Consider the reaction: 2HBr(g)H2(g) + Br2(l) Using standard absolute entropies at 298K, calculate the entropy change for the system when 1.96 moles of HBr(g) react at standard conditions. S°system = J/K Submit Answer
Answers
Given the reaction is:2HBr(g)H2(g) + Br2(l)We need to calculate the entropy change for the system when 1.96 moles of HBr(g) react at standard conditions, using standard absolute entropies at 298K.
So, the solution is as follows:Let's write the chemical equation as follows:H2(g) + Br2(l)→ 2HBr(g)The given absolute entropies are:S°(H2) = 130.7 J/K molS°(Br2) = 152.2 J/K molS°(HBr) = 198.8 J/K molHence the entropy change of the system can be calculated by using the following formulaΔS°= ∑nS°(products) - ∑mS°(reactants) ΔS°= [2 × S°(HBr) - (S°(H2) + S°(Br2))]ΔS°= [2 × 198.8 J/K mol - (130.7 J/K mol + 152.2 J/K mol)] = + 174.7 J/K molSince ΔS° is positive,
this means that there is an increase in entropy. The reaction becomes more disordered as HBr is produced. Thus, this is the long answer to the problem statement.
To know more about moles visit:
https://brainly.com/question/15209553
#SPJ11
Related Questions
what’s the answer ?????

Answers
option A is the correct one
It represents Mp orBp of a substance at a specific pressure
2H2O(g) -- 2H2(g) + O2(g)
What total volume of gas (at STP) is produced by the electrolysis of 4 moles of H2O?
Answers
Explanation:
\(v = vdm \times n\)
Vdm=22.4dm.
mole(n)=4 mol
therefore the total volume
\(v = 22.4 \times 4 \\ v = 89.6dm\)
How do micro-organisms change large
organic molecules to forms that can be
absorbed and used inside the cell
Answers
Micro-organisms change large organic molecules to forms that can be absorbed and used inside the cell through the process of decomposition.
What is Decomposition?Different organic compounds decompose when plant residues are added back to the earth. Decomposition is a biological process in which complicated organic molecules found in dead matter are physically broken down and biochemically converted into less complex organic and inorganic molecules.
The biological activity and the process of cycling carbon in the soil are both aided by the ongoing increase of decomposing plant residues to the soil's surface. These processes are also aided by the decomposition of soil organic matter and the development and decay of roots. Carbon cycling is the ongoing transformation of organic and inorganic carbon compounds in the earth, plants, and the atmosphere by micro- and macroorganisms.
How does decomposition take place?The organic molecules in organic matter are broken down during decomposition into smaller organic molecules that need additional breakdown or into mineralized minerals. Different organic substances can be broken down by microorganisms in different ways. Amino acids and sugars are among the first organic substances to break down because they are simple to do so. Lignin, phenols, and waxes will last the longest in the earth, while cellulose will degrade more slowly.
In this question, the process that occurs when micro-organisms change large organic molecules to forms that can be absorbed and used inside the cell is decomposition.
To know more about Decomposition, visit,
https://brainly.com/question/8009068
#SPJ1
A sample of black mineral hematite, an oxide of iron found in many iron ores, contains 34.97g of iron and 15.03g of oxygen. what is the empirical formula of hematite?
Answers
The empirical formula : Fe₂O₃
Further explanationGiven
34.97g of Iron
15.03g of Oxygen
Required
The empirical formula
Solution
Mol ratio :
Fe(Iron) : 34.97 g : 56 g/mol = 0.624
O(Oxygen) : 15.03 g : 16 g/mol = 0.939
Divide by 0.624 :
Fe : O = 1 : 1.5
Fe : O = 2 : 3
Calculate the decrease in temperature when 6.00 L at 25.0 °C is
compressed to 2.50 L.
Answers
Answer:
-149.0K
Explanation:
Charles's law states that the volume of a gas is directly proportional to the absolute temperature under constant pressure.
The equation is:
V1/T1 = V2/T2
Where V1 is initial volume = 6.00L
T1 is absolute initial temperature = 25.0°C + 273 = 298K
V2 is final volume = 2.50L
T2 is our incognite
Replacing:
6.00L/298K = 2.50L/T2
T2 = 2.50L * 298K / 6.00L
T2 = 124.17K
The temperature is 124.17K - 273.15K =
-149.0K
An endothermic dissolution process a. absorbs energy as heat and has positive enthalpy of solution. b. releases energy as heat and has positive enthalpy of solution. c. absorbs energy as heat and has negative enthalpy of solution. d. releases energy as heat and has negative enthalpy of solution.An endothermic dissolution process a. absorbs energy as heat and has positive enthalpy of solution. b. releases energy as heat and has positive enthalpy of solution. c. absorbs energy as heat and has negative enthalpy of solution. d. releases energy as heat and has negative enthalpy of solution.
Answers
Answer:
a. absorbs energy as heat and has positive enthalpy of solution.
Explanation:
A reaction can either be exothermic or endothermic. An endothermic reaction, as the dissolution described in the question, is that which absorbs heat energy from the surroundings in order to start the reaction.
Because an endothermic reaction makes heat lost from the surroundings, the enthalpy (∆H) of the solution will be positive (+). ∆H is got by finding the difference between the enthalpy of the reactants and products and since the enthalpy of a product in endothermic reaction is more, the enthalpy change (∆T) will be positive.
What does radioactive mean
Answers
Answer:
emitting or relating to the emission of ionizing radiation or particles
Explanation:
Radiation can damage the DNA in our cells.
In other words!
the giving off of rays of energy or particles by the breaking apart of atoms of certain elements (as uranium)
Complete this Lewis structure for S2O32− by adding lone pairs and formal charges.Add lone-pair electrons and formal charges to this structure.
Answers
Oxygen is more electronegative compared to Sulfur. So, negative charge can be accomodate by oxygen rather than sulfur.
What is electron example?The smallest element component of an atom, the electron has a negative charge. In a neutral atom, there are an equal amount of protons and electrons. One atom and one particle are all that the hydrogen atom has. But in the other hand, the uranium atom possesses 92 protons, which means 92 electrons.
What is electrons and protons?The electron, quark, and nucleo are the three subatomic particles that make up an atom. The core nucleus of the atom, which contains neutrons and protons, is what makes up an atom. Its electrons that surround the nucleus. Protons have a positive charge, revolutionaries have a negative surface charge, and neutrons are neutral.
To know more about Electrons visit:
https://brainly.com/question/25674345
#SPJ4

Which quantity of particles is correctly represented 1 mol of H2?
Answers
(?)Fe+(?)H20 - - -> (?)Fe3O4+(?)H2
Answers
Answer:
what is this question??
which process moves ions from an area of high concentration?
Answers
The process that moves ions from an area of high concentration to an area of lower concentration is called diffusion.
Diffusion is the movement of particles or substances from an area of high concentration to an area of lower concentration until the concentration of the substance is uniform throughout the space.
Key points:
Diffusion occurs naturally as a result of the random thermal motion of particles.The rate of diffusion is proportional to the concentration gradient (difference in concentration between two areas) and the temperature.Diffusion occurs in liquids, gases, and through membranes.Diffusion plays a role in many biological processes, such as the movement of oxygen and carbon dioxide in the respiratory system, and the absorption of nutrients in the digestive system.Diffusion can also be used in various industrial and technological processes, such as the separation of components of a mixture, and the purification of substances.Learn more about Diffusion here:
https://brainly.com/question/8880115
#SPJ4
Will has been told to check the oxygen level in a confined space, before workers are allowed to enter. Will should verify that the space has at least ____ % oxygen by volume.
Answers
If you need a full explanation, let me know :]
Suppose a 22.092 g sample of a 1:1 mixture of acetylferrocene and ferrocene was separated by column chromatography, and the recovered fractions weighed 9.017 g (acetylferrocene) and 8.075 g (ferrocene), what was the percent recovery of acetylferrocene?
Answers
Answer:
81.6%
Explanation:
mass of acetylferrocene and ferrocene mixture = 22.092 g
mass ratio acetylferrocene and ferrocene mixture = 1 : 1
The sum of the ratios = 2, therefore the mass of each compound will be half the mass of the mixture
mass of each compound in the sample mixture = 1/2 * 22.09 2= 11.046 g
mass of recovered acetylferrocene = 9.017 g
percentage recovery = mass recovered/mass in sample * 100%
percentage recovery of acetylferrocene = (9.017 g / 11.046 g) * 100%
percentage recovery of acetylferrocene = 81.6%
How much potassium nitrate could be dissolved into 2 L of water
Answers
Answer:
640 grams
Explanation:
look up Solubility table in wikipedia for potassium nitrate (KNO3)
32 grams of potassium nitrate (KNO3) water solubility at 20 degrees celsius (room temperature) can be dissolved in 100 milliliters (0.1 L) of water.
2 liters = 2000 milliliters
32 grams / 100 milliliters = x grams / 2000 milliliters
cross-multiply
100 * x = 32 * 2000
x = (32 * 2000) / 100
x ≈ 640 grams
chatgpt
Given 1 inch equals 2.54 cm how many centimeters are in an average hand that is 9. 50 inches
Answers
Answer:
It's not that hard to do figure it out yourself
Explanation:
too simple
1. True or false, phosphorus-33 is an isotope
Answers
Explanation:
it's answer is false
hope it helps you
how many grams of carbon are required to produce 75 L of CH4 (g) at STP
Answers
Answer: 62.4g according to a quizlet
Explanation:
convert the following to the unit shown and show your dimensional analysis
135 mm Hg = _________ atm
Answers
The value of the given pressure in atm is 0.18 atm.
What is a conversion factor?A conversion factor, which is a ratio, is a statement of the relationship between two different units of measurement or physical quantities. It is used to convert a quantity from one unit to another while keeping the quantity's numerical value.
We have to note that;
1 atm = 760 mmHg
x atm = 135 mm Hg
Hence;
x = 1 atm* 135mmHg/760 mmHg
x = 0.18 atm
Conversion factors are widely used in various fields, including physics, chemistry, engineering, and finance.
Learn more about conversion factor:https://brainly.com/question/23510660
#SPJ1
heating up a reaction increases the speed of a reaction until the ____
Answers
Heating up a reaction increases the speed of a reaction until the enzyme denatures.
What is enzyme denaturation?Enzyme denaturation occurs when a biological protein catalyst does not work anymore due to a high temperature that alters its tridimensional conformation.
This cellular process (denaturation) is well known to be one of the main causes of enzymatic failure.
In conclusion, heating up a reaction increases the speed of a reaction until the enzyme denatures.
Learn more about enzymes here:
https://brainly.com/question/1596855
#SPJ12
Definition: The possible state for an electron of an atom that is proportional to its distance to the nucleus.
Answers
Answer:
"Energy Level" is the correct approach.
Explanation:
An electric charge electron density during an atomic seems to be the potential condition for this with the electron and therefore is proportional to its density from the nucleus. The ground state seems to be a particle, nucleus, and perhaps molecule with the corresponding energy level as well as its electrodes. The electron travels to something like a higher or excited energy state whenever there's an improvement in electricity, and has also been stimulated.65 million years ago there was a mass extinction that is best known for killing the dinosaurs. There was also a large
deposit of dust that contained substances only found in asteroids, What most likely killed off the dinosaurs?
Answers
Answer:
65 million years ago there was a mass extinction that is best known for killing the dinosaurs. There was also a large
deposit of dust that contained substances only found in asteroids, What most likely killed off the dinosaurs?
Explanation:
Answer:
b
Explanation:
Heat energy is necessary for evaporation to occur. Energy is used to break the bonds that hold water molecules together, which is why water easily evaporates at the boiling point. In fact the process of the evaporation removes heat from the environment . Which of these every day examples illustrates how evaporation removes heat from the environment ?
Answers
Answer:
As a result of the network of hydrogen bonding present between water molecules, a high input of energy is required to transform one gram of liquid water into water vapor, an energy requirement called the heat of vaporization. Water has a heat of vaporization value of 40.65 kJ/
Explanation:
Perform Calculatlons Using Ksp Question For the following equilibrium; HgBr2(s) ~= Hg?+(aq) + 2Br"(aq) If Ksp 6.2 x 10-20, what is the molar solubility of mercury (II) bromide (HgBrz)? Report your answer in scientific notation with the correct number of significant figures Provide your answer below:'
Answers
The molar solubility of mercury (II) bromide (HgBr2) is 7.00 × 10^-7 M. This is the long answer to the problem.
To perform calculations using Ksp for the given equilibrium HgBr2(s) ↔ Hg2+(aq) + 2Br-(aq) and find the molar solubility of mercury (II) bromide (HgBr2) we have to use the solubility product expression.Ksp for HgBr2 is 6.2 × 10^-20Ksp = [Hg2+][Br-]^2 Since the initial concentration of HgBr2 is given as s, and after dissociation, the concentration of Hg2+ becomes s, while the concentration of Br- becomes 2s.[Hg2+] = s M and [Br-] = 2s MThus,Ksp = [Hg2+][Br-]^2= s(2s)^2= 4s^3= 6.2 × 10^-20Molar solubility of HgBr2 is given as s, therefore;s = (6.2 × 10^-20/4)1/3s = 7.00 × 10^-7 M
To know more about molar solubility visit:-
https://brainly.com/question/31043999
#SPJ11
PLEASE HELP ILL EVEN MARK AS BRAINLIEST
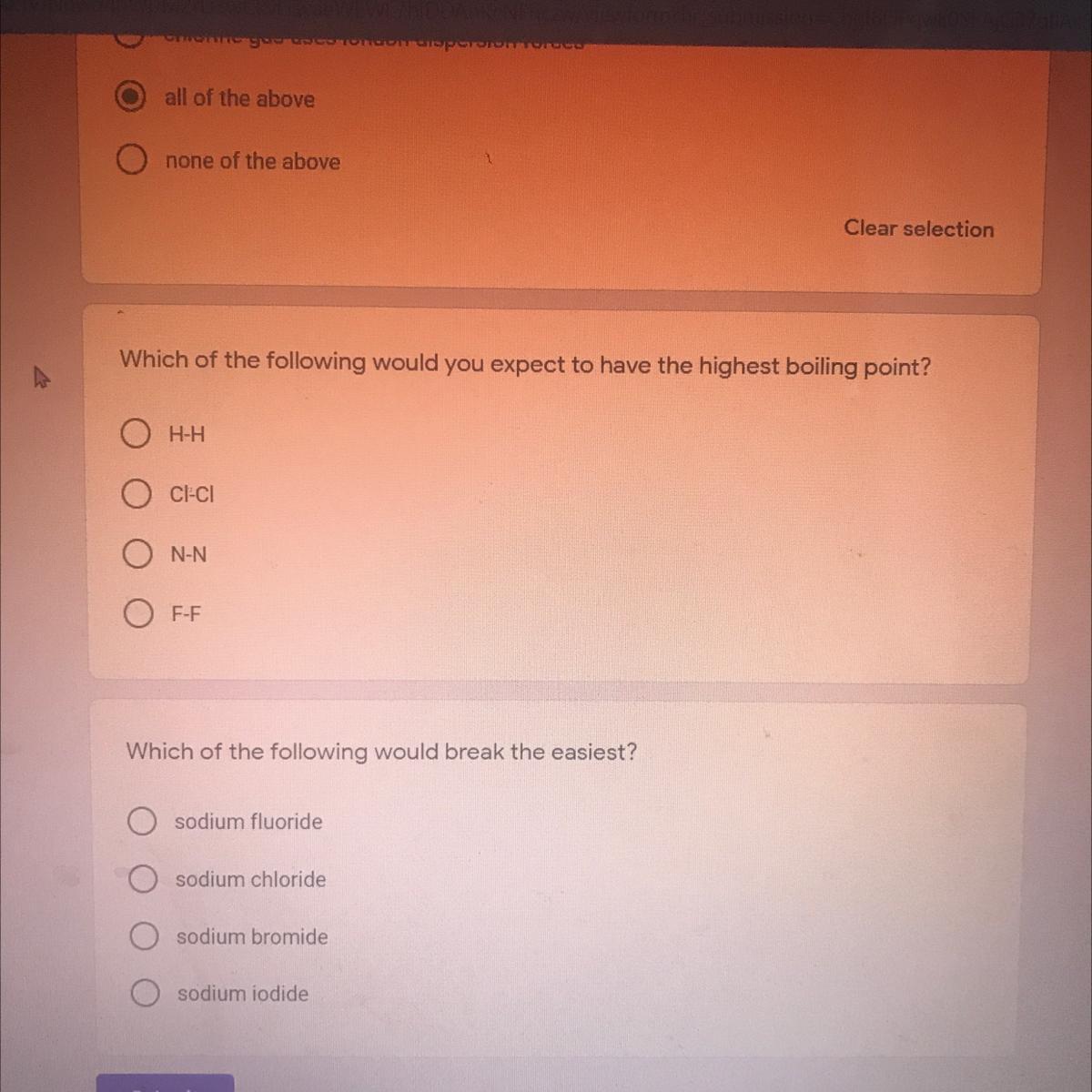
Answers
Answer:
Cl: -29.272*
Explanation:
The other temperatures go from lowest to highest, H: -423* F, N: -320* F, F: -306.6* F
when 5.00 × 1022 molecules of ammonia react with 4.00 × 1022 molecules of oxygen according to the chemical equation shown below, how many grams of nitrogen gas are produced?
Answers
When 5.00 ×\(10^{22\) molecules of ammonia react with 4.00 ×\(10^{22\)molecules of oxygen, approximately 2.32 grams of nitrogen gas are produced.
To determine the number of grams of nitrogen gas produced when 5.00 × \(10^{22\) molecules of ammonia react with 4.00 × \(10^{22\) molecules of oxygen, we need to use the given balanced chemical equation and perform a stoichiometric calculation.
The balanced chemical equation for the reaction is:
\(4 NH_3 + 5 O_2\) → \(4 NO + 6 H_2O\)
From the balanced equation, we can see that 4 moles of \(NH_3\) react to produce 4 moles of nitrogen gas.
First, let's calculate the number of moles of \(NH_3\):
5.00 × \(10^{22\) molecules of \(NH_3\) * (1 mole \(NH_3\) / 6.022 × 10^23 molecules \(NH_3\)) = 0.083 moles \(NH_3\)
Since the stoichiometric ratio between \(NH_3\) and \(N_2\) is 4:4, we can conclude that 0.083 moles of \(NH_3\) will produce 0.083 moles of \(N_2\).
Finally, to calculate the mass of nitrogen gas, we need to use the molar mass of nitrogen gas , which is approximately 28.0134 g/mol.
Mass of \(N_2\) = Number of moles of \(N_2\) * Molar mass of \(N_2\)
Mass of \(N_2\) = 0.083 moles * 28.0134 g/mol = 2.32 grams
Therefore, when 5.00 × \(10^{22\) molecules of ammonia react with 4.00 × \(10^{22\) molecules of oxygen, approximately 2.32 grams of nitrogen gas are produced.
Learn more about stoichiometric ratio here:
https://brainly.com/question/6907332
#SPJ11
PLS HELP
When studying mythology, Jaalib reads a description that says a particular figure is "vernal." What can he guess about
the figure?
O
they are related to spring
O
they are related to autumn
O
they are related to the tides
they are related to the moon
Answers
Answer: They are related to spring.
Explanation:
It should be right, I got this question correct.
PLEASE HELP ASAP WILL GIVE BRAINLIEST
explain in terms of subatomic particles why the X+ ion is smaller than the X atom.
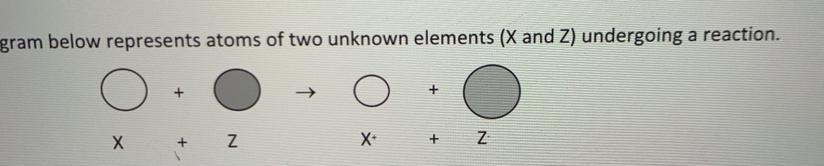
Answers
Answer:
X+ ion cation is smaller than neutral atom because when one or more electrons are lose by an atom it usually result in lose of shell.
And when electron is removed positive/ negative charged ratio increas which causes more effective nuclear charge.
The more effective nuclear charge the less its size.
Explanation:
how many milligrams of atropine sulfate are needed to make 30 ml of a 1:200 solution
Answers
15mg of atropine sulfate is needed to make 30ml of a 1:200 solution
To find the mass of atropine sulfate, first, the ratio should be converted into a percentage. The given ratio is 1:200
Percentage mass = (1/200) x 100
= 1/2%
Percentage mass = 0.5%
Let us consider the mass of atropine sulfate required to be x
Therefore,
0.5/100 ml = x / 30ml
x = (0.5/100)30
x = 0.15g
On converting 0.15g to milligrams we get,
x = 0.15 x 1000
x = 150mg
Therefore, the required mass of atropine sulfate to make 30 ml of a 1:200 solution is 150mg.
To learn more about how to find the concentration of mass click below:
https://brainly.com/question/23437000
#SPJ4
How many grams of co2 ( carbon dioxide gas ) will exert a pressure of 769 mmhg at a volume of 52.3l at 425k?
66.7 g
1.51 g
26.2 g
1153 g
Answers
volume, n is the number of moles, R is the ideal gas constant, and T is the temperature. 26.2 g.
To determine the number of grams of CO2, we can use the ideal gas law equation, which states PV = nRT, where P is the pressure, V is the volume, n is the number of moles, R is the ideal gas constant, and T is the temperature.
Rearranging the equation, we get n = PV / RT.
Given:
P = 769 mmHg = 769 torr
V = 52.3 L
T = 425 K
Converting pressure to atm: 769 torr / 760 torr/atm = 1.012 atm.
Using the value of R = 0.0821 L·atm/(K·mol), we can calculate the number of moles:
n = (1.012 atm) * (52.3 L) / ((0.0821 L·atm/(K·mol)) * (425 K))
≈ 26.2 mol
Finally, since the molar mass of CO2 is approximately 44 g/mol, we can calculate the mass:
Mass = n * molar mass
≈ 26.2 mol * 44 g/mol
≈ 1153 g.
Therefore, the correct answer is 1153 g.
learn more about volume here:
https://brainly.com/question/13338592
#SPJ11
When an electron in an unknown atom transitions from the n=3 to n=1 energy state, which statement correctly describes how the kinetic energy (KE), and potential energy (PE) of the excited electron changes in response to this transition to the n=1 energy state? KE decreases and PE increases KE decreases and PE decreases KE increases and PE increases KE increases and PE decreases
Answers
When an electron in an unknown atom transitions from the n=3 to n=1 energy state, the correct statement describing the change in kinetic energy (KE) and potential energy (PE) of the excited electron is:
KE increases and PE decreases.
As the electron transitions from a higher energy level (n=3) to a lower energy level (n=1), it moves closer to the nucleus. In the process, the electron loses potential energy because it is now in a more stable, lower energy state. Since potential energy decreases, kinetic energy must increase to conserve the total energy of the electron. Therefore, KE increases and PE decreases during this transition.
Learn more about the electron transition: https://brainly.com/question/29221248
#SPJ11