Answers
Related Questions
The powerful, warm, fast Atlantic Ocean current is called the
A.Atlantic Curren
B.Rip Tide
C.Gulf Stream
D.El Nino
60 points!
Answers
Answer:
C
Explanation:
Warm water is heated by the Gulf Stream, a warm air current that originates in the Gulf of Mexico. As the warm water moves north, it forces cooler water to sink and move south.
the reason of which I do not believe it is a Rip Tide is because rip tides have been found in both several rivers and oceans, if not all.
how many molecules are in 0.610 moles of neon gas?
Answers
hello, can you help me identify the name of these 4 molecules as well as the structural formula and the skeletal formula? thank you

Answers
Answer:1. metyletevinyl
4. (Z)-3-hydroxypropenal
Explanation:
HQ5.40
Homework Answered Due Today, 11:59 PM
The reaction 3H₂(g) + N₂(g) → 2NH3(g) has an enthalpy of reaction of -92.6 kJ/mol. If 1 g of hydrogen and 2 g of nitrogen are
reacted, how much heat is produced (kJ)?
Answers
The amount of heat energy produced when 1 g of hydrogen and 2 g of nitrogen are reacted, is -6.61 KJ
How do i determine the heat energy produced?First, we shall obtain the limiting reactant. Details below:
3H₂ + N₂ -> 2NH₃
Molar mass of N₂ = 28 g/molMass of N₂ from the balanced equation = 1 × 28 = 28 g Molar mass of H₂ = 2 g/molMass of H₂ from the balanced equation = 3 × 2 = 6 gFrom the balanced equation above,
28 g of N₂ reacted with 6 g of H₂
Therefore,
2 g of N₂ will react with = (2 × 6) / 28 = 0.43 g of H₂
We can see that only 0.43 g of H₂ is needed in the reaction.
Thus, the limiting reactant is N₂
Finally, we the amount of heat energy produced. Details below:
3H₂ + N₂ -> 2NH₃ ΔH = -92.6 KJ
Molar mass of N₂ = 28 g/molMass of N₂ from the balanced equation = 1 × 28 = 28 gFrom the balanced equation above,
When 28 grams of N₂ reacted, -92.6 KJ of heat energy were produced.
Therefore,
When 2 grams of N₂ will react to produce = (2 × -92.6) / 28 = -6.61 KJ
Thus the heat energy produced from the reaction is -6.61 KJ
Learn more about heat energy:
https://brainly.com/question/31429264
#SPJ1
what organ is very important in human body
Answers
Answer:
The brain is arguably the most important organ in the human body. It controls and coordinates actions and reactions, allows us to think and feel, and enables us to have memories and feelings—all the things that make us human.
Explanation:
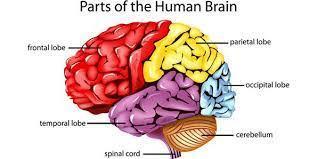
Answer:
The brain is arguably the most important organ in the human body. It controls and coordinates actions and reactions, allows us to think and feel, and enables us to have memories and feelings—all the things that make us human.
Explanation:
A (aq) + 2 B (aq) ⇌ 2 C (aq) For the reaction above and the ICE table below, what value should be placed in the Change for the concentration of A? Make sure you use the correct sign.

Answers
Answer:
-0.01 M
Explanation:
Let's consider the following reaction.
A(aq) + 2 B(aq) ⇌ 2 C(aq)
We will make an ICE chart.
A(aq) + 2 B(aq) ⇌ 2 C(aq)
I 0.10M 0.10M 0M
C -x -2x +2x
E 0.10-x 0.10-2x 2x
According to the chart, the concentration of C at equilibrium is:
2x = 0.02
x = 0.01
The value in the change row for A is:
-x = -0.01 M
Name a liquid substance that could be used in the laboratory for: dissolving dry mortar on floor tiles; (i) removing KMnO, stains; drying acid anhydrides
Answers
A liquid substance that could be used in the laboratory for dissolving dry mortar on floor tiles is vinegar; (i) removing KMnO₄, stains is sodium metabisulfite solution; drying acid anhydrides is concentrated sulfuric acid.
What are solvents?Solvents are substances usually liquids, but may also be gases or solids that dissolve other substances known as solutes.
Solvents are usually used as cleansing agents.
One possible liquid substance that could be used for dissolving dry mortar on floor tiles is a mild acid solution, such as diluted hydrochloric acid or vinegar.
KMnO₄ stains are often difficult to remove, but one substance that can be used is sodium metabisulfite (Na₂S₂O₅) solution. Sodium metabisulfite acts as a reducing agent and can effectively neutralize and remove KMnO₄ stains.
Concentrated sulfuric acid is commonly used in the laboratory as a drying agent. It has a strong affinity for water and can efficiently absorb moisture, including water present in acid anhydrides.
Learn more about solvents at: https://brainly.com/question/25326161
#SPJ1
PLEASEEE HELP ?!?!?!
which of the following processes provide evidence of the particulate nature of matter
I. Diffusion
II. Filtration
III. Osmosis
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Answers
Answer:
B
Explanation:
I think the answer is Diffusion and Osmosis
The processes that provide evidence of the particulate nature of matter would be diffusion and osmosis.
Matter is defined as anything with mass/weight and able to occupy space. Matters could be molecules of solid, liquid, or gases. The molecules of each category of matter have their specific characteristics.Molecules of liquids and gases are able to diffuse. Diffusion is defined as the movement of molecules from the region of high concentration to the region of low concentration. Molecules of solids in the dissolved form are also able to diffuse. Water molecules are also able to move from the region of high to the region of low water potentials by osmosis in the presence of a semi-permeable membrane.Thus, both diffusion and osmosis back up the particulate nature of matter.
More on the particulate nature of matter can be found here: https://brainly.com/question/15230454?referrer=searchResults
I need friends and I’m in middle school :)
Answers
Answer:
Ok?
Explanation:
Two solutions, one with a mass of 450 g and the other with a mass of 350 g, are mixed. A chemical reaction occurs and 125 g of solid crystals are produced that settle on the bottom of the container. What is the mass of the remaining solution?
Answers
475 g is the correct response to the query. This is true because the combined mass of two solutions with masses of 450 g and 350 g equals 800 g. 125 g of solid crystals are created and fall to the bottom of the container as a result of a chemical reaction.
As a result, the mass of the residual solution is equal to 475 g, or 800 g less 125 g. It is significant to observe that the masses of the two solutions that were combined originally do not equal those of the solid crystals or the leftover solution.
This is because the two solutions' chemical reaction when combined results in a transition of the matter that is resulting in the production of a new substance.
Learn more about mass at:
https://brainly.com/question/11954533
#SPJ1
At STP the number of liters of O2 required to react with 11.2 liters of CH4 to form only CO2 and H2O is ________ liters.
Answers
The number of liters of oxygen, O₂, required at STP to react with 11.2 liters of CH₄ to form only CO₂ and H₂O is 22.4 liters.
What is the number of liters of O₂ required at STP to react with 11.2 liters of CH₄ to form only CO₂ and H₂O?The number of liters of O₂ required at STP to react with 11.2 liters of CH₄ to form only CO₂ and H₂O is determined from the balanced equation of the reaction as follows:
The balanced equation of the reaction of O₂ at STP CH₄ to form only CO₂ and H₂O is given below:
CH₄ + 2 O₂ (g) ----> CO₂ (g) + 2 H₂O (g)
From the balanced equation of the reaction of O₂ at STP CH₄, 2 moles of O₂ reacts with one mole of CH₄.
At STP, the volume of one mole of CH₄ is equal to 22.4 liters
The number of mole of CH₄ in 11.2 liters of CH₄ will be 11.2 / 22.4 = 0.5 moles
The moles of oxygen required will be 0.5 liters * 2 /1 = 1 mole of oxygen.
At STP, the volume of one mole of O₂ is equal to 22.4 liters.
Hence, 22.4 liters of oxygen are required.
Learn more about the volume of gases at STP at: https://brainly.com/question/26364483
#SPJ1
The law of partial pressures was developed by ___________.
Answers
Answer:
John Dalton
Explanation:
Aluminium having atomic mass 27 g mol crystallises in face centred pack
Answers
The number of unit cells in the given quantity is 2.23x10²³ unit cells
:Given Aluminum has an atomic mass of 27g mol-1
>Crystal is face-centered cubic structure
>number of aluminium atoms in l0 g mass
so w =10g
> the number of unit cells in the given quantity is to be calculated
Number of atoms of Al = (mass /molar mass ) x Nₐ
=(w/M)xNa , where Na =6.022x 10²³
There are 4 atoms present in the unit cell it will crystallize in a face-centered cubic crystal
.>.On solving the above values
we get the number of unit cells as= (10/27)x 6.022x10²³ unit cells
The number of unit cells in the given quantity is 2.23x10²³ unit cells
this question is incomplete.
kindly find the correct question in link below
https://brainly.in/question/44357676
#SPJ9
how do one get this solution
-log10 (2* 10^-2)
Answers
The result of the computation when you follow the steps is 1.699.
A logarithm is a mathematical function that represents the exponent or power to which a specific base must be raised to obtain a given number. In simpler terms, it answers the question: "To what power must we raise a base number to obtain a certain value?"
What you should do is that on your calculator, you could press the logarithm key and then put in the value that has been shown and then the result would be displayed on your calculator.
Learn more about logarithm:https://brainly.com/question/30226560
#SPJ1
A solution has [H+] = 2.35 × 10-3 M. Find the [OH-] for this solution
Answers
Answer:
[OH-] for this solution is 4.255*10^-12
Explanation:
We are given
[H+] = 2.35 × 10-3 M
we need to find the concentration of [OH-]
we know from Equilibrium
[H+][OH-] = 10^-14
[OH-] = 10^14/2.35*10^10^-3
[OH-] = 0.4255*10^-11
[OH] = 4.255*10^-12
Therefore the Concentration of [OH-] for this solution is 4.255*10^-12
Predict the missing component in the nuclear equation.

Answers
Since what we have is an alpha decay, the missing component is 234/90 Th
What is Alpha decay?Alpha decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle, which is a helium nucleus consisting of two protons and two neutrons. During alpha decay, the atomic number of the parent nucleus decreases by two, and the mass number decreases by four.
Apha particles can be stopped by a thin layer of material such as paper or skin, and they do not penetrate very far into matter.
Learn more about Alpha decay:https://brainly.com/question/27870937
#SPJ1
a student pipettes 5 ml of vinegar into a erleyenmayeer flask adds indictator and 25 ml of distilled water and titrates it with 0.1098M naoh. calcualte the concentraion of acetic acid in the vinegar if the intitial volume reading on the burrette was 1.35 ml and the final reading is 37.83ml
Answers
The concentration of the solution is obtained as 0.134 M.
What is the concentration of the acetic acid?We know that titration is a method of analysis that depends on the volume of the solutions in order to determine the concertation.
We know that;
Total volume of the vinegar = 5 ml + 25 mL = 30 mL
Concentration of the sodium hydroxide = 0.1098M
Volume of the sodium hydroxide used = 37.83ml - 1.35 ml = 36.48 mL
Using the formula;
CAVA/CVB = NA/VB
CAVANB = CBVBNA
CA= CBVBNA/VANB
CA = 0.1098 * 36.48 * 1/30 * 1
CA = 0.134 M
Lear more about concentration:https://brainly.com/question/10725862
#SPJ1
There were 80 dodos living on an island. After five years the number of dodos had decreased to 55. The number of dodos alive each year decreased exponentially. How many more years (to the nearest year) was it before there were just two dodos left on the island?
Answers
The island was it before there were just two dodos left in 44 years.
The bird was first seen by Portuguese sailors around 1507 and was exterminated by humans and imported animals. The Dodo was extinct by 1681 the Reunion solitaire by 1746, and Rodriguez solitaire around 1790. The last dodo bird went extinct on the island of Mauritius more than 300 years before him. The Indian Ocean.
The last dodo bird was killed in 1681. Although the dodo bird's demise story is well documented no complete specimen of the bird has been preserved. There are only fragments and sketches. The dodo bird is just one of Mauritius's endangered bird species. Overharvesting of birds, combined with habitat loss and loss of competition from newly introduced animals, was just too much for the dodo to survive. The last dodo was killed in 1681 this species was forever endangered.
Learn more about The dodo birds here:-https://brainly.ph/question/248833
#SPJ1
the mixture of carbon dioxide and water tastes salt why
Answers
Answer:
When carbon dioxide reacts with water carbonic acid is formed, from which hydrogen ions dissociate increasing the acidity of the systemCarbon dioxide emissions to the atmosphere can therefore increase the acidity of land, sea and air
Explanation:
Question 1 (4 points)
What is the molecular weight of Magnesium nitride, Mg3N2 (OR Mg3 N2). Report
your answer to two decimal places.
Do not include units with your answer.
The atomic weight of Mg is 24.31 grams/mole
The atomic weight of N is 14.01 grams/mole
Answers
1.49 g na2so4 mixed with 3.42g al(so4)3 calculate difference of number of cation and anions is
Answers
The difference in the number of cations and anions in the given mixture is approximately 0.0005 mol.
The molar mass of Na2SO4 (sodium sulfate) can be calculated as follows:
2(Na) + 1(S) + 4(O) = 2(22.99 g/mol) + 32.07 g/mol + 4(16.00 g/mol) = 142.04 g/mol
The number of moles of Na2SO4 :
moles of Na2SO4 = mass of Na2SO4 / molar mass of Na2SO4
moles of Na2SO4 = 1.49 g / 142.04 g/mol ≈ 0.0105 mol
Similarly, the molar mass of Al2(SO4)3 (aluminum sulfate) :
2(Al) + 3(S) + 12(O) = 2(26.98 g/mol) + 3(32.07 g/mol) + 12(16.00 g/mol) = 342.15 g/mol
The number of moles of Al2(SO4)3:
moles of Al2(SO4)3 = mass of Al2(SO4)3 / molar mass of Al2(SO4)3
moles of Al2(SO4)3 = 3.42 g / 342.15 g/mol ≈ 0.0100 mol
For Na2SO4, the ratio of cations (Na+) to anions (SO42-) is 2:1. So, the number of cations in 0.0105 mol of Na2SO4 is 2 * 0.0105 mol = 0.0210 mol, and the number of anions is 0.0105 mol.
For Al2(SO4)3, the ratio of cations (Al3+) to anions (SO42-) is 2:3. So, the number of cations in 0.0100 mol of Al2(SO4)3 is 2 * 0.0100 mol = 0.0200 mol, and the number of anions is 3 * 0.0100 mol = 0.0300 mol.
Calculating the difference in the number of cations and anions:
Difference = (Number of Cations in Na2SO4 + Number of Cations in Al2(SO4)3) - (Number of Anions in Na2SO4 + Number of Anions in Al2(SO4)3)
Difference = (0.0210 mol + 0.0200 mol) - (0.0105 mol + 0.0300 mol)
Difference = 0.0410 mol - 0.0405 mol
Difference ≈ 0.0005 mol
Therefore, the difference in the number of cations and anions in the given mixture is approximately 0.0005 mol.
For more questions on cations
https://brainly.com/question/14309645
#SPJ8
Which diagram shows how CH3Br and OH must collide in order to react and form CH2OH? (Marking brainliest!)

Answers
Answer:
Its B
Explanation:
I just took the quiz.
When \(CH_{3} Br\) and OH collide in order to react and form \(CH_{3} OH\) , such reaction will be best represented by diagram (B).
What is reaction?A chemical reaction would be a procedure that causes one group of chemical components to change chemically into another. In a chemical reaction, one or even more substances also known as reactants would be changed into one or more additional materials known as products. Chemical elements and chemical compounds make up substances.
What is collide?A colloid would be a mixture wherein the component made up of insoluble particles that are scattered at a microscopic scale is suspended within another component.
When
\(CH_{3} Br + OH\) → \(CH_{3} OH + Br\).
By the Collison of methyl bromide with OH ion then methyl alcohol will be formed.
Therefore, when \(CH_{3} Br\) and OH collide in order to react and form \(CH_{3} OH\) , such reaction will be best represented by diagram (B).
Therefore, the correct answer will be option (B).
To know more about Collison
https://brainly.com/question/14992935?
#SPJ2
Rust is to iron as tarnished is to
Answers
Answer:
Tarnished is to Image or reputation
What does light do in the photoelectric effect?
O A. Light turns metal into electricity.
B. Light knocks electrons off metal atoms.
C. Light reacts with metal atoms.
D. Light is turned into electricity by the metal.
Answers
Answer:
it's B: light knocks electrons off metal atoms
1. A compound is made up of 11 percent oxygen and 89 percent gold. What would be this compound's
empirical formula?
Answers
Answer:
Empirical formula is Au₂O₃
Explanation:
Given data:
Percentage of oxygen = 11%
Percentage of gold = 89%
Empirical formula = ?
Solution:
Solution:
Number of gram atoms of O = 11 / 16 = 0.69
Number of gram atoms of Au = 89 / 197 = 0.45
Atomic ratio:
Au : O
0.45/0.45 : 0.69 /0.45
1 : 1.5
Au : O = 1 : 1.5
Au : O = 2( 1 : 1.5)
Empirical formula is Au₂O₃.
A sample of argon has a volume of 1.20 L at STP. If the temperature is increased to 22.0°C and the pressure is lowered to 0.800 atm, what will the new volume be, in L?
Answers
Answer:
You have a pressure change and temperature change - you can use the combined gas law:
P1V1/T1 = P2V2/T2
Where P1 = 1am
P2 = 0.8atm
T1 = 273K
T2 = 273 + 22 = 295K
V1 = 1.2lL
V2 = x
Explanation:
Initial temperature of metal =
°℃
Initial temperature of water =
°℃
Final temperature of both =
√°C
Subtract to find the temperature changes
for the water and the metal.
AT (water) =
AT (metal)=-C
Answers
The temperature changes for the water and the metal can be calculated by subtracting their initial temperatures from the final temperature.
AT (water) = √°C - °℃
AT (metal) = √°C - °℃
The above equations give the temperature changes for the water and the metal, respectively. The specific values of the temperatures and the final temperature are not provided, so the actual temperature changes cannot be determined without knowing these values.
In general, the temperature change of a substance is given by the difference between the final and initial temperatures. When a warmer object comes into contact with a cooler one, heat energy is transferred from the warmer object to the cooler one until they reach thermal equilibrium, where their temperatures become equal.
The magnitude of the temperature change depends on factors such as the specific heat capacity of the substances involved and the amount of heat exchanged between them.
To accurately calculate the temperature changes, the specific heat capacities of water and the metal would be needed. Additionally, the masses or quantities of the substances would be necessary to determine the amount of heat exchanged. Without these specific values, it is not possible to provide a precise numerical answer.
for such more questions on temperature
https://brainly.com/question/4735135
#SPJ8
Describe echolocation. Give an example of an animal that uses echolocation.
Answers
dolphin and whales use echolocation
PLSSSSSSSSSS HELP!!! I REALLY NEED IT SO MUCH :( I PROMICE TO GIVE BRAINLIEST AND FIVE STARS AND A THANKS! 6TH GRADE SCIENCE! i used all my points for this!
You measure the mass of an apple using a balance. You get these three measurements:
Trial 1: 167.0 g
Trial 2: 166.9 g
Trial 3: 167.2 g
You know that the true mass of the apple is 172 g. Describe your results in terms of precision and accuracy. Explain your answer.
Answers
Answer:
100 POINTS! (50 split up) PLSSSSSSSSSS HELP!!! I REALLY NEED IT SO MUCH :( I PROMICE TO GIVE BRAINLIEST AND FIVE STARS AND A THANKS! 6TH GRADE SCIENCE! i used all my points for this!
You measure the mass of an apple using a balance. You get these three measurements:
Trial 1: 167.0 g
Trial 2: 166.9 g
Trial 3: 167.2 g
You know that the true mass of the apple is 172 g. Describe your results in terms of precision and accuracy. Explain your answer in a paragraph for the best grade.
Explanation:
The carbon cycle is the exchange of carbon between the lands, the oceans the atmosphere and the earth's interior
O True
O False
Answers
Answer:
True
Explanation:
Carbon is the backbone of life on Earth. Most of Earth's carbon, about 65,500 billion metric tons is stored in rocks. The rest is in the ocean, atmosphere, plants, soil, and fossil fuels. Carbon flows between each reservoir in an exchange called the carbon cycle, which has slow and fast components.