Describe a simple experiment that can be perfomed in tha laboratory to demonstrate the formation of iron3chloride from iron fillings
Answers
Answer:
laboratory use the organic substances dissolve in water methanol or ethanol then Sinha to rise iron chloride solution is added a transient permanent coloration usually purple green or blue indicate the presence of a funnel or an hall
Explanation:
Related Questions
one practical radioactive system used to date lava flows involves: group of answer choices the gas argon-40, which decays to the gas potassium-40. the gas argon-40, which decays to solid potassium-40. the solid potassium-40, which decays to solid argon-40. the solid potassium-40, which decays to the gas argon-40. the solid potassium-40, which decays to the solid moosemossium-41.
Answers
The practical radioactive system used to date lava flows involves the gas argon-40, which decays to the gas potassium-40. Option A is correct.
This is known as the potassium-argon dating method, which is commonly used to date volcanic rocks and minerals. Potassium-40 decays into argon-40 with a half-life of 1.25 billion years, and the ratio of argon-40 to potassium-40 can be used to determine the age of the sample.
A radioactive system refers to a group of radioactive atoms that decay over time according to a specific decay scheme. The decay of radioactive isotopes occurs at a constant rate, known as the half-life, which is the time it takes for half of the original atoms to decay.
Radioactive systems are used in various applications, including dating geological materials, medical imaging, and nuclear energy production. Different radioactive isotopes have different half-lives and decay schemes, which determine their usefulness for different applications.
Hence, A. is the correct option.
To know more about radioactive here
https://brainly.com/question/1770619
#SPJ4
--The given question is incomplete, the complete question is
"One practical radioactive system used to date lava flows involves: group of answer choices A) the gas argon-40, which decays to the gas potassium-40. B) the gas argon-40, which decays to solid potassium-40. C) the solid potassium-40, which decays to solid argon-40. D) the solid potassium-40, which decays to the gas argon-40. E) the solid potassium-40, which decays to the solid moosemossium-41."--
what is the purpose of beta oxidation? what is the purpose of beta oxidation? catabolism of fatty acids feedback regulation control of atp accumulation oxidation of pyruvate oxidation of glucose
Answers
The purpose of beta-oxidation in living organisms is the catabolism of fatty acids. The correct option is A.
What is the beta oxidation of fatty acids?The beta-oxidation of fatty acids refers to the process by which fatty acid molecules are broken down into acetyl-CoA molecules by the enzymes of beta-oxidation. The process begins at the beta carbon atom of the fatty acid, hence the name beta-oxidation.
The beta-oxidation of fatty acids occurs in the mitochondria and involves a series of four repetitive steps catalyzed by four separate enzymes.
Beta-oxidation involves even-number fatty acid molecules.
The product of beta-oxidation, acetyl-CoA can then be used by the citric acid cycle to produce reducing equivalents that enter the electron transport chain for the synthesis of ATP molecules.
Hence, the role of beta-oxidation in cells is to produce acetyl CoA for ATP synthesis.
Learn more about beta-oxidation at: https://brainly.com/question/12099307
#SPJ1
what is the anion of cobalt III sulfide
chemical formula:
Co2S3
Answers
The only ion that is seen to be a negative ion in cobalt III sulfide is the sulfide ion.
What is an anion?The term anion just means a negative ion. We know that when we have an ionic compound, the ionic compound would have a positive ion which would have a plus sign and a negative ion which would have a minus sign.
We have to look at the compound that is called cobalt sulfide. there are two ions here, the cobalt III ion and the sulfide ion. The cobalt III ion is positive while the sulfide ion is negative.
Learn more about anion:https://brainly.com/question/15578817
#SPJ1
How much force is needed to accelerate a 1,000kg car at a rate of 8m/s2
Answers
Answer:
Force = Mass × Acceleration
Thus, F = 1,000 × 8
F = 8,000N
Thus force needed is 8,000N
Answer:3000N
Explanation:
80 calories must be lost to make water change into ice at 0° C. True False
Answers
which metal is more reactive? Mg or Ba
Answers
Answer:
These metals are less reactive than the neighbouring alkali metal. Magnesium is less active than sodium; calcium is less active than potassium; and so on. These metals become more active as we go down the column.
Explanation:
Magnesium is more active than beryllium; calcium is more active than magnesium; and so on.
Walter White cooked 20 pounds of methamphetamine. Gustavo Fring wants to know how much will he earn from this batch. He asks Jesse Pinkman to calculate the total profit he will make. Each pound of methamphetamine costs $4500. How much will Mista White make from this batch?
Answers
Answer:
TOTAL: $90000
Explanation:
A marathon is approximately 42.195 km Convert the distance to
centimeters.
Answers
Answer:
4219500 centimeters
Explanation:
I hope this helped!
FOUR ways to obtain a brighter lamp?
Answers
Increasing LED brightness with a lampshade or reflection and choosing LED lights with high luminous efficiency can be useful to obtain a brighter lamp.
What is lamp?Lamp, lighting device, initially a jar housing a flame soaked in flammable substance, and later such light-producing devices as gas and electrical lamps.
The light was developed at least 70,000 years ago. Originally, it was a sunken rock filled in moss or other absorbent substance, saturated in animal fat, and burned. The ways to obtain a brighter lamp are
Increasing LED brightness with a lampshade or reflection.
To boost brightness, use greater color temperature LED lights.
Choosing LED lights with high luminous efficiency.
With increased voltage, LED lights get brighter.
Therefore, in above ways we can get brighter lamp.
To learn more about lamp, here:
https://brainly.com/question/9498978
#SPJ1
Which of the following is not a type of mechanical wave?
Question 3 options:
surface
concave
transverse
longitudinal
Answers
Answer:
concave
Explanation:
Help ASAP give brainest if right And please hurry

Answers
Answer:
Conduction
Convention
Radiation
in that order
Answer:
Conduction
Convention
Radiation
Explanation:
T/F: the step in any reaction sequence determines the rate law for the overall reaction. this step is called the rate- step.
Answers
The step in any reaction sequence that determines the rate law for the overall reaction is called the rate-determining step. TRUE.
This step is also known as the slowest step in the reaction sequence. The rate law for the overall reaction is determined by the reactants and the rate-determining step. Therefore, it is important to identify the rate-determining step in order to determine the rate law for the overall reaction.
True, the step in any reaction sequence that determines the rate law for the overall reaction is called the rate-determining step. This step has the slowest rate among all the steps in the reaction sequence and thus governs the overall rate of the reaction.
Learn more about reaction sequence
brainly.com/question/29607707
#SPJ11
Which joint in the human body is similar to the chicken wing joint?
Answers
The joint in the human body which is similar to the chicken wing joint is referred to as the elbow joint.
What is a Joint?This is referred to as a point where two or more bones meet and are usually lined with cartilages so as to reduce the effect of friction as it causes wear and tear of the skeletal structures which are important for our movement.
The chicken has a structure called wing as its hands which is therefore the reason why it is similar to that of the elbow joint in humans and makes it the correct choice.
Read more about Joint here https://brainly.com/question/1007674
#SPJ1
an acid chloride is much more reactive toward nucleophiles than an ester. this is because cl– is a much _____ base than ro– and is therefore a better _____ group.
Answers
An acid chloride is much more reactive toward nucleophiles than an ester because Cl– is a much weaker base than RO– and is therefore a better leaving group.
In the context of nucleophilic substitution reactions, a nucleophile attacks the carbonyl carbon of an acid chloride or an ester, displacing the leaving group and forming a new bond to the carbonyl carbon. In the case of an acid chloride, the chloride ion is a better leaving group than the alkoxide ion in an ester, because it is less basic and more stable. This means that when a nucleophile attacks an acid chloride, the chloride ion is more easily displaced, leading to a faster and more efficient reaction.
Additionally, the electronegativity of the chlorine atom in an acid chloride is higher than that of the oxygen atom in an ester. This means that the carbonyl carbon in an acid chloride is more electrophilic and more prone to attack by a nucleophile than the carbonyl carbon in an ester.
In summary, the higher reactivity of acid chlorides toward nucleophiles compared to esters is due to the weaker basicity and better leaving group ability of the chloride ion, as well as the higher electrophilicity of the carbonyl carbon in an acid chloride.
To know more about esters, refer
https://brainly.com/question/28118164
#SPJ11
A 14.00 g sample of hydrate copper(II) sulfate, CuSO4 * nH2O, is heated to drive off the water. 5.051 g of H2O was released from the sample. What is the value of "n" in the hydrate formula
Answers
The value of "n" in the hydrate formula CuSO4 * nH2O is 5.
To determine the value of "n," we need to calculate the molar ratio between the released water and the hydrate copper(II) sulfate.
First, we need to convert the mass of water released to moles. The molar mass of water (H2O) is approximately 18.015 g/mol. Therefore, 5.051 g of water is equal to 5.051 g / 18.015 g/mol ≈ 0.2804 mol.
Next, we calculate the molar ratio between water and copper(II) sulfate. The molar mass of copper(II) sulfate (CuSO4) is approximately 159.609 g/mol. From the balanced chemical equation, we know that one mole of copper(II) sulfate is associated with "n" moles of water.
Assuming that the molar ratio is 1:1 between CuSO4 and H2O, we can set up the following equation:
0.2804 mol H2O = 14.00 g CuSO4 * (1 mol H2O / (159.609 g CuSO4 * n))
By rearranging the equation, we can solve for "n":
n = 14.00 g CuSO4 / (159.609 g CuSO4/mol) = 0.0877 mol
Since "n" represents the number of water molecules, it must be a whole number. Therefore, the closest whole number to 0.0877 is 5.
Therefore, the value of "n" in the hydrate formula CuSO4 * nH2O is 5.
for such more questions on formula
https://brainly.com/question/26388921
#SPJ8
A copper wire 10 m long must experience a voltage drop of less than 1.0 V when a current of 3.0 A passes through it. Using the data in Table 12.1, compute the minimum diameter of the wire. Table 12.1 Room-Temperature Electrical Conductivities for Nine Common Metals and Alloys Metal Electrical Conductivity (22-m)!) Silver 6.8 * 107 Copper 6.0 x 107 Gold 4.3 x 107 Aluminum 3.8 x 107 Brass (70 Cu-30 Zn) 1.6 × 107 Iron 1.0 x 107 Platinum 0.94 x 107 Plain carbon steel 0.6 x 107 Stainless steel 0.2 x 107 mm
Answers
The minimum diameter of the copper wire is 0.798 mm.
A copper wire 10 m long must experience a voltage drop of less than 1.0 V when a current of 3.0 A passes through it. Using the data in Table 12.1, compute the minimum diameter of the wire.
Table 12.1 Room-Temperature Electrical Conductivities for Nine Common Metals and Alloys Metal Electrical Conductivity (22-m)!) Silver 6.8 * 107 Copper 6.0 x 107 Gold 4.3 x 107 Aluminum 3.8 x 107 Brass (70 Cu-30 Zn) 1.6 × 107 Iron 1.0 x 107 Platinum 0.94 x 107 Plain carbon steel 0.6 x 107 Stainless steel 0.2 x 107 mm
Calculation:-
D = √4IL/πv sigma
D = √ 4× 3 × 10/π × 1× 6 × 10⁷
= 7.98 × 10-⁴.
D = 0.798 mm.
Gold is a chemical detail with the symbol Au (from Latin: aurum) and atomic number seventy nine. This makes it one of the better atomic range factors that occur naturally.
it is a shiny, barely orange-yellow, dense, smooth, malleable, and ductile metal in a pure form. Chemically, gold is a transition metallic and a collection eleven element. it's far one of the least reactive chemical elements and is stable underneath trendy conditions.
Learn more about copper wire here:-
brainly.com/question/24856041
#SPJ4
Polar bears are adapted to stay warm by growing thick fur. These organisms most likely live in a what
Answers
Answer:
They live in the open snow
Explanation:
fill in the blanks am giving brainliest and thanx
Answers
Answer:
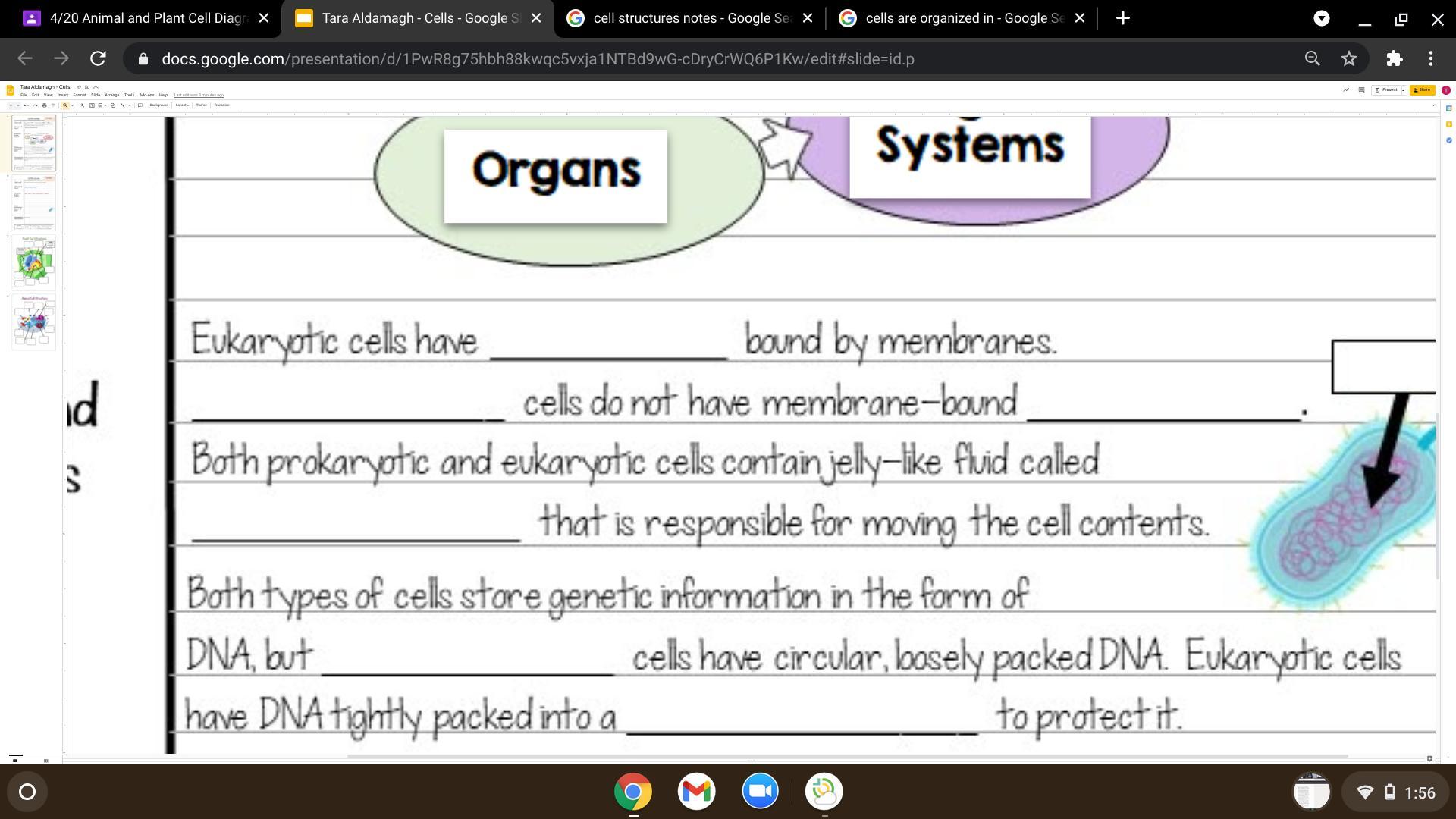
Organelles, prokaryotic, organelles, cytoplasm, prokaryotic, nucleus
Explanation:
Organelles are membrane bound only in eukaryotic ccells.
Prokaryotic cells unlike eukaryotic cells, do not have membrane bound organlles.
Something that is found in both prokaryotic and eukaryotic cells is the cytoplasm, which is fluid that is inside the whole cell.
Unlike eukaryotic cells, prokaryotic cells have loose DNA, which floats around the cell quite literally.
The only way DNA is stored in eukaroyotic cells is in the nucelus, which is only found in eukaryotic cells, not prokaryotic.
Hope this helps ;)
A weather balloon calibrated at 0.00 °C to have a volume of 20.0 L has
what volume at -40.0 °C assuming pressure is held constant?
Answers
Answer:
17.1 Liters
Explanation:
It's a gas law question (more specifically a Charles's Law question). Formula is V1/T1 = V2/T2. You're given V1 and T1 and T2. However, in order to use the equation, temperature needs to be in Kelvins (by subtracting the degrees C from 273) for the numbers to work (among other reasons, the 0 degrees C will always give you an answer of zero or undefined). Placing all temps in kelvins makes the answers come out right. So 20L/273K = xL/233K gives you the answer when you cross-multiply.
Considering the Charles's law, a weather balloon calibrated at 0.00 °C to have a volume of 20.0 L has 17.07 L at -40.0 °C, assuming pressure is held constant.
The gas laws are a set of chemical and physical laws that allow determining the behavior of gases in a closed system. The parameters evaluated in these laws are pressure, volume, temperature and moles.
Charles's law is one of the gas laws. It relates the volume and the temperature of a certain quantity of ideal gas, kept at a constant pressure.
This law states that, at constant pressure, the volume of a gas is directly proportional to its temperature. In other words, for a given sum of gas at constant pressure, as the temperature increases, the volume of the gas increases, and as the temperature decreases, the volume of the gas decreases.
Mathematically, Charles's law says that the quotient that exists between the volume and the temperature will always have the same value:
\(\frac{V}{T}=k\)
Being an initial state 1 and a final state 2, it is true:
\(\frac{V1}{T1}=\frac{V2}{T2}\)
In this case, you know:
V1= 20 LT1= 0 C=273 KV2= ?T2= -40 C= 233 KReplacing:
\(\frac{20 L}{273 K}=\frac{V2}{233 K}\)
Solving:
\(V2=233 K x\frac{20 L}{273 K}\)
V2=17.07 L
Finally, a weather balloon calibrated at 0.00 °C to have a volume of 20.0 L has 17.07 L at -40.0 °C, assuming pressure is held constant.
Learn more:
https://brainly.com/question/4147359?referrer=searchResultsIs evaporation a chemical or physical change?
Answers
Evaporation, the liquid turns into a gas, but it still retains its original chemical composition. It is not a new substance, but rather a change in state from a liquid to a gas. This change is caused by the escape of molecules from the surface of the liquid into the air, which results in a decrease in the number of molecules in the liquid and an increase in the number of molecules in the air.
Therefore, evaporation is considered a physical change, not a chemical change. This is because the substance undergoing evaporation retains its original chemical composition, and the change is reversible by condensation, which is the process by which a gas turns into a liquid.
Evaporation is when a liquid turns into a gas at a temperature below its boiling point. It is a common phenomenon that occurs in everyday life, such as when water evaporates from a puddle or a wet towel.
The question of whether evaporation is a chemical or physical change can be confusing for some students. However, it is important to understand the difference between these two types of changes in order to understand evaporation.
A chemical change is a change that results in the formation of a new substance with different properties than the original substances. Chemical changes are often accompanied by a change in energy, such as a release or absorption of heat. Examples of chemical changes include burning, rusting, and digestion.
On the other hand, a physical change is a change in a substance's physical properties, such as its state, size, or shape, without forming a new substance. Physical changes are reversible, meaning that the original substance can be restored to its original state by reversing the change. Examples of physical changes include melting, freezing, and boiling.
Know more about evaporation: https://brainly.com/question/2013258
#SPJ11
Choose the pair of substances that are most likely to form a homogeneous solution.
A. F2 and C2H5OH
B. H2O and CH3OH
C. LiCl and C10H20
D. CH4 and C2H5OH
Answers
A) F2 and C2H5OH (Heterogeneous mixture)B) H2O and CH3OH (Homogeneous mixture)C) LiCl and C10H20 (Heterogeneous mixture)D) CH4 and C2H5OH (Heterogeneous mixture).
The pair of substances that are most likely to form a homogeneous solution is B. H2O and CH3OH. A homogeneous mixture is one in which the components are evenly distributed throughout the mixture. These types of solutions have uniform properties and the same composition throughout.A heterogeneous mixture is one in which the components are not evenly distributed. This means that the mixture has distinct regions or phases that are visibly different from one another. The following are the most likely pairs of substances to form a homogeneous solution:A) F2 and C2H5OH (Heterogeneous mixture)B) H2O and CH3OH (Homogeneous mixture)C) LiCl and C10H20 (Heterogeneous mixture)D) CH4 and C2H5OH (Heterogeneous mixture).
learn more about Heterogeneous
https://brainly.com/question/24898889
#SPJ11
if you decide to do acid/base reaction which mixture will be more appropriate for the experiment?
Answers
The choice of mixture will depend on the specific goals of the experiment and the chemical properties of the substances being used. It is important to carefully consider these factors before choosing the appropriate mixture for the acid/base reaction.
When deciding on an acid/base reaction, it is important to choose the appropriate mixture for the experiment. The mixture chosen will depend on the specific reaction being conducted and the desired outcome.
For example, if the goal of the experiment is to neutralize an acid, a basic solution would be the appropriate mixture. This is because the basic solution will react with the acid to form water and a salt, neutralizing the acid.
On the other hand, if the goal of the experiment is to create a chemical reaction, an acid solution may be the appropriate mixture. This is because the acid will react with a base to form a salt and water, creating a chemical reaction.
Overall, the choice of mixture will depend on the specific goals of the experiment and the chemical properties of the substances being used. It is important to carefully consider these factors before choosing the appropriate mixture for the acid/base reaction.
To know more about acid/base reaction refer to
https://brainly.com/question/15209937
#SPJ11
What is the mass number of sodium

Answers
Answer:
Explanation:
Sodium mass number 23, 11 electrons
Magnesium: neutrons = 12
aluminum : atomic number = 13
phosporus : protons = 15
Answer:
23
Explanation:
You need to add protons and neutrons to get mass
Suppose 50.0 grams of compound AB reacts completely with 100.0 grams of compound CD, how many grams of products will be formed, according to the Law of Conservation of Matter?
a. 50
b. 100
c. 150
d. 200
Answers
Answer:
Option C = 150
Explanation:
First of all we will understand what is law of conservation of mass.
Law of conservation of mass:
This law stated that, mass can neither be created nor destroyed in a chemical equation.
This law was given by French chemist Antoine Lavoisier in 1789. According to this law mass of reactant and mass of product must be equal, because masses are not created or destroyed in a chemical reaction.
For example:
In given photosynthesis reaction:
6CO₂ + 6H₂O + energy → C₆H₁₂O₆ + 6O₂
there are six number of carbon atoms, eighteen oxygen atoms and twelve hydrogen atoms on the both side of chemical equation so this reaction followed the law of conservation of mass because mases are same on both side.
In given chemical reaction:
AB + CD → X
50 g + 100 g = 150 g
Thus option c is correct.
Is silicon(IV) oxide the same as silicon dioxide
Answers
Answer:
Yes, it is
Explanation:
Silicon (IV) oxide, commonly known as silica is a substance that majorly constitutes SAND. It has the chemical formula, SiO2, which contains a combination of silicon and oxygen.
The IUPAC name of SiO2 is Silicon IV oxide, which can also be referred to as Silicon dioxide because the structure the compound contains two (-di) oxygen atoms. Hence, based on the question, silicon(IV) oxide is the same as silicon dioxide.
The Sun is a constant supply of energy to Earth.
A. True
B. False
Answers
The sun gives the earth thermal energy, which warmth the planet and effects the weather and the seasons. Also the sun is the beginning of the food chain, because it allows for plants to grow.
We also get our energy either directly or in directly from the sun, either from our food that we eat, or through things like the burning of fossil fuels, which was once plant-matter millions of years ago.
Need help???????!!!!?????!!???

Answers
Answer:
a. 87.33 g
b. 2.30 g
Explanation:
1 mole of LiCl has 42.394 g
=> 2.060 x 42.394 = 87.33164 g or 87.33 g
1 mole of C2H2 has 26.04 g
=> 0.0885 x 26.04 = 2.30454 g or 2.30 g
2. How have observations of the natural world helped
in the development of calendars?
Answers
Answer:
just wanted points
Explanation:
i wasted your time srry
Why is there a higher rate of skin cancer in Australia than in other parts of the world?
Answers
Answer:
Read below
Explanation:
Melanoma -- the most virulent form of skin cancer -- is caused by harmful ultraviolet light from the sun. Australia's proximity to Antarctica, where there is a hole in the ozone layer that filters out UV rays, also increases the risk.
Answer:
In Austrailia there is the hieghest rate of skin cancer because there is so much sunlight in Austrailia and there some people that have sensitive skin and they get skin cancer easily/ fast.
Explanation: This answer is correct because my teacher gave me this answer.
Hope this was helpful if not plz don't report my answer plz.
Science questions about pure substances and mixtures
Answers
The composition of the element categorised elements as a pure substance and mixture.
What is element?
Element, any substance that cannot be decomposed into simpler substances by ordinary chemical processes.
A pure substance consists only of one element or one compound. a mixture consists of two or more different substances, not chemically joined together.
Learn more about elements here:
https://brainly.com/question/14347616
#SPJ4