Answers
Answer:
1.342g of picolinic acid and 6.743mL of 1.0M NaOH diluting the mixture to 100.0mL
Explanation:
The pKa of the picolinic acid is 5.4.
Using Henderson-Hasselbalch formula for picolinic-picolinate buffer:
pH = pKa + log [Picolinate] / [Picolinic]
Where [] could be taken as moles of each species
5.61 = 5.4 + log [Picolinate] / [Picolinic]
0.21 = log [Picolinate] / [Picolinic]
1.62181 = [Picolinate] / [Picolinic] (1)
Now, both picolinate and picolinic acid will be:
0.100L * (0.109mol / L) =
0.0109 moles = [Picolinate] + [Picolinic] (2)
First, as we will start with picolinic acid, we need add:
0.0109 moles picolinic acid * (123.10g/mol) = 1.342g of picolinic acid
Now, replacing (2) in (1):
1.62181 = 0.0109 moles - [Picolinic] / [Picolinic]
1.62181 [Picolinic] = 0.0109 moles - [Picolinic]
2.62181 [Picolinic] = 0.0109 moles
[Picolinic] = 4.157x10⁻³ moles
And:
[Picolinate] = 0.0109 - 4.157x10⁻³ moles =
6.743x10⁻³ molesTo obtain these moles of picolinate ion we need to make the reaction of the picolinic acid with NaOH:
Picolinic acid + NaOH → Picolinate + Water
That means to obtain 6.743x10⁻³ moles of picolinate ion we need to add 6.743x10⁻³ moles of NaOH
6.743x10⁻³ moles of NaOH that is 1.0M are, in mL:
6.743x10⁻³ moles * (1L / 1mol) = 6.743x10⁻³L * 1000 =
6.743mL of the 1.0M NaOH must be addedThus, we obtain the desire moles of picolinate and picolinic acid to obtain the buffer we want, the last step is:
Dilute the mixture to 100mL, the volume we need to prepareFollowing are the calculation to the mass of picolinic acid:
Let pKa of picolinic acid \(= 5.52\)
form the buffer: \(\bold{pH = pKa + \log(\frac{A-}{ HA})}\)
Acid concentration\(= 0.109\)
We will require the conjugate, which would be formed by interaction of picolinic acid and NaOH.
calcultion to the need:
\(pH = pKa + \log(\frac{A-}{ HA})\\\\5.61 = 5.52 + \log(\frac{A-}{ HA})\)
solve for A- by using antilog
\(0.09 = \log(\frac{A-}{ HA})\\\\1.230= \frac{A-}{HA}\)
When
\(HA = 0.109\\\\A- = 0.134\)
If \(V = 100 ml\)then
\(n A- = M\timesV = 0.134 \times 0.1 = 0.0134 \ mol\ of\ A-\)
needed
\(HA + NAOH \to H_2O + Na^+ \ and \ A-\)
therefore,
ratios =1:1
we need 0.0134 mol of NaOH
\(n = M\times V \\\\V = \frac{0.0134}{1} = 0.0134 \ liter \ of\ NaOH\)
but you also want \(0.109 M\) of free picolinic acid so
\(n = M\times V = 0.109\times 0.1 = 0.0109\ mol \ of \ Acid\)
Therefore:
\(n \ acid = 0.0109 + 0.0134\ mol = 0.0243 \ mol\ of \ acid\)
Preparation:
by add volume (19.07) in NaOH
M = 1.0 to the beaker, and by add water until the beaker marks 1 L
Then add 0.0243 mol of picolinic acid
\(\to m = 0.0243 \times 123 = 2.9889\ grams\) of picolinic acid
stir and pH will be that of buffer.
Learn more:
brainly.com/question/17156849
Related Questions
What is the difference between osmosis and diffusion?
Osmosis is a kind of diffusion that involves movement of water.
Diffusion uses energy, but osmosis does not.
Diffusion only occurs in animal cells, and osmosis only occurs in plant cells.
Osmosis is movement of proteins, and diffusion is movement of water.
Answers
Answer:
Osmosis is the movement of solvent particles across a semipermeable membrane from a dilute solution into a concentrated solution. ... Diffusion: Diffusion is the movement of particles from an area of higher concentration to lower concentration. The overall effect is to equalize concentration throughout the medium.
Osmosis is a special case of diffusion. Water, like other substances, moves from an area of higher concentration to one of lower concentration. ... In this example, the solute cannot diffuse through the membrane, but the water can. Water has a concentration gradient in this system.
98.96g/mol of CH2O what will be the chemical formula
Answers
Let's break down the molar mass of CH2O:
- Carbon (C) has a molar mass of approximately 12.01 g/mol.
- Hydrogen (H) has a molar mass of approximately 1.01 g/mol.
- Oxygen (O) has a molar mass of approximately 16.00 g/mol.
Now, let's calculate the molar mass of CH2O:
(1 x molar mass of C) + (2 x molar mass of H) + (1 x molar mass of O)
= (1 x 12.01 g/mol) + (2 x 1.01 g/mol) + (1 x 16.00 g/mol)
= 12.01 g/mol + 2.02 g/mol + 16.00 g/mol
= 30.03 g/mol
The molar mass of CH2O is approximately 30.03 g/mol, which is different from the given molar mass of 98.96 g/mol.
It seems that there might be an error or misunderstanding in the given molar mass value. The correct chemical formula for a compound with a molar mass of 98.96 g/mol cannot be determined based on the information provided.
What are some things you do to help you learn and remember new words and what they mean?
Answers
The following are some things you do to help you learn and remember new words and they mean:-
Learn in chunks and scripts.Visualize what the word or phrase looks like.Reading is one of the only methods to teach vocabulary and normal learning is the approach that gives college students the possibility to exercise and grasp the stages of crucial reading that result in studying success and progressed word utilization.
One of the maximum impactful mastering techniques is allotted practice spacing out your analyzing over several brief intervals of time over several days and weeks. The only exercise is to work a quick time on every elegance every day.
Learn more about learning new words here:- https://brainly.com/question/8039201
#SPJ1
A 3.743 g sample of a new organic material is combusted in a bomb calorimeter. The temperature of the calorimeter and its contents increase from 23.97 ∘C to 27.16 ∘C.
The heat capacity (calorimeter constant) of the calorimeter is 41.93 kJ/ ∘C, what is the heat of combustion per gram of the material?
heat of combustion: _________ kJ/g
Answers
The heat of combustion of the material is obtained as 35.5 kJ/g.
What is the heat of combustion?The heat of combustion refers to the heat that is given off when a substance is burnt in oxygen. Now we are told that a 3.743 g sample of a new organic material is combusted in a bomb calorimeter. The temperature of the calorimeter and its contents increase from 23.97 ∘C to 27.16 ∘C.
Thus;
Heat evolved can be obtained by the use of the formula;
H = cdT
c = heat capacity
dT= temperature change
H = 41.93 kJ/ ∘C * (27.16 ∘C - 23.97 ∘C)
H = 133.8 kJ
The heat of combustion per gram of the material is;
133.8 kJ/ 3.743 g = 35.5 kJ/g
Learn more about heat of combustion:https://brainly.com/question/14317568
#SPJ1
The heat of combustion per gram of the material is 35.74 kJ/g
What is the heat of combustion per gram of the substance?The heat of combustion per gram of the substance is calculated as follows:
Heat change = specific heat capacity * temperature change
mass of sample = 3.743 g
The heat capacity of the calorimeter = 41.93 kJ/ ∘C
temperature change = 27.16 - 23.97 = 3.19
Heat change = 41.93 * 3.19
Heat change = 133.76 kJ
The heat of combustion per gram = 133.76 kJ / 3.743 g
The heat of combustion per gram = 35.74 kJ/g
Learn more about the heat of combustion at: https://brainly.com/question/25712824
#SPJ1
PLEASE HELP 85 POINTS!
Aluminum chloride is a white compound that contains one aluminum atom and three chlorine atoms.
Which model is the best representation of aluminum chloride?
O A.
B.
Al
Cl
Al
Cl
O C.
D.
Cl
Al
Al
Cl

Answers
Answer:
Model B (in image) would the best representation of aluminum chloride.
Explanation:
It's the only model the has three chlorine atoms and one aluminum atom.
Additionally, Cl can only bond once, so it can't be bonded to multiple different atoms. Also, Al needs to have more than one bond to achieve the octet rule.
Samples of water are taken from two swimming pools. One has a ph of 4, the other a ph of 7.4. In which pool would you rather go swimming
Answers
Between water with a pH of 4 and one with a pH of 7.4, I will rather go swimming in the one with a pH of 7.4.
What is pH?The pH of a substance is the degree of acidity of alkalinity of the substance.
The pH scale ranges from 0 to 14. A pH of 7 is considered neutral.
Any pH below 7 is acidic. The further the pH is the downside of 7, the more acidic the substance.
Any pH above 7 is considered basic. The further the pH is the upside of 7, the more basic or alkaline the substance is.
Substances with very high or very low pH are usually corrosive and will harm the human body. The human body's pH ranges between 7.35 to 7.45.
Thus, swimming in water with a pH of 4 would be totally harmful to the body. Swimming in water with a pH of 7.4 would be safe.
More on pHs can be found here: https://brainly.com/question/15289741
#SPJ1
How many grams are in 0.0823 moles of Ar? given; unknown:
Answers
Answer:
3.2877204
Explanation:
Name the substance in red blood cells that carbon monoxide from cigarette smoke combines with.
Answers
The substance in red blood cells that carbon monoxide from cigarette smoke combines with is called hemoglobin.
Hemoglobin is a protein molecule found in red blood cells that is responsible for transporting oxygen from the lungs to the rest of the body. Hemoglobin consists of four subunits, each containing a heme group. The heme group contains an iron ion, which binds to oxygen molecules in the lungs.
Carbon monoxide (CO) from cigarette smoke can bind to the iron ion in the heme group with a much greater affinity than oxygen. When CO binds to hemoglobin, it forms a stable complex called carboxyhemoglobin (COHb).
This reduces the amount of hemoglobin available for binding with oxygen, which can lead to a reduction in the amount of oxygen that can be transported to the tissues.
The formation of COHb can also affect the release of oxygen from hemoglobin in the tissues. Hemoglobin releases oxygen in response to a decrease in oxygen concentration, which is sensed by the heme group. However, when CO binds to the heme group, it alters the shape of the hemoglobin molecule and reduces its ability to release oxygen.
As a result, the presence of carbon monoxide in the blood can lead to a range of health problems, including headaches, dizziness, shortness of breath, and even death in severe cases.
The best way to prevent carbon monoxide poisoning is to avoid exposure to smoke from tobacco products and other sources of combustion, such as gas heaters and stoves.
For more question on red blood cells click on
https://brainly.com/question/1407525
#SPJ11
3: Given 12.3 grams of NH3, how many moles of N₂ were needed?
Answers
0.361 moles of N₂ were required to produce 12.3 g of NH₃, using the balanced chemical equation N₂ + 3H₂ → 2NH₃.
The balanced chemical equation for the reaction is N₂ + 3H₂ → 2NH₃. We can use the balanced equation and the molar mass of NH₃ to calculate the number of moles of N₂ required to produce 12.3 g of NH₃,
1 mol NH₃ = 2 mol N₂ (from the balanced equation)
molar mass of NH₃ = 17.03 g/mol
moles of NH₃ = 12.3 g / 17.03 g/mol
moles of NH₃ = 0.722 mol
moles of N₂ = (0.722 mol NH₃) / 2
moles of N₂ = 0.361 mol
Therefore, 0.361 moles of N₂ were needed to produce 12.3 grams of NH₃.
To know more about moles, visit,
https://brainly.com/question/29367909
#SPJ1
Complete question - For the reaction, N₂ + 3H₂ → 2NH₃. Given 12.3 grams of NH3, how many moles of N₂ were needed?
PLEASE HELP!!!
How many calories are in 4,180 joules?
Answers
Answer:
To convert joules to calories, you can use the conversion factor:
1 calorie = 4.184 joules
To find out how many calories are in 4,180 joules, divide the given value by the conversion factor:
4,180 joules / 4.184 joules per calorie = 0.9 calories (approximately)
Therefore, there are approximately 0.9 calories in 4,180 joules.
a molecule is the ___________particle of a substance that can normally exist independenty
Answers
Answer:
a molecule is the smallest particle of a substance that can normally exist independenty
If have .106g of Gold, how many atoms of Gold do you have?
Answers
Answer:
32.52×10¹⁹ atoms
Explanation:
Given data:
Mass of gold = 0.106 g
Number of atoms of gold = ?
Solution:
We will calculate the number of moles first.
Number of moles = mass/molar mass
Number of moles = 0.106 g/ 197 g/mol
Number of moles = 5.4×10⁻⁴ mol
Number of atoms:
1 mole contain 6.022×10²³ atoms
5.4×10⁻⁴ mol × 6.022×10²³ atoms /1 mol
32.52×10¹⁹ atoms
3,3-dibromo-4-methylhex-1-yne
Answers
Explanation:
see the attachment. hope it will help you...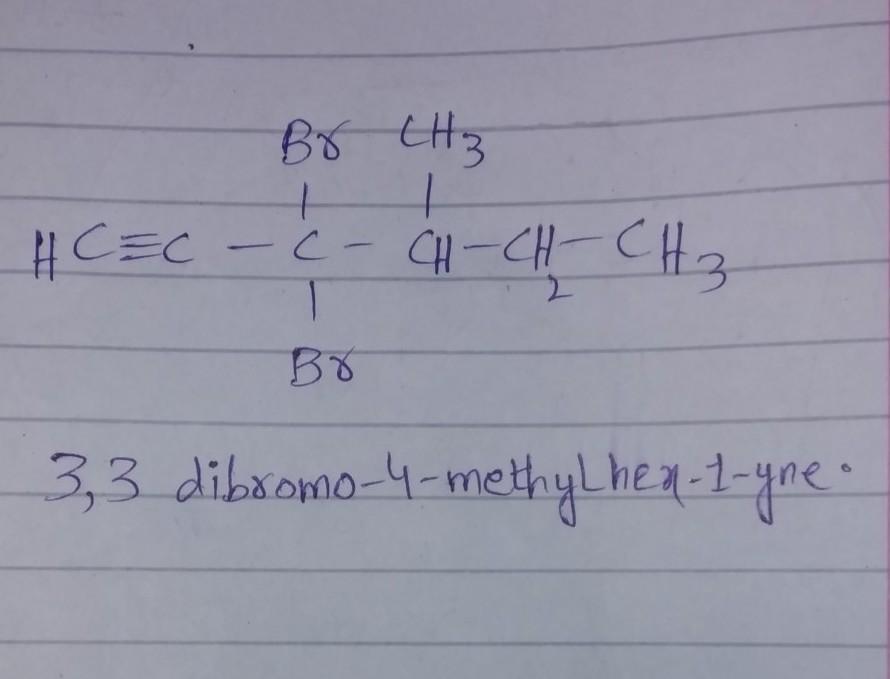
Carrie measured mass and another property, X, of three pure samples of the same compound. She recorded her data in the table.
Which of the following best describes property X?
Answers
The property X is an extrinsic property.
What is an extensive property?The term extensive property refers to the type of property that depends on the amount of the substance present. We must recall that an intensive property does not in any way depend on the number or the amount of the substance present in the sample.
If we have a good look at the table that is being referred to here, we would see that the mass of the substance tends to increase as a certain un named property of the substance X is increasing. This implies that the property X is affected by the mass of the object which is the quantity of matter in the object. This gives us an idea that the property that we are referring to can not be an intrinsic property since it varies with the mass of the substance that is under study as shown.
We can then conclude that the property X is an extrinsic property.
Learn more about extrinsic property: https://brainly.com/question/28588297
#SPJ1
Missing parts;
Carrie measured mass and another property, X, of three pure samples of the same compound. She recorded her data in the table. Which of the following best describes property X?
A. X is an intensive property because it does not vary with the size of the sample.
B. X is an extensive property because it does not vary with the size of the sample.
C. X is an extensive property because it varies directly with the size of the sample.
D. X is an intensive property because it varies directly with the size of the sample.

Substance Specific Heat Capacity J/Kgo C Ammonia 4700 Ethanol 2440 Gasoline 2220 Water 4186 A liquid has a mass of 18.39g. If 655J of heat are required to change the temperature from 32.8oC to 18.2oC, what is the identity of the liquid? A) ammonia B) ethanol C) gasoline D) water
Answers
Answer:
B) ethanol
Explanation:
I got a 100% on my test plus its the answer on usatestprep
Which of the following is the best explanation of why an ionic compound formula is a ratio of atoms
ООО
The sharing of electrons is always in a simple ratio
The number of atoms in an ionic compound change... there can be several possible combinations
When ions form they are attracted to each other and "cxmp" together to form a crystal
lonic compounds conduct electricity when dissolved in water.
Answers
standard form to scientific notation: 0.004078

Answers
Someone help me I don’t know

Answers
Answer:
What's the gas given in the question??
Energy produced by the sun and stars comes from
Answers
Answer:
It's heat energy
Explanation:
which is transmitted through radiation. The sun warms us.
Which is the order of these solutions from strong acid to strong base?
Solutions:
household ammonia
battery acid
baking soda
stomach acid
antacid
A-stomach acid household ammonia battery acid antacid baking soda
B-battery acid stomach acid antacid baking soda household ammonia
C-battery acid stomach acid baking soda antacid household ammonia
D-stomach acid battery acid antacid baking soda household ammonia
Answers
Answer:
First is battery acid with a pH of 1, second is stomach acid with a pH of 1.5-3.5 (depends), third is antacid with a pH of 7, fourth is baking soda with a pH of 9, and finally, fifth is household ammonia with a pH of 11.
Here is a picture of the pH levels to better help you understand.

Answer:
b
Explanation:
Write the empirical formula of at least four binary ionic compounds that could be formed from the following ions:
Mg2+, V5+, Br-, S2-
Answers
Answer: \(MgS\), \(MgBr_2\), \(V_2S_5\) and \(VBr_5\) are four binary ionic compounds that could be formed.
Explanation:
For formation of a neutral ionic compound, the charges on cation and anion must be balanced. The cation is formed by loss of electrons by metals and anions are formed by gain of electrons by non metals.
Mg is having an oxidation state of +2 called as \(Mg^{2+}\) cation and \(S^{2-}\) is an anion with oxidation state of -2. Thus they combine and their oxidation states are exchanged and written in simplest whole number ratios to give neutral \(MgS\). Also \(Mg^{2+}\) cation and \(Br^{-}\) can combine and their oxidation states are exchanged and written in simplest whole number ratios to give neutral \(MgBr_2\).
Also Vanadium is having an oxidation state of +5 called as \(V^{5+}\) cation and \(S^{2-}\) is an anion with oxidation state of -2. Thus they combine and their oxidation states are exchanged and written in simplest whole number ratios to give neutral \(V_2S_5\). Also \(V^{5+}\) cation and \(Br^{-}\) anion can combine and their oxidation states are exchanged and written in simplest whole number ratios to give neutral \(VBr_5\).
Carbon-14 has a half-life of 5730 years. If an original sample was 100g of C¹4 and it is now 0.781g of C14, how old is your sample?
Answers
Answer:
40,113 years
Explanation:
To find the age of the sample, you need to use the half-life formula:
\(N(t)=N_0(\frac{1}{2})^{t/h\)
In this formula:
------> N(t) = current mass (g)
------> N₀ = initial mass (g)
------> t = time passed (yrs)
------> h = half-life (yrs)
You can plug the given values into the equation and rearrange the formula to find "t".
N(t) = 0.781 g t = ? yrs
N₀ = 100 g h = 5730 yrs
\(N(t)=N_0(\frac{1}{2})^{t/h\) <----- Half-life formula
\(0.781=100(\frac{1}{2})^{t/5730}\) <----- Insert values
\(0.00781=(\frac{1}{2})^{t/5730}\) <----- Divide both sides by 100
\(log_{1/2}(0.00781)=log_{1/2}((\frac{1}{2})^ {t/5730})\) <----- Take \(log_{1/2}\) of both sides
\(7.00 = \frac{t}{5730}\) <----- Solve \(log_{1/2}\)
\(40,113 = t\) <----- Multiply both sides by 5730
The given sample is 40,113 years .
What do you mean by half-life ?Half-life, in radioactivity, is the interval of time required for one-half of the atomic nuclei of a radioactive sample to decay.
Half-life formula,
\(\rm N(t)\;=N_0(\dfrac{1}{2})^\frac{t}{t1/2}\) .......(1)
where,
N(t)=current mass
N₀=initial mass
t=time period
h=half -life
Given,
N(t)=0.781g, t=? yrs, N₀=100g, h=5730 years
\(\rm N(t)\;=N_0(\dfrac{1}{2})^\frac{t}{t1/2}\)
put the values, in ......(1)
0.781=100(1/2) \(t/5730\\\)
log₁/₂(0.00781)=log₁/₂ ( 1/2)\(t/5730\)
7=t/5730
40,113=t
Hence, the given sample is 40,113 years .
Learn more about half-life ,here:
https://brainly.com/question/16387602
#SPJ1
Three elements have the electron configurations 1s2 2s2 2p6 3s2 3p6, 1s2 2s2 2p6 3s2, and
1s2 2s2 2p6 3s2 3p6 4s1. The first ionization energies of the three elements (not in the same order) are 0.4189, 0.7377, and 1.505 MJ/mol. The atomic radii are 1.60, 0.94, and 2.35 .Identify the three elements and match the appropriate values of ionization energy and atomic radius to each configuration.
Answers
Explanation:
First element
1s2 2s2 2p6 3s2 3p6
Atomic Number = 18
Element = Argon
Second Element
1s2 2s2 2p6 3s2
Atomic Number = 12
Element = Magnesium
Second Element
1s2 2s2 2p6 3s2 3p6 4s1
Atomic Number = 19
Element = Potassium
In the periodic table;
The general trend is for ionization energy to increase moving from left to right across an element period. It would decrease down the group.
Both Argon and Magnesium are on the same period. Potassium is o the period below.
This leads us to;
Magnesium - 0.7377 MJ/mol
Argon - 1.505 MJ/mol
Potassium - 0.4189 MJ/mol
The atomic radius of atoms generally decreases from left to right across a period. It would increase down the group.
This leads to;
Magnesium - 1.60
Argon - 0.94
Potassium - 2.35
5. What part of soil is made up of decayed organic materials? (1. clay 2. bedrock 3. humus 4. sand
please help me .
Answers
Answer:humis
Explanation:because I looked it up
Answer:
It is C humus! also this is correct!
Explanation:
Which statement accurately describes a light-year?(Select One) It is the shape of a galaxy. It is the distance that light travels in a year. It is a yearly measurement of light given off by stars. It is the amount of time it takes light to travel one million kilometers.
Answers
The statement which accurately describes a light-year is " It is the distance that light travels in a year."
What is light year?A light-year would be the actual distance a light beam is able to travel in one year on Earth, that would be roughly 6 trillion miles.
What is light ?The region of the electromagnetic spectrum that the human eye perceives as light, as well as visible light, has been made up of electromagnetic radiation.
The statement which accurately describes a light-year is " It is the distance that light travels in a year."
To know more about light and light year.
https://brainly.com/question/1302132
#SPJ3
Answer: B
Explanation:
Based on the ideal gas law, there is a simple equivalency that exists between the amount of gas and the volume it occupies. At standard temperature and pressure (STP; 273.15 K and 1 atm , respectively), one mole of gas occupies 22.4 L of volume. What mass of methanol ( CH3OH ) could you form if you reacted 7.82 L of a gas mixture (at STP) that contains an equal number of carbon monoxide ( CO ) and hydrogen gas ( H2 ) molecules?
Answers
You could form approximately 5.8 grams of methanol (CH3OH) from the given gas mixture at STP.
What is the mass?
Using the Ideal gas equation;
n = PV / RT
n = (1 atm * 7.82 L) / (0.0821 L·atm/(mol·K) * 273.15 K)
From the question;
nCO = n / 2
nH2 = n / 2
Then;
n = (1 atm * 7.82 L) / (0.0821 L·atm/(mol·K) * 273.15 K)
n = 0.362 mol
nCO = n / 2 = 0.362 mol / 2 = 0.181 mol
nH2 = n / 2 = 0.362 mol / 2 = 0.181 mol
By stoichiometry;
methanol= nCO = 0.181 mol
Mass of methanol = 0.181 mol * 32.04 g/mol
= 5.8 g
Learn more about Ideal gas equation:https://brainly.com/question/30935329
#SPJ1
Help me with this question

Answers
Answer:
your cool
Explanation:
owa owa owa owa owa bob
How many grams of lead will be produced if 2.54g of PbS is burned with 1.88g of O2? write the equation
Answers
If 2.54 g of PbS is burned with 1.88 g of O2, approximately 2.20 grams of Pb will be produced.
The balanced equation for the reaction of lead sulfide (PbS) with oxygen (O2) to produce lead (Pb) and sulfur dioxide (SO2) is as follows:
2PbS + 3O2 -> 2Pb + 2SO2
From the balanced equation, we can see that the stoichiometric ratio between PbS and Pb is 2:2 or 1:1. This means that for every 1 mole of PbS, 1 mole of Pb is produced.
To calculate the number of moles of PbS, we need to divide the given mass (2.54 g) by its molar mass:
Molar mass of PbS = 207.2 g/mol (Pb) + 32.07 g/mol (S) = 239.27 g/mol
Moles of PbS = 2.54 g / 239.27 g/mol = 0.0106 mol
Since the stoichiometric ratio between PbS and Pb is 1:1, the number of moles of Pb produced is also 0.0106 mol.
To calculate the mass of Pb, we multiply the number of moles by its molar mass:
Molar mass of Pb = 207.2 g/mol
Mass of Pb = 0.0106 mol x 207.2 g/mol = 2.20 g
This calculation is based on the stoichiometric ratio between PbS and Pb, where 1 mole of PbS produces 1 mole of Pb. By converting the given mass of PbS to moles and then multiplying by the molar mass of Pb, we can determine the mass of Pb produced.
For more such question on PbS. visit :
https://brainly.com/question/27964828
#SPJ8
The mass of 3.02 x 10^22 molecules of water is ___.
Answers
Answer:
0.9 g (approximately)
Explanation:
determine the ratio of the diatomic element with equal percentage abundance
Answers
Answer:
I ratio is
47 : 43 ratio