exist in four main states as shown
1. NaCl(aq)
2. NaCl(l)
3. NaCl(g)
4. NaCl(s)
Mow in a subject of its arrangement of particles we can see that as its state changes from gaseous through to solid it gains in form and arrangement of particles
Therefore
The arrangement of particles of NaCl is most ordered in a sample of
NaCl(s)
i.e solid NaCl
For more information on this visit
https://brainly.com/question/1641336
A 93-L sample of dry air cools from 145°C to -22°C while the pressure is maintained at 2.85 atm. What is the final volume?
Answers
This question is an example of Charles' law.
Charles' law- This law states that at constant pressure, the volume of a given amount of gas is directly proportional to the temperature (in kelvin K). Therefore, with the increase in volume of the gas, the temperature increases whereas with decrease in volume of the gas, the temperature decreases.
Formula of Charles' law-
V₁/T₁=V₂/T₂
where,
V₁= Initial volume
V₂= Final volume
T₁= Initial temperature
T₂= Final temperature
Given,
V₁= 93mL
T₁ = 144°C + 273.15 = 417 K
T₂ = -22°C + 273.15 = 251 K
Therefore, V₂ = V₁T₂/T₁
= (93mL × 251 K)/ 417 K = 56 mL
Learn more about Charles' Law here-
https://brainly.com/question/16927784
#SPJ4
If 27g of N2 reacts with 5.4g of H2, how much NH3 will be
produced?
Answers

Can someone do a True or false for these

Answers
Answer:
all i can accurately say is that 2 and 4 are both true
Raquel has collected $3. 80 in nickels and dimes. She has exactly 48 nickels. How many dimes does she have?.
Answers
Answer:
14 dimes
Explanation:
48 nickels = 48 x .05 = $2.40
$3.80 - $2.40 = $ 1.40
$1.40 / .10 = 14 dimes
What pillar of sustainability is broken by recycling
electronics in India? Should the US make a law that electronics can
only be recycled in the US?
Answers
The pillar of sustainability broken by recycling electronics in India is environmental sustainability. Implementing a law that restricts electronics recycling to the US would not necessarily be the most effective solution, as it overlooks the complex global dynamics of electronic waste management.
Recycling electronics in India often involves improper disposal methods, such as burning or dismantling without proper safety measures. This leads to environmental pollution, including the release of hazardous substances into the air, soil, and water, thus violating the principle of environmental sustainability.
However, simply mandating that electronics can only be recycled in the US may not be the most optimal solution. Electronic waste is a global issue, and restricting recycling to a single country disregards the fact that electronic products are manufactured and consumed worldwide. A more comprehensive approach to addressing electronic waste would involve international cooperation, strict regulations, and monitoring of recycling practices to ensure they meet environmental standards.
Efforts should focus on improving recycling practices globally, including promoting responsible electronic waste management, developing sustainable recycling infrastructure in multiple countries, and encouraging the adoption of safe and environmentally friendly recycling practices. This approach would foster global sustainability and address the challenges associated with electronic waste disposal more effectively than a geographically limited restriction.
To learn more about sustainability, here
https://brainly.com/question/32771548
#SPJ4
Which of the following acids will not dissociate completely in water? Pick only one. HCl HClO4 HClO HNO3
Answers
HClO will not dissociate completely in water among the given option.
When acids dissolve in water, they can dissociate into ions. Strong acids dissociate completely, while weak acids only partially dissociate. To determine which acid will not dissociate completely, we need to identify the weak acid among the options.
HClO is a weak acid known as hypochlorous acid. It does not dissociate completely in water. Instead, it partially dissociates into H⁺ and ClO⁻ ions.
On the other hand, HCl, HClO₄, and HNO₃ are strong acids and dissociate completely in water, producing H⁺ ions. These strong acids are considered to be fully ionized in aqueous solutions.
learn more about acids here:
https://brainly.com/question/29796621
#SPJ11
What most likely happens during this reaction

Answers
Answer:
I think that it is A I am sorry if I am wrong
Explanation:
Choose the paramagnetic species from below.
Ar
O
Ti4+
All of the above are paramagnetic.
None of the above are paramagnetic.
Answers
The correct answer is option (c) Ti4+.
The species which are attracted to a magnetic field are known as paramagnetic species. If we talk about the given options, then we can see that there are only 3 species that are given. Out of these three, only Ti4+ is paramagnetic. How can we determine whether a species is paramagnetic or not? The species which contain unpaired electrons are paramagnetic in nature. If there are all paired electrons, then the species are diamagnetic. If we talk about Ti4+, then it contains 2 unpaired electrons, which makes it paramagnetic. This is the reason why the correct answer is Ti4+.In Ar, all the electrons are paired, which makes it diamagnetic. In O, there are 2 unpaired electrons, which makes it paramagnetic. How can we determine whether a species is paramagnetic or not? The species which contain unpaired electrons are paramagnetic in nature. If there are all paired electrons, then the species are diamagnetic.
Learn more about paramagnetic species at brainly.com/question/29990302
#SPJ11
Can I find a tutor help me wit this question?

Answers
Chemistry =>Introduction to Chemistry => Scientific Method
A scientific method corresponds to a methodology to obtain new knowledge.
We must start from an idea, an assumption of how a compound or a process behaves, this is our hypothesis.
Following this, we must identify what can affect our process, what are the variables, and what will be the response variable, for this we carry out an experiment.
Once the experiments have been carried out, we must analyze the results, draw conclusions as to why the behavior occurs, and if our hypothesis is true or not.
It is useless for us to obtain new knowledge if we do not share it, We have to share the results, in this way other people can start from that knowledge to create another,
Therefore, the answer will be:
1. Make a hypothesis
2. Conduct an experiment
3. Analyze the experiment data
4. Communicate the results
after millikan performed his oil drop experiemnt, he concluded that
Answers
After Millikan performed his oil drop experiment, he concluded that the fundamental unit of electric charge is the same for all charged particles and that it is a constant value.
He also determined the value of this unit of charge, which is now known as the elementary charge and is equal to approximately 1.602 x 10^-19 coulombs. Millikan's experiment also confirmed the existence of electrons and provided a way to measure their charge and mass. This experiment was a major breakthrough in understanding the nature of electricity and laid the foundation for many further discoveries in the field of particle physics.
More on Millikan: https://brainly.com/question/31906412
#SPJ11
what happens to nitrogen during the process of denitrification?
Answers
Denitrification is the process by which nitrates and nitrites in the environment are reduced to nitrogen gas (\(N_2\)) by removing and returning bioavailable nitrogen to the atmosphere.
Denitrification is a natural process that occurs in the environment and is primarily carried out by certain types of bacteria, such as Pseudomonas, Thiobacillus, and Clostridium. During this process, the bacteria break down nitrates and nitrites into nitrogen gas using a series of oxidation-reduction reactions. The nitrates and nitrites are first converted to nitric oxide (\(NO\)) and nitrous oxide (\(N_2O\)) gases which are then converted to nitrogen gas, which is released into the atmosphere. This process is important in the nitrogen cycle and is essential for the removal of nitrogen from the environment.
To learn more about nitrogen click here https://brainly.com/question/2396742
#SPJ4
what are the valency and valence electrons of
1) Calcium
2) Magnesium
3)Oxygen
4)Argon
Answers
Answer:
calcium - valency -: ( 2) valence electron -: 2magnesium - valency -:(2) valence electron -: 2 oxygen - valency. -: (2) valence electron -:. 8argon -. valency -:. (doesn't have because it is a noble gas so that it doesn't have valency). valence electron-:. 8.....Explanation:
hopes it help you a lot
pls. (mark me as brainlist ).......
The pH of a 0.100 M solution of a weak acid, HA, is 3.50. Calculate the percent ionization of the acid in 0.100 M solution.
a. 0.016%
b. 0.078%
c. 0.32%
d. 0.68%
e. 1.6%
Answers
The percent ionization of the acid in 0.100 M solution is c. 0.32%
To calculate the percent ionization of a weak acid (HA) in a 0.100 M solution, we will first determine the ionization constant (Ka) and then the percent ionization. Here's a step-by-step explanation:
1. From the given pH, determine the H+ ion concentration: pH = -log[H+]
3.50 = -log[H+]
[H+] = 10^(-3.50) = 3.16 x 10^(-4) M
2. Write the ionization reaction of the weak acid, HA:
HA ⇌ H+ + A-
3. Set up the Ka expression:
Ka = [H+][A-]/[HA]
4. Determine the concentrations of HA, H+, and A-:
- Initial concentrations: [HA] = 0.100 M, [H+] = 0, [A-] = 0
- At equilibrium: [HA] = 0.100 - x, [H+] = x, [A-] = x (Here, x represents the change in concentrations due to ionization.)
5. Plug in the H+ concentration obtained from the pH:
x = 3.16 * 10^(-4)
6. Calculate the Ka:
Ka = (3.16 *10^(-4))(3.16 * 10^(-4))/(0.100 - 3.16 * 10^(-4))
Ka ≈ 1.00 *10^(-5)
7. Calculate the percent ionization:
Percent ionization = (Ionized acid concentration / Initial acid concentration) x 100
Percent ionization = (3.16 x 10^(-4) / 0.100) x 100 ≈ 0.316%
The closest option to the calculated value is c. 0.32%, which is the answer.
learn more about weak acid Refer: https://brainly.com/question/22104949
#SPJ11
What happens to the atomic mass of the elements moving from left to right within a period?
A. The atomic mass stays the same within a period.
B. The atomic mass fluctuates throughout the period.
C. The atomic mass decreases.
D. The atomic mass increases.
Answers
Answer:
The atomic mass increases.
Explanation:
if 20 ml of a 1.0 m hydrochloric acid solution will neutralize 30 ml of sodium hydroxide, what is the molarity of the sodium hydroxide?
Answers
The molarity of the sodium hydroxide is 0.67 M.
The balanced chemical equation for the neutralization reaction between hydrochloric acid and sodium hydroxide is:
HCl + NaOH → NaCl + H2O
From the equation, we know that 1 mole of HCl reacts with 1 mole of NaOH. Therefore, the number of moles of HCl in 20 ml of a 1.0 M solution is:
moles of HCl = (20 ml) x (1.0 mol/L) x (1 L/1000 ml) = 0.02 mol
Since 1 mole of HCl reacts with 1 mole of NaOH, the number of moles of NaOH that reacts with the HCl is also 0.02 mol. The molarity of the NaOH solution can be calculated by dividing the number of moles of NaOH by the volume of the solution used:
Molarity of NaOH = (0.02 mol) / (30 ml x 1 L/1000 ml) = 0.67 M
The molarity of the sodium hydroxide solution is 0.67 M.
To know more about molarity, visit;
https://brainly.com/question/30404105
#SPJ11
How many miles of Al2O3 are produced by the reaction 200.g Al
Answers
The moles of Al₂O₃ produced by the reaction of 200g of Al with O₂ is 3.7 . 4 moles of Al reacts with 3 moles of O₂ to produce 2 moles of Al₂O₃ according to the reaction.
What is a mole?A mole is exactly 6.022 × 10²³, which is the number of particles defined by Avogadro. For all intents and purposes, the mass of one mole of a substance in grams and the mass of one molecule in Daltons are essentially equivalent.
The amount of anything with the same number of particles as 12.000 kg of carbon-12 is known as a mole. Avogadro's Number is that number of particles, which is approximately 6.02 × 10²³. 6.02 × 10²³ carbon atoms make up a mole of carbon.
In the reaction:
4Al(s) + 3O₂(g) → 2Al₂O₃(s)
According to stoichiometry, 4 moles of Al reacts with 3 moles of O₂ to produce 2 moles of Al₂O₃.
Number of moles of Al;
Moles = \(\frac{200}{27}\) = 7.4 moles
4 moles of Al produce 2 moles of Al₂O₃.
So, the moles of Al₂O₃ produced by 7.4 moles of Al will be;
Moles = \(\frac{2}{4}\) × 7.4 = 3.7 moles of Al₂O₃.
To know more about stoichiometry, visit;
https://brainly.com/question/30215297
#SPJ1
What is the partial pressure of oxygen in a mixture that contains 0.30 moles of O2, 0.70 moles of N2, and 0.25 moles of Ar with a total pressure of 1.1 atm? a) 0.33 atm b) 0.26 atm c) 0.35 atm d) 0.30 atm e) 0.80 atm
Answers
The partial pressure of oxygen in the mixture is approximately 0.26 atm, which corresponds to option b) 0.26 atm.
How to find partial pressure of a gas molecule?
The partial pressure of oxygen in a mixture that contains 0.30 moles of \(O_{2}\), 0.70 moles of \(N_{2}\), and 0.25 moles of Ar with a total pressure of 1.1 atm can be calculated using the following steps:
1. Calculate the total number of moles in the mixture: 0.30 moles of \(O_{2}\) + 0.70 moles of \(N_{2}\) + 0.25 moles of Ar = 1.25 moles.
2. Determine the mole fraction of \(O_{2}\) in the mixture: 0.30 moles of \(O_{2}\) / 1.25 moles = 0.24.
3. Multiply the mole fraction of \(O_{2}\) by the total pressure to find the partial pressure of \(O_{2}\): 0.24 * 1.1 atm = 0.264 atm.
The partial pressure of oxygen in the mixture is approximately 0.26 atm, which corresponds to option b) 0.26 atm.
To know more about Partial Pressures:
https://brainly.com/question/29574925?
#SPJ11
The equilibrium constant Kc for the reaction
N2 (g) + 3H2 (g) -> 2NH3 (g)
at 450°C is 0.159. Calculate the equilibrium composition
when 1.00 mol N2 is mixed with 3.00 mol H2 in a 2.00-L
vessel.
part 1: Enter the equilibrium concentration for N2.
part 2: Enter the equilibrium concentration for H2
part 3: Enter the equilibrium concentration for NH3.
Answers
Answer:
[N2] = 0.3633M
[H2] = 1.090M
[NH3] = 0.2734M
Explanation:
Based on the reaction of the problem, Kc is defined as:
Kc = 0.159 = [NH3]² / [N2] [H2]³
Where [] are the equilibrium concentrations.
The initial concentrations of the reactants is:
N2 = 1.00mol / 2.00L = 0.500M
H2 = 3.00mol / 2.00L = 1.50M
When the equilibrium is reached, the concentrations are:
[N2] = 0.500M - X
[H2] = 1.50M - 3X
[NH3] = 2X
Where X is reaction quotient
Replacing in the Kc equation:
0.159 = [2X]² / [0.500 - X] [1.50 - 3X]³
0.159 = 4X² / 1.6875 - 13.5 X + 40.5 X² - 54 X³ + 27 X⁴
0.268313 - 2.1465 X + 6.4395 X² - 8.586 X³ + 4.293 X⁴ = 4X²
0.268313 - 2.1465 X + 2.4395 X² - 8.586 X³ + 4.293 X⁴ = 0
Solving for X:
X = 0.1367. Right solution.
X = 1.8286. False solution. Produce negative concentrations
Replacing:
[N2] = 0.500M - 0.1367M
[H2] = 1.50M - 3*0.1367M
[NH3] = 2*0.1367M
The equilibrium concentrations are:
[N2] = 0.3633M[H2] = 1.090M[NH3] = 0.2734MThis is how fluorine appears in the periodic table.
Which is one piece of information that "9" gives about an atom of fluorine?
The atomic mass is different than the atomic number, and the number of neutrons is the difference between the atomic mass and the atomic number.
the atomic number
the atomic mass
the mass of protons
the number of neutrons
Answers
The only piece of information we can deduce from the number "9" about a Fluorine atom is its atomic number.
Fluorine is an element with the atomic symbol F and the number 9. It is found in group 17 (group VIIa), at the top of the halogen family, on the opposite side of Oxygen and Neon. The lightest, riskiest, and most reactive of all the halogens is fluorine, which is positioned above chlorine on the periodic table.
With an electronegativity of 3.98, the fluorine atom is the most electronegative element in the periodic table. Its electron configuration is [He] 2s²2p⁵, or 1s²2s²2p⁵. It is extremely challenging to isolate and will ferociously shred an electron off practically any other atom.
Seven valence electrons make up fluorine. It is particularly reactive and electronegative because it only requires one more electron to complete its second shell.
To know more about halogen
brainly.com/question/11156152
#SPJ4
How many solutions does the equation (―3)(+ 1)(+ 5) = 0 have? What are the solutions?
Answers
Answer:
- 15 = 0
Explanation:
==> (―3)(+ 1)(+ 5) = 0
==> -3 × +1 × +5 = 0
==> -3×+5=0
==> -15=0
What is the [OH-] if the pH is 7
Answers
Answer:
neutral [H3O+] = [OH−] pH = 7 7.2: pH and pOH
Explanation:
At pH 7, the substance or solution is at neutral and means that the concentration of H+ and OH- ion is the same.
A calorie is defined as the amount of energy required to raise the temperature of one lb of water by one degree celsius.
a. Trueb. False
Answers
Answer: The answer is true
Explanation:
a buffer contains equal amounts of a weak acid and conjugate base and has a ph of 5.25. how will the concentration of conjugate base in the buffer change after the addition of a small amount of strong base?
Answers
The concentration of conjugate base in the buffer change after the addition of a small amount of strong base The concentration of conjugate base will increase. HA+BOH ⇒ H2O +AB
Buffer capacity refers to the amount of added acid or base that can be neutralized by a buffer. This is determined by the concentration of the conjugate acid and conjugate base. As these concentrations increase, the buffering capacity increases. A buffer is a solution that can withstand changes in pH due to the addition of acidic or basic components.
Can neutralize small amounts of added acids or bases and keep the pH of the solution relatively stable. When a strong base is added to a buffer the hydroxide ions are consumed by the weak acid, forming water, and consumed by the weak conjugate base of the acid. The number of weak acids decreases and the number of conjugate bases increases.
Learn more about The concentration here:-https://brainly.com/question/17206790
#SPJ4
P4 (s) + 6 H2 (g) → 4 PH3 (g)
Use the balanced equation above.
How many moles of PH3 are produced from 6.59 moles of hydrogen gas?
Type your answer...
Answers
Answer:
4.39 moles
Explanation:
According to the balanced chemical equation:
P4 (s) + 6 H2 (g) → 4 PH3 (g)
6 moles of hydrogen gas (H2) react with 1 mole of phosphorus (P4) to produce 4 moles of phosphine gas (PH3). This means that the mole ratio of H2 to PH3 is 6:4 or 3:2.
To find out how many moles of PH3 are produced from 6.59 moles of H2, we can use the mole ratio as a conversion factor:
6.59 moles H2 x (4 moles PH3 / 6 moles H2) = 4.39 moles PH3
Therefore, 6.59 moles of H2 react to produce 4.39 moles of PH3.
In an inverted trophic pyramid, _______ biomass is present in the secondary carnivores than in the primary producers. Compared to terrestrial systems, aquatic systems are _______ likely to feature inverted pyramids.
Answers
In an inverted trophic pyramid, greater biomass is present in the secondary carnivores than in the primary producers. Compared to terrestrial systems, aquatic systems are more likely to feature inverted pyramids.
In an inverted trophic pyramid, greater biomass is present in the secondary carnivores. This unique phenomenon deviates from the traditional pyramid shape typically found in trophic structures. Generally, biomass decreases as one ascends the trophic levels, with primary producers having the most biomass and tertiary consumers having the least.
Compared to terrestrial systems, aquatic systems are more likely to feature inverted pyramids. This is mainly due to the rapid turnover rate of phytoplankton, which are the primary producers in aquatic ecosystems. As phytoplankton have a short lifespan and high reproductive rate, they are consumed rapidly by zooplankton, the primary consumers. Consequently, the biomass of primary producers in aquatic systems is relatively lower at any given time.
In contrast, terrestrial ecosystems usually have a more stable and long-lasting primary producer base, such as trees and plants, resulting in the traditional pyramid shape.
In summary, an inverted trophic pyramid is characterized by a greater biomass in secondary carnivores compared to primary producers, a pattern more commonly found in aquatic ecosystems than in terrestrial systems. This distinctive structure results from the rapid turnover rate of primary producers in aquatic environments.
To know more about trophic pyramid, refer to the link below:
https://brainly.com/question/32272701#
#SPJ11
Please help How many moles of a gas sample are in 14.0 L container at 269 K and 358 kPa? The gas constant is 8.31 L kPa/ mol K Round you answer to one decimal place and enter the number only with no units.
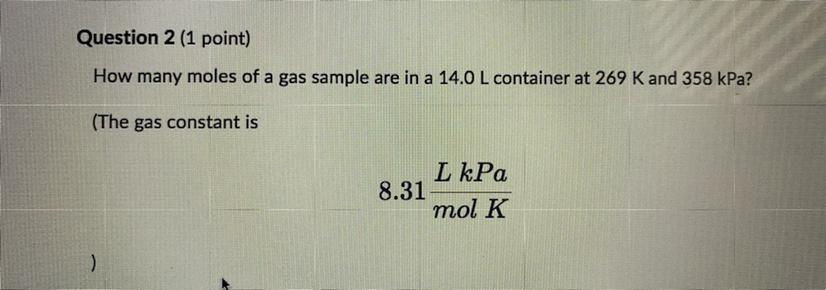
Answers
We are going to assume that the gas mentioned behaves like an ideal gas. The equation that describes the behavior of an ideal gas is as follows:
\(PV=nRT\)Where,
P is the pressure of the gas, 358kPa
V is the volume of the gas, 14.0L
R is the gas constant, 8.31 L kPa/mol K
T is the temperature of the gas, 269K
Now, we will clear the number of moles, n.
\(n=\frac{PV}{RT}\)We replace the known data:
\(\begin{gathered} n=\frac{358kPa\times14.0L}{8.31\frac{L.kPa}{mol.K}\times269K} \\ n=\frac{358\times14.0}{8.31\times269}mol \\ n=2.24mol \end{gathered}\)Answer: In the sample of gas there are 2.24 moles
Which finding would most concerning when a assessing a woman at 12 hours postpartum after a C-section delivery
Answers
The finding would most concerning when a assessing a woman at 12 hours postpartum after a C-section delivery is signs of infection.
Signs of infection can include fever, chills, foul-smelling discharge from the incision site, redness or swelling around the incision, and abdominal pain. Infections after C-section delivery can lead to serious complications, including sepsis and wound dehiscence. Therefore, it is important to monitor for signs of infection and promptly treat any infections that are detected. Other important factors to assess in the postpartum period after C-section delivery include pain levels, mobility, vital signs, and the amount and color of vaginal bleeding.
It is crucial to provide close monitoring and support for women who have undergone a C-section delivery to ensure that they have a safe and healthy recovery. Encouraging early ambulation and providing pain management, as well as clear instructions for wound care and monitoring for signs of infection, can help prevent complications and promote a smooth recovery. So therefore when assessing a woman at 12 hours postpartum after a C-section delivery, there are several findings that would be concerning, but the most concerning finding would be signs of infection.
Learn more about C-section at
https://brainly.com/question/31675959?
#SPJ11