Considering the temperature vs. time graph below, how does the temperature at the beginning of a change of state compare with the temperature at the end of the change?

Answers
The temperature is always the same. Hence, option A is correct.
What is temperature?The degree of hotness or coldness is measured on a definite scale.
Assume we have something in the solid phase. As we increase the temperature, the particles on the solid increase their kinetic energy, thus, the particles move more.
This causes the volume of the object increases (for example when we heat up the metal and it dilates). This keeps happening until we reach a critical point when we are near a change of phase.
At this point, the energy given is not used to increase the temperature of the object but is used to "break" bonds in such a way that the particles are freer than before. When all these bonds are "broken" the change of phase is completed, and in the case of the solid, we go from solid phase to liquid phase.
An example of this is that we can have liquid water and solid water both at 0°C, so if you have a thermometer in your home, an experiment that you can do is:
Put water in the refrigerator.
Note when the liquid water reaches 0°C
As the water starts to solidify, keep recording its temperature, you will see that it does not change (a lot, it may change a little bit) until all the water changes phase.
Hence, the temperature is always the same.
Learn more about the temperature here:
brainly.com/question/11804615
#SPJ1
Related Questions
how can you tell which Element is it, by just the diagram? help
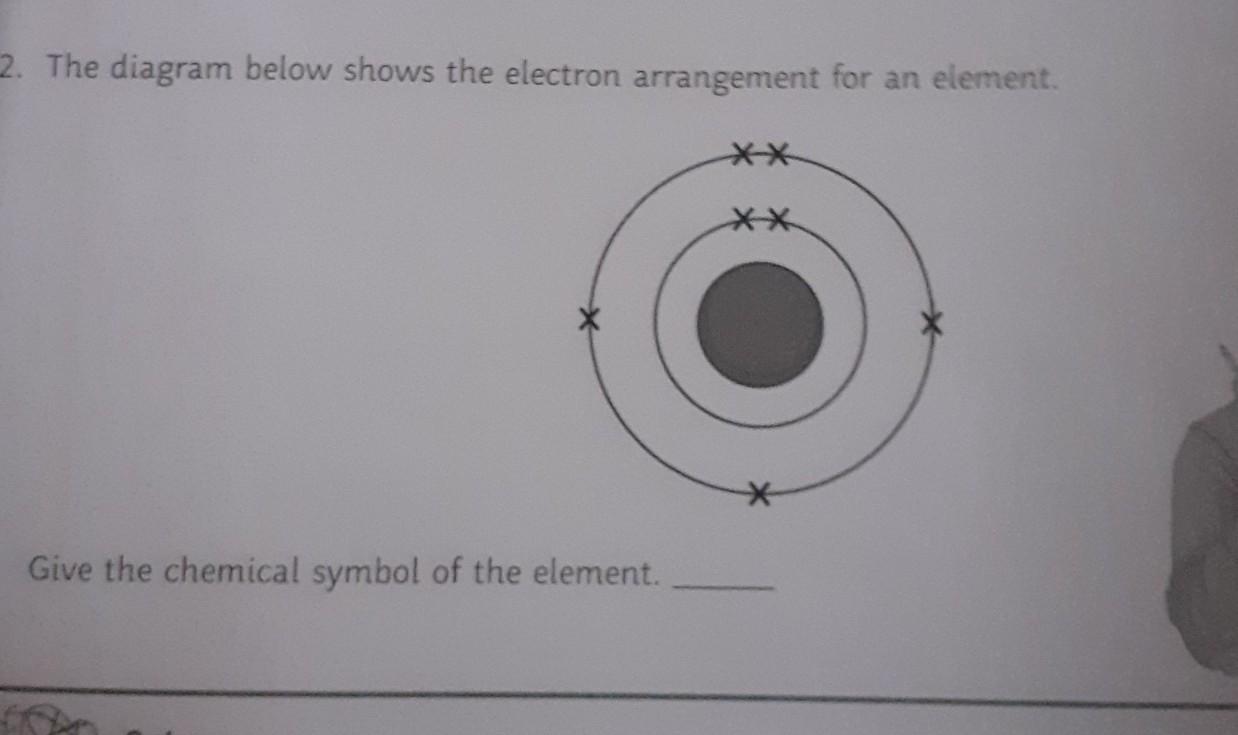
Answers
Answer:
Nitrogen (N2)
Explanation:
it has a total of 7 electrons occupying it's shells... that's due to its atomic number of 7
: In this problem, you will answer some basic questions about the electron configuration notation used to show the number of electrons in each subshell of an atom of a particular element. Why should the As subshell be filled before the 3d? The As subshell has greater spherical symmetry than the 3d subshell. The 4s subshell is farther from the nucleus than the 3d subshell. The 4s subshell is at lower energy than the 3d subshell. The As subshell holds fewer electrons than the 3d subshell. Write the electron configuration for the Na^+ ion, which has ten electrons. Enter 3S^3 for 3s^3, etc. Separate the subshells by spaces. 1*s^2, 2*2, 2*p^6, 3*s^1 Write the electron configuration for the Br^- ion, which has thirty-six electrons. Enter 3s^3 for 3s^3 (e.g., 1s^2 2s^2).
Answers
1. The 4s subshell should be filled before the 3d subshell because the 4s subshell is at lower energy than the 3d subshell.
Electrons fill the subshells in order of increasing energy.
2. To write the electron configuration for the Na^+ ion, which has ten electrons, follow these steps:
a. Begin with the lowest energy subshell, which is 1s.
b. Fill the subshells with electrons in increasing energy order: 1s, 2s, 2p, 3s, and so on.
c. Stop when you've added ten electrons.
The electron configuration for the Na^+ ion is: 1s^2 2s^2 2p^6
3. To write the electron configuration for the Br^- ion, which has thirty-six electrons, follow the same steps as above, but stop when you've added thirty-six electrons.
The electron configuration for the Br^- ion is: 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^6
To know more about electronic configuration, visit: https://brainly.com/question/30424681
#SPJ11
what best describe the process of percipitation in the water cycle
Answers
Answer:
Precipitation is water released from clouds in the form of rain, freezing rain, sleet, snow, or hail. It is the primary connection in the water cycle that provides for the delivery of atmospheric water to the Earth. Most precipitation falls as rain.
Explanation:
Which of the following statements is not an intensive
property?
A chemical reaction requires 10.00 g of carbon.
The density of water at 25°C is 1.00 g/mL..
Solid copper (II) sulfate pentahydrate is blue colored.
The boiling point of water is 373.15 K.
Answers
The intensive properties is ;
The density of water at 25°C is 1.00 g/mL..
What is an intensive property?Intensive properties are properties of a substance that do not depend on the amount of the substance present, but only on its temperature, pressure, and other conditions.
We have to note that the density does not depend on the amount of the substance that is present then it follows that the density that we have is an intensive property. It does not depend on the amount of the substances that we have here.
Learn more about intensive properties:https://brainly.com/question/30136831
#SPJ1
help me please I really need it

Answers
a) Magnesium, 2.8.2, Group number 2,Metals, They are not non metals.
b) Fluorine, 2.2.5, Group number7, Metal No, Non-Metal yes
c) Carbon, 2,2,2. Group number 4, Metal No, Non-Metal yes
_____________________________________Best Regards,'Borz'The ability of a substance to be hammered or rolled into thin sheets is called ________
Answers
Answer: Malleability
Explanation: is a physical property of metals that defines their ability to be hammered, pressed, or rolled into thin sheets without breaking. In other words, it is the property of a metal to deform under compression and take on a new shape.
Guys please help me this is for a final
What happens when you change only the number of electrons inside of an atom
Answers
Answer: Changing the number of electrons will change the overall charge on an atom.
Explanation:
An atom that loses electrons will become positively charged and an atom with added electrons will become negatively charged. ... A charged atom is called an ion.
35. a
When aqueous iron (III) chiondes added to aqueous potassium iodide a chemical con
ours and lodine is formed
Which statement is correct?
A todide sons are oxidised, they gain electrons in this reaction
lodide ions are oxidised, they lone electrons in this reaction
C trond) chionde is oxidised in this reaction
D Neither iodide ions nor iron (III) chlonde is ondised in this reachon

Answers
Everything else being equal, a gradual increase in atmospheric CO2 would most likely bring
about: (1) no change in global climate (2) a decrease in evaporation from the earth's oceans
(3) a marked decrease in plant growth
(4) an increase in surface temperature
Answers
Everything else being equal, a gradual increase in atmospheric CO2 would most likely bring about (4) an increase in surface temperature.
CO2 is a greenhouse gas, which means that it absorbs infrared radiation and contributes to the warming of the Earth's atmosphere. As the concentration of CO2 in the atmosphere increases, it traps more heat and leads to an increase in surface temperature. This effect is known as the greenhouse effect.
The increase in temperature can have many consequences, including changes in precipitation patterns, rising sea levels, and more frequent and severe weather events. However, it is important to note that other factors, such as changes in solar radiation and volcanic activity, can also affect the Earth's climate.
Learn more about temperature here:
https://brainly.com/question/11464844
#SPJ11
Can someone help me with number 4? Please and thank you

Answers
Answer:
1st Blank: 1 Co
2nd Blank: 2 Na2S
3rd Blank: 4 Na
4th Blank: 1 CoS2
Explanation:
Trust me
The diagram represents the general equation for photosynthesis.
What does the X represent?
hydrogen
oxygen
glucose
carbon dioxide
Answers
Answer:
The diagram represents the general equation for photosynthesis.
What does the X represent?
Explanation:
Carbon dioxide
If the centre of an atom contains 8 particle that are charged, how many particles are revolving round this centre?
Answers
Explanation:
charged particles=8 which is proton and proton=no.of electron. That's why 8 particles are revolving round this center. And this atom structure is of oxygen
If the center of an atom has 8 charged particles that are protons as the neutrons are neutral there will be 8 negative charges that are electrons revolving around the center nucleus.
What is an atom?An atom is defined as the smallest unit of matter which forms an element. Every form of matter whether it is solid,liquid or gas consists of atoms . Each atom has a nucleus which is composed of protons and neutrons and shells in which the electrons revolve.
The protons are positively charged particles and neutrons are neutral and hence the nucleus is positively charged. The electrons which revolve around the nucleus are negatively charged particles and hence the atom as a whole is neutral and stable due to presence of oppositely charged particles.
Atoms of the same element are similar as they have number of sub- atomic particles which on combination do not alter the chemical properties of the substances.
Learn more about atom,here:
https://brainly.com/question/13654549
#SPJ2
patterns of reactivity quick check
Answers
Reactivity decreases as you move from left to right. The farther to the left and down the periodic chart you move, easier it is for electrons to be given or taken away, hence resulting in higher reactivity.
What is the pattern of reactivity in periodic table?Reactivity decreases as we move down the column. As you learn more about the table, you will be able to find that this pattern is true for other families.
The atoms get bigger, as the atomic number increases. The chemical properties change slightly when compared to the element right above them on the table. The non-metal elements in Group 7 that are known as the halogens, get less reactive as you move to the down of the group.
To know more about pattern of reactivity, refer
https://brainly.com/question/6759644
#SPJ4
what is the effect on the boiling point of a solution produced by an insoluble nonvolatile substance ?
Answers
Answer:
nothing
Explanation:
hope this helps ;)
What do you notice when you get into a car that has been sitting in the sun for a while?
Answers
When you get into a car that has been sitting in the sun for a while, there are several noticeable things that may occur. Here are some of the common observations:
1. Heat: One of the first things you'll notice is the intense heat inside the car. This is because the sun's rays have been absorbed by the car's exterior and trapped inside, creating a greenhouse effect. The temperature inside the car can become significantly higher than the temperature outside.
2. Hot Surfaces: The surfaces inside the car, such as the seats, dashboard, steering wheel, and metal parts, can become extremely hot to the touch. This is due to the absorption of heat from the sun. It's important to be cautious and avoid direct contact with these hot surfaces to prevent burns or discomfort.
3. Odor: The interior of the car may have a distinct smell when it has been sitting in the sun for a while. This is often referred to as the "hot car smell." It is caused by the combination of materials, such as upholstery, plastic, and carpet, heating up and emitting a specific odor.
4. Fading or Discoloration: Prolonged exposure to sunlight can cause fading or discoloration of materials inside the car. For example, the upholstery, dashboard, and other surfaces may gradually lose their original color and become faded or discolored over time.
5. Glare: When you first enter a car that has been sitting in the sun, you may notice a strong glare from the sunlight reflecting off the windshield and other glass surfaces. This glare can make it difficult to see clearly and may require the use of sunglasses or adjusting the sun visors to minimize the brightness.
It's important to note that these observations may vary depending on factors such as the intensity of the sunlight, the duration the car has been in the sun, and the materials used in the car's interior. Regular maintenance and taking precautions, such as using sunshades or parking in shaded areas, can help minimize some of these effects.
to know more about the greenhouse effect here:
brainly.com/question/31595505
#SPJ11
Like any other equilibrium constant, kw is also affected by temperature. the kw at 75 degrees celsius is 1.995 x 10⁻¹³. What is the poh of water at this temperature?
Answers
Like any other equilibrium constant, kw is also affected by temperature. the kw at 75 degrees Celsius is 1.995 x 10⁻¹³. Therefore, 13 is the pOH of water at this temperature.
What is pOH?The concentration of hydroxide ions (OH-) is measured by pOH. It is utilized to express a solution's alkalinity.
Aqueous solutions having pOH less than 7 at 25 degrees Celsius are alkaline, pOH larger than 7 are acidic, while pOH equal with 7 are neutral. pH and hydrogen ion concentration is used to compute pOH.
Kw = [OH⁻] [H⁺]
1.995 x 10⁻¹³ = Kw
[OH⁻] =[H⁺]
Kw = [OH⁻]²
[OH⁻] =10⁻¹³
pOH = -log [OH⁻]
= - log 10⁻¹³
= 13
Therefore, 13 is the pOH of water at this temperature.
To know more about pOH, here:
https://brainly.com/question/4092108
#SPJ1
1) In this experiment, you will be mixing aqueous solutions of sodium carbonate and calcium chloride to produce solid calcium carbonate.
Na CO2 (aq) +CaCl(aq) — 2 NaCl(aq) +CaCO3
Order the steps required to predict the volume (in mL) of 0. 200 M sodium carbonate needed to produce 2. 00 g of calcium carbonate. There is an excess of calcium chloride.
Comp
Identi
volum
2
>
Calcu
Check
>
Step 1
Convert mass of calcium carbonate 10 moles of calcium carbonate
Step 2
Compare moles of calcium carbonate to moles of sodium carbonate based on balanced equation to calculate moles of sodium carbonate required
Step 3
Compute the volume of sodium carbonate solution required
Step 4
Convert the volume of sodium carbonate solution required from liters to milliers
2) Na2CO3(aq) + CaCl2(ag) + 2NaCl(aq) + CaCO3(-)
Calculate the volume (in mL) of 0. 200 M Na2CO, needed to produce 2. 00 g of CaCO3(s). There is an excess of CaCl.
Molar mass of calcium carbonate = 100. 09 g/mol
Volume of sodium carbonate - 100 mL
METHODS
RESET
MY NOTES
A LAB DATA
Answers
Based on the information provided, the correct order of steps required to predict the volume of 0.200 M sodium carbonate needed to produce 2.00 g of calcium carbonate is:
Step 1: Identify the molar mass of calcium carbonate (CaCO3)
Step 2: Convert the given mass of calcium carbonate to moles using its molar mass
Step 3: Use the balanced chemical equation to determine the mole ratio between calcium carbonate and sodium carbonate
Step 4: Calculate the amount of moles of sodium carbonate required
Step 5: Convert the moles of sodium carbonate to volume in liters using its molarity
Step 6: Convert the volume in liters to milliliters
Therefore, the correct order of steps is:
Step 1: Identify
Step 2: Convert
Step 3: Compute
Step 4: Convert
Step 5: Convert
Step 6: Check
Using the given information, the calculation can be done as follows:
Step 1: The molar mass of calcium carbonate (CaCO3) is given as 100.09 g/mol.
Step 2: The given mass of calcium carbonate is 2.00 g. Therefore, the number of moles of calcium carbonate can be calculated as follows:
2.00 g / 100.09 g/mol = 0.01998 mol ≈ 0.020 mol
Step 3: According to the balanced chemical equation, the mole ratio between calcium carbonate and sodium carbonate is 1:1. Therefore, the amount of moles of sodium carbonate required is also 0.020 mol.
Step 4: The molarity of the sodium carbonate solution is given as 0.200 M. Therefore, the volume of sodium carbonate solution required in liters can be calculated as follows:
0.020 mol / 0.200 mol/L = 0.100 L = 100 mL
Step 5: The volume in liters needs to be converted to milliliters:
0.100 L x 1000 mL/L = 100 mL
Step 6: Check the answer to make sure it is reasonable and makes sense.
To know more about sodium carbonate, visit: brainly.com/question/13565765
#SPJ4
What happens when we add water to acid and acid to water?
Answers
Explanation:
When the water add acid there have the reaction then the molecules are evolve highly reactive which have produce bubble which cause our face directly but acid add in water there have less reactive molecules evolve ant there doesnot produce bubble
Mr. Summers observed that kids with hot dogs are happy. This observation led to a hypothesis that hot dogs must make kids happy. Before Mr. Summers can test this hypothesis, what must he do?
a. create a problem
b. make an observation
c. analyze data
d. design the experiment
Answers
Given what we know, we can confirm that since Mr. Summers has to test a hypothesis, his next step should be to design an experiment.
Why design an Experiment?The next step is to design an experiment.This is because Mr. Summers has already made an observation and created a problem.He must now gather data to be analyzed. In order to do this, he must first design and perform an experiment.Therefore, we can confirm that Mr. Summers must design an experiment given that this is the best way to gather data in order to be analyzed in the future and draw a valid conclusion.
To learn more about Hypothesis visit:
https://brainly.com/question/2695653?referrer=searchResults
Which of the following is the primary medium for beach erosion?
ice
gravity
wind
water
Answers
Answer:
Water is the primary erosion
hope it helps :)
Water is added to a test-tube containing dilute NaOH of pH 11. What could be the pH of the resulting solution?
Answers
Answer:
NaOH atomic mass is 39.977
pH is 11 due to dilution must be multiplied by 0.11 to find pH
39.977 x 0.11 = 0.43979 x 100%
43% pH
Lithium and Fluoride be neutral?
2sentence
Answers
Answer:
Ionic
Explanation:
Lithium is an alkali metal and form an ionic bond by donating an electron.
Fluorine is a halogen and forms ionic bonds by accepting an electron.
Diagramming the formation of an ionic bond between lithium and fluorine looks exactly like the diagrammed bond between sodium and chlorine in the video below. Lithium behaves just like the sodium (Na) and fluorine will act like the chlorine (Cl).
5. a) Please derive expressions for \alpha_{0}, \alpha_{1} , and \alpha_{2} implicated in the speciation of rm{H}_{2} rm{CO}_{3} into rm{HCO}_{3}^{-} and r
Answers
The expression for α0, α1, and α2 implicated in the speciation of H2CO3 into HCO3- and CO32- are:
H_{2} CO_{3}(aq) \rightleftharpoons HCO_{3} ^{-}(aq)+H^{+}(aq)$ (1) HCO_{3} ^{-}(aq) \rightleftharpoons CO_{3} ^{2-}(aq)+H^{+}(aq)(2)These reactions can be defined using the acid dissociation constant (Ka) of H2CO3 as follows:
Ka1 = [HCO3-][H+]/[H2CO3]Ka2 = [CO32-][H+]/[HCO3-]According to Brønsted-Lowry theory, H2CO3 can donate two protons and can behave as a diprotic acid.From these definitions, the acid dissociation constants for H2CO3's first (Ka1) and second (Ka2) dissociation reactions can be expressed as shown below:
Ka1 = α1[ H+][ HCO3-]/[ H2CO3] Ka2 = α2[ H+][ CO32-]/[ HCO3-]where α1 and α2 are the activity coefficients of the intermediate ions. α0 represents the activity coefficients of H2CO3. Let's look at each of these coefficients in turn.α0: the activity coefficient of the molecular species, H2CO3α1 the activity coefficient of HCO3- ionα2 the activity coefficient of CO32- ion.About AcidAn acid is a molecule or ion that can donate a proton, or, alternatively, can form a covalent bond with an electron pair. The first category of acids is the proton donor or Brønsted acid. In the special case of an aqueous solution, the proton donor forms the hydronium ion H₃O⁺ and is known as an Arrhenius acid. PH is the degree of acidity or alkalinity of a solution, expressing the negative logarithm of the concentration of H ions with a base number of 10. Neutral solutions have a PH of 7, acids less than 7, bases greater than 7. In waters that are not polluted, PH is controlled by CO2 ions, Carbonates and Bicarbonates.
Learn More About Acid at https://brainly.com/question/25148363
#SPJ11
¿Qué energías son usadas en la fabricación de las llantas desde la obtención de materia prima caucho natural, hasta el producto terminado ?
Answers
Answer:
yo te diria que el elastico amigo es esa
1. give an example of a type of real-world item that is organized or sorted in a specific way.
Answers
One example of a real-world item that is organized or sorted in a specific way is a library's book collection. The books are typically sorted using the Dewey Decimal Classification system, which categorizes them based on subject matter.
There are many types of real-world items that are organized or sorted in specific ways. One example is a library. Libraries organize books according to various systems, such as the Dewey Decimal System or the Library of Congress Classification System. These systems allow books to be organized by subject matter, author, and other criteria, making it easier for patrons to locate specific books or browse for new ones. In addition, libraries often have specific sections for different types of materials, such as reference books, periodicals, and audiovisual materials.
This organization helps users to find the specific type of material they need, while also allowing library staff to manage the collection more efficiently. Overall, many real-world items are organized or sorted in specific ways in order to make them more manageable and user-friendly. Whether it's a library, a grocery store, or another type of organization, these systems help people find what they need and make the most of the resources available to them.
To know more about matter visit :-
https://brainly.com/question/28945834
#SPJ11
Which eukaryotic cell does the organelle shown belong to?
CM
Answers
Answer:
The Eukaryotic cell that the organelle shows below belongs to the Nucleus.
Explanation:
In fact, the mere presence of a nucleus is considered one of the defining features of a Eukaryota cell.
why do we not know what was on the far side of the moon until 1960's
Answers
Answer:
Like Earth, it gets plenty of sunlight. We don’t see the far side because “the moon is tidally locked to the Earth,” said John Keller, deputy project scientist for NASA’s Lunar Reconnaissance Orbiter project. “The moon does rotate, but it rotates at the same speed that it rotates around the Earth.”
Explanation:
An aqueous solution of non-electrolyte A, with a molecular
mass of 60, contains 6 grams of non-electrolyte A in 500 mL
and has a density equal to 1.05 g per mL. The molality of the
solution is
Answers
Answer:0.19m
Explanation:
Molality (m) of the solution is = 0.192 mol/kg. Molality (also molal concentration) is represented by symbol m, it measures the number of moles of solute (here a non-electrolyte solute A) present in 1 kg of solvent. Here, moles of solute can be calculated by dividing given mass of non-electrolyte A with molecular weight of A.
Molecular mass of non-electrolyte A = 60g
Mass of non-electrolyte A = 6g
Volume of solution = 500mL
Density of solution = 1.05g
Calculating molality of solutionTo calculate molality of solution using formula of molality:
Molality = moles of solute / kilograms of solvent (mol/kg). Here we need to calculate mass of the solvent in kg that is not directly given in the question. Hence, it is required to calculate mass of the solution first and then subtract the mass of solute from it. Also, to calculate the mass of solution, density and volume are given. So, the calculations are done as follows;
Firstly,
To calculate mass of solution using formula of density; D (ρ) = mass/volume. Density (ρ) is the mass of substance divide by it's volume. Therefore, Mass of solution = Density × volume
Mass of solution = 1.05 × 500
Mass of solution = 525 g
Now, Mass of solvent is calculated as Mass of solute (non-electrolyte A) subtracted from mass of solution. Therefore,
Mass of solvent = 525 - 6 = 519 g (0.519 kg)
Moles of solute are calculated as given weight of solute divided by molecular weight of solute. Hence, moles of solute = 6/60
Moles of solute = 0.1
Molality of solution is calculated as moles of solute divided by mass of solvent or kilograms of solvent (mol/kg)
Molality of solution = 0.1/ 0.519
Molality (m) = 0.192 mol/kg
Learn more about molality here
https://brainly.com/question/20018472
#SPJ2
to act as an effective coolant in a car's radiator, a substance has to have the capacity to absorb a great deal of heat. which physical property is the best indicator for a good coolant?
Answers
To act as an effective coolant in a car's radiator, a substance has to have the capacity to absorb a great deal of heat. The physical property is the best indicator for a good coolant is specific heat.
Specific heat capacity of the substance is the amount of heat energy required raise the temperature of the substance. A coolant should have the high specific heat capacity. The large amount of heat is required for a coolant to raise their temperature and if the coolant have high heat capacity it can absorb large amount of heat without heating much. The specific heat is the physical property of a substance.
Thus, To act as an effective coolant in a car's radiator, a substance has to have the capacity to absorb a great deal of heat. The physical property is the best indicator for a good coolant is specific heat.
To learn more about specific heat here
https://brainly.com/question/11297584
#SPJ4
17. Interpret each chemical formula Mn₂(CO3)3. Determine how many atoms of each
element make up the compound.
Answers
The chemical formula Mn₂(CO₃)₃ represents a compound composed of manganese (Mn) and carbonate (CO₃) ions and contains 2 manganese atoms, 9 carbon atoms, and 9 oxygen atoms.
Understanding the Component of a Chemical FormulaTo determine the number of atoms of each element in the compound, we need to break down the formula and analyze the subscripts.
Breaking down the formula
- Mn₂ indicates that there are two manganese atoms in the compound.
- (CO₃)₃ indicates that there are three carbonate ions in the compound. Each carbonate ion consists of one carbon atom (C) and three oxygen atoms (O).
Analyzing the carbonate ion
Since there are three carbonate ions in the compound, we need to multiply the number of atoms in each ion by three:
- There are three carbon atoms (C) in each carbonate ion, so in total, there are 3 x 3 = 9 carbon atoms.
- There are three oxygen atoms (O) in each carbonate ion, so in total, there are 3 x 3 = 9 oxygen atoms.
Summing up the atoms
- Manganese (Mn): 2 atoms
- Carbon (C): 9 atoms
- Oxygen (O): 9 atoms
Therefore, the compound Mn₂(CO₃)₃ contains 2 manganese atoms, 9 carbon atoms, and 9 oxygen atoms.
Learn more about chemical formula here:
https://brainly.com/question/11574373
#SPJ1