consider the carbonate anion. what is the central atom? enter its chemical symbol. how many lone pairs are around the central atom? what is the ideal angle between the carbon-oxygen bonds? compared to the ideal angle, you would expect the actual angle between the carbon-oxygen bonds to be ...
Answers
The central atom in the carbonate anion is carbon (C).
There are no lone pairs around the central carbon atom.
The ideal angle between the carbon-oxygen bonds is approximately 120 degrees.
The actual angle between the carbon-oxygen bonds is expected to be close to the ideal angle, but it may deviate slightly depending on specific factors and interactions.
The carbonate anion, denoted as CO3^2-, consists of three oxygen atoms (O) covalently bonded to a central carbon atom (C).
The central atom in the carbonate anion is carbon (C).
Regarding the lone pairs around the central carbon atom, there are no lone pairs present. All three oxygen atoms in the carbonate anion contribute a double bond (two shared electrons) with the central carbon atom, resulting in a total of six shared electrons.
The ideal angle between the carbon-oxygen bonds in a carbonate ion, as predicted by the VSEPR (Valence Shell Electron Pair Repulsion) theory, is approximately 120 degrees. This angle arises from the repulsion between the three electron-rich oxygen atoms bonded to the central carbon atom. The VSEPR theory suggests that electron pairs, whether they are bonding or non-bonding, will arrange themselves in a way that minimizes their repulsion.
However, due to the presence of a double bond between carbon and each oxygen atom, the actual angle between the carbon-oxygen bonds in the carbonate ion deviates slightly from the ideal angle. The actual angle is approximately 120 degrees but can vary due to factors such as electron delocalization or the presence of other substituents.
Learn more about bond angle at: https://brainly.com/question/25425872
#SPJ11
Related Questions
the ksp of baf2 is 1.7 x 10-6 mol/l in water at 25oc. what is the concentration of fluoride ions in equilibrium with solid barium fluoride? (assume that the only relevant reaction is the solubility-product equilibrium.)
Answers
The Ksp of BaF2 is given as 1.7 x 10^-6 mol/L in water at 25oC. To find the concentration of fluoride ions in equilibrium with solid barium fluoride, we can use the solubility-product equilibrium equation:
Ksp = [Ba2+][F-]^2
Since the only relevant reaction is the solubility-product equilibrium, we can assume that the concentration of Ba2+ and F- are equal. Therefore, we can rewrite the equation as:
Ksp = [F-]^3
Next, we can solve for the concentration of F-:
[F-] = (Ksp)^(1/3)
[F-] = (1.7 x 10^-6)^(1/3)
[F-] = 1.19 x 10^-2 mol/L
Therefore, the concentration of fluoride ions in equilibrium with solid barium fluoride is 1.19 x 10^-2 mol/L.
To know more about equilibrium equation click on below link :
https://brainly.com/question/15118952#
#SPJ11
The diagram shows the layers of Earth. Convection currents in which region influence the movement of tectonic plates?
Illustration depicts vertical section of four layers of the earth. Left to right, Inner Core labeled 1, Outer Core labeled 2, Mantle labeled 3, Lithosphere labeled 4.
A.
1
B.
2
C.
3
D.
4
Answers
Convection currents in the mantle, symbolized by region 3 in the diagram, play a significant role in tectonic plate movement.
What are tectonic plates?The Earth's lithosphere, or outermost layer, which comprises the crust and highest section of the mantle, is made up of massive, solid chunks called tectonic plates. These plates are floating on top of the underlying, more fluid asthenosphere and fit together like jigsaw pieces. The African Plate, Eurasian Plate, Pacific Plate, and North American Plate are only a few of the larger tectonic plates. There are also numerous smaller ones.
Because of the heat produced by the core, the mantle, which is the deepest layer of the Earth, is made up of hot, molten rock that is continually in motion. Convection currents are produced by this movement, which transport heat from the core to the surface and back down again. These currents cause the hard lithospheric plates to move and shift over time as they are being dragged along by the currents.
A number of geological events, such as the generation of new crust at mid-ocean ridges, the movement of continents, and the development of volcanic hotspots, are caused by the convection currents in the mantle. For the purpose of foreseeing and minimizing natural disasters, such as earthquakes and volcanic eruptions, it is crucial to comprehend the dynamics of these currents.
To know more about tectonic plates, visit:
brainly.com/question/19317822
#SPJ9

Answer:
C. 3
Explanation:
plato
What does kinetic energy measure?
A particle shape
B, particle volume
C. particle motion
D. particle color
Answers
Answer:
c
Explanation:
kinetic energy is the energy of motion. if it helps potential energy is stored energy. like charging up a battery before it gets used in something.
The number of electrons in ATOM 1 is ______

Answers
What happens when a bike rusts?
Select all that apply.
Responses
A new substance is formed.A new substance is formed. , ,
A physical change happens.A physical change happens. , ,
The atoms in the reactants rearrange to form the product.The atoms in the reactants rearrange to form the product. , ,
The atoms in the reactants stay the same in the product.The atoms in the reactants stay the same in the product. , ,
The atoms on the surface of the bike react with atoms in the air.

Answers
2.) the atoms in the reactants rearrange to form the product.
3.) the atoms on the surface of the bike react with atoms in the air.
The Adálie penguins feed on krill that live on the underside of ice sheets. Which of the following would help Adálie penguins survive during the melting of Antarctic sea ice? (4 points)
Migrating farther into the sea
Breeding during summer months
Growing strong toe nails to grip ice
Spending more time raising their young
Answers
Answer:
Migrating farther into the sea
Explanation:
Answer:
Migrating farther into the sea
Explanation:
I aced the test
0.1 mL of an original sample is diluted into 9.9 mL of water, and then 0.1 mL of this is spread on a plate. 54 colonies grew. What was the original cell density of the sample
Answers
The original cell density of the sample was 54,000 cells/mL. This means that for every 1 mL of the original sample, there were 54,000 cells. It is important to note that this calculation assumes that each colony arose from a single cell and that the cells were evenly distributed throughout the original sample. If there were clumps or aggregates of cells, this calculation may not be accurate.
To calculate the original cell density of the sample, we need to use the following formula:
Original cell density = (number of colonies / volume plated) * (1 / dilution factor)
Here, the volume plated is 0.1 mL, and the dilution factor is 1:100 (since we diluted 0.1 mL of the original sample into 9.9 mL of water). Therefore, the dilution factor is 1/100 = 0.01.
Substituting these values into the formula, we get:
Original cell density = (54 colonies / 0.1 mL) * (1 / 0.01)
Simplifying this, we get:
Original cell density = 54,000 cells/mL
For more such questions on aggregates
https://brainly.com/question/16380585
#SPJ11
for 280.0 ml of pure water, calculate the initial ph and the final ph after adding 0.028 mol of naoh .
Answers
The initial pH of pure water is 7.0, and after adding 0.028 mol of NaOH to 280.0 ml of water, the final pH is approximately 13.0 due to an increase in hydroxide ion concentration.
The initial pH of pure water is 7.0, as it is considered neutral. After adding 0.028 mol of NaOH to 280.0 ml of pure water, the final pH can be calculated.
Pure water has a neutral pH of 7.0, which means it has an equal concentration of hydrogen ions (H+) and hydroxide ions (OH-). When NaOH is added to water, it dissociates into Na+ and OH- ions. The OH- ions react with the H+ ions in the water, resulting in an increase in the concentration of hydroxide ions and a decrease in the concentration of hydrogen ions.
To calculate the final pH, we need to determine the concentration of OH- ions after the addition of NaOH. Since 0.028 mol of NaOH is added to 280.0 ml of water, the concentration of OH- ions can be calculated using the molarity formula:
Molarity = Moles of solute / Volume of solution (in liters)
Converting the volume of water to liters (280.0 ml = 0.280 L), we can calculate the molarity of the OH- ions:
Molarity of OH- = (0.028 mol) / (0.280 L) = 0.10 M
The concentration of OH- ions corresponds to the pOH value, which is the negative logarithm (base 10) of the hydroxide ion concentration:
pOH = -log [OH-] = -log (0.10) ≈ 1.0
Since pH + pOH = 14 (for neutral solutions), the final pH can be calculated:
pH = 14 - pOH = 14 - 1.0 = 13.0
Therefore, the final pH after adding 0.028 mol of NaOH to 280.0 ml of pure water is approximately 13.0.
To learn more about Molarity click here: brainly.com/question/2817451
#SPJ11
. How will this affect the amount of water and the amount of oxygen in the area?
Answers
Diffusion and aeration, photosynthesis, respiration, and decomposition all have an ongoing impact. In addition to fluctuating dissolved oxygen levels caused by temperature, salinity, and pressure fluctuations.
What impact does water have on oxygen?In comparison to warm water, cold water can hold more dissolved oxygen. The concentration of dissolved oxygen is highest in the winter and early spring when the water temperature is low. The concentration of dissolved oxygen is frequently lower in the summer and fall when the water temperature is high.
Why does water temperature impact how much oxygen is there in it?Water molecules receive energy as a result of rising water temperatures, which in turn causes gas and water molecules to gain more energy. This increased energy dissolves the water and oxygen molecules' weak molecular bonds, allowing oxygen to escape.
To know more about decomposition visit-
https://brainly.com/question/8009068
#SPJ1
Is oil a reasonable energy source? Why or why not?
Answers
Answer:
No, it is not.
Explanation:
Oil is a non-renewable source of energy. ... Burning oil can pollute the air. Much of our oil has to be imported and it is becoming more and more expensive as reserves reduce and imports increase. Producing electricity from crude oil is expensive compared to other fossil fuels such as coal or gas.
*You Can put this in your own words
which graph above best represents the relationship between the volume and the pressure of a gas?
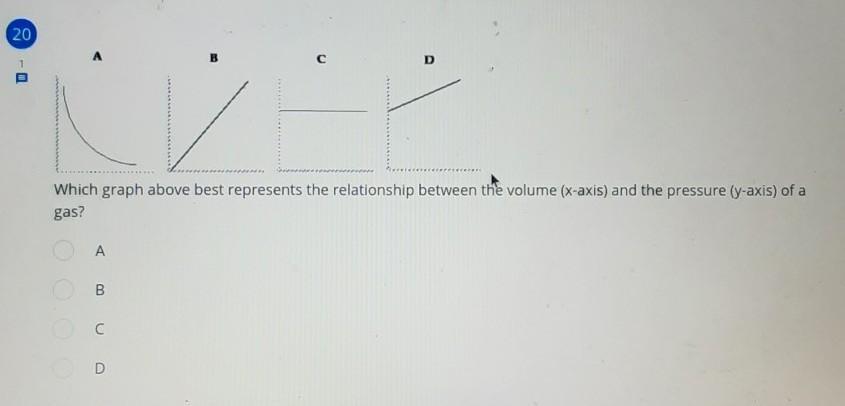
Answers
The graph (A) best represents the relationship between the volume and the pressure of a gas.
What is pressure?The force was applied perpendicular to an object's surface per unit area across which that force is spread is known as pressure. The relationship between pressure and volume can be understand with the aid of ideal gas law.
PV=nRT.
where, P is pressure, V is volume.
It can be seen that when temperature will kept constant then pressure will be inversely proportional to the volume. It can be shown as:
P ∝ 1/V.
Therefore, graph A represent relationship between pressure (P) and volume (V).
To know more about pressure.
https://brainly.com/question/14760196.
#SPJ2
12. Ammonium carbonate and aluminum acetate
Molecular Equation:3 (NH4)₂CO3(aq) + 2 Al(C₂H³O₂)³(aq)
Complete lonic Equation:
Net Ionic Equation:
Answers
Complete Ionic Equation: 6 NH4^+(aq) + 3 CO3^2-(aq) + 2 Al^3+(aq) + 6 C2H3O2^-(aq) → 6 NH4^+(aq) + 2 Al(C2H3O2)3(aq) + 3 C2O3^2-(aq)
Net Ionic Equation: 3 CO3^2-(aq) + 2 Al^3+(aq) → 2 Al(C2H3O2)3(aq) + 3 C2O3^2-(aq)
Note: The complete ionic equation breaks down all of the soluble ionic compounds into their respective ions to show the spectator ions that do not participate in the reaction. The net ionic equation only includes the reactants that participate in the reaction and excludes the spectator ions.
How do the properties of a compound compare to the properties of the elements that make it up?
Answers
Answer:
A compound contains atoms of different elements chemically combined together in a fixed ratio. An element is a pure chemical substance made of same type of atom. Compounds contain different elements in a fixed ratio arranged in a defined manner through chemical bonds
When collecting temperature as a function of time for the reaction of KOH with HCL, which time is most significant
Answers
Answer:
At the completion of reaction.
Explanation:
The time when the reaction take places is the most significant time for measuring temperature of the solution because on this time the temperature will decrease or increase. Some reactions releases heat energy upon completion we called them exothermic reactions whereas some absorb heat energy from the surrounding, decreases the temperature which is known as endothermic reaction so measuring temperature at the completion of reaction is the correct time.
If you have 83.2g of Fe and 110.0g of H,0 then which one is the limiting reactant and which one is in excess.
Answers
Answer:
Explanation:
For every two moles of H2O, one mole of H2 is produced. 3) Na runs out first. It is the limiting reagent. Water is the excess reagent.
In an experiment, the gram atomic mass of magnesium was determined to be 14.7. Compared to the accepted value of 24.3, the percent error for this determination was
39.5
1.65
24.7
98.4
Answers
Answer:
The answer is 39.5 %Explanation:
The percentage error of a certain measurement can be found by using the formula
\(P(\%) = \frac{error}{actual \: \: number} \times 100\% \\\)
actual mass = 24.3
error = 24.3 - 14.7 = 9.6
We have
\(p(\%) = \frac{9.6}{24.3} \times 100 \\ = 39.506172839...\)
We have the final answer as
39.5 %Hope this helps you
What does the nucleus of an atom do to its own electrons? To the electrons of a nearby atom?
Answers
An atom is made up of energy levels that contain electrons which are negatively charged and the nucleus which contains neutrons and protons that are negatively charge .
Due the positive charge of the nucleus of an atom, an atom always want to attract its electrons and keep them near it however it weakly attracts the other electrons of a nearby atom.
A sphere of radius 0.457 m, temperature 32.2 ∘
C, and emissivity 0.924 is located in an environment of temperature 82.9 ∘
C. At what rate does the sphere (a) emit and (b) absorb thermal radiation? (c) What is the sphere's net rate of energy exchange? (a) Number (b) Number Units Units
Answers
a) The sphere emits thermal radiation at a rate of 139.75 Watts.
b) The sphere absorbs thermal radiation at a rate of 37.66 Watts.
c) The sphere's net rate of energy exchange is 102.09 Watts.
What are the rates of thermal radiation emission, absorption, and net energy exchange for the sphere?To calculate the rates of thermal radiation emission and absorption, we can use the Stefan-Boltzmann law, which states that the rate of thermal radiation emitted or absorbed by an object is proportional to its surface area, temperature, and the Stefan-Boltzmann constant.
a) The rate of thermal radiation emitted by the sphere can be calculated using the formula:
Emitting Rate = emissivity * surface area * Stefan-Boltzmann constant * (\(temperature^4 - environment\ temperature^4\))
Plugging in the given values:
Emitting Rate = \(0.924 * (4\pi * (0.457)^2) * 5.67 \times 10^{-8} * ((32.2 + 273.15)^4 - (82.9 + 273.15)^4)\)
Emitting Rate ≈ 139.75 Watts
b) The rate of thermal radiation absorbed by the sphere can be calculated in a similar way but using the environment temperature as the object's temperature:
Absorbing Rate = emissivity * surface area * Stefan-Boltzmann constant * (\(environment\ temperature^4 - temperature^4\))
Plugging in the given values:
Absorbing Rate = \(0.924 * (4\pi * (0.457)^2) * 5.67 \times 10^{-8} * ((82.9 + 273.15)^4 - (32.2 + 273.15)^4)\)
Absorbing Rate ≈ 37.66 Watts
c) The net rate of energy exchange is the difference between the emitting rate and the absorbing rate:
Net Rate = Emitting Rate - Absorbing Rate
Net Rate = 139.75 Watts - 37.66 Watts
Net Rate ≈ 102.09 Watts
Therefore, the sphere emits thermal radiation at a rate of 139.75 Watts, absorbs thermal radiation at a rate of 37.66 Watts, and has a net rate of energy exchange of 102.09 Watts.
Note: The units for all the rates are Watts.
Learn more about thermal radiation emission
brainly.com/question/28517392
#SPJ11
What would be another good title for this passage mood and tides, the moons of our solar system, the moon phase is in order, benefits of knowing the moon phases
Answers
Here is the picture to the reading of the question

If the work function of tin (Sn) is 281 nm, what equation could you use to determine the amount of energy required to eject a photoelectron from a sample of tin?
Answers
Answer:
See explanation
Explanation:
The work function is given by;
Wo = hc/λ
where;
h = 6.6 * 10^-34 Js
c = 3 * 10^8 ms-1
λ = 281 nm or 281 * 10^-9 m
Wo = 6.6 * 10^-34 * 3 * 10^8 / 281 * 10^-9
Wo = 7.0 * 10^-19 J or 4.375 eV
Note that the work function is the minimum energy required to eject an electron from a sample of tin.
Density of mercurio is
Answers

Answer:
The density of mercury is 13.6 g/mL, which is equal to 1360 kg/m3.
If a pure sample of an oxide of sulfur contains 40. percent sulfur and 60. percent oxygen by mass, then the empirical formula of the oxide is:1. SO32. SO43. S2O64. S2O8
Answers
The empirical formula of the oxide of sulfur is 1.) SO₃, based on the given mass percentages of sulfur and oxygen in the sample.
First, we need to assume that we have a 100-gram sample of the oxide. From the problem, we know that the sample contains 40 grams of sulfur and 60 grams of oxygen.
Next, we need to find the moles of each element in the sample. To do this, we divide the mass of each element by its molar mass. The molar mass of sulfur is 32.06 g/mol, and the molar mass of oxygen is 16.00 g/mol.
Number of moles of sulfur = 40 g / 32.06 g/mol = 1.247 mol
Number of moles of oxygen = 60 g / 16.00 g/mol = 3.750 mol
Next, we need to divide the number of moles of each element by the smallest number of moles. In this case, sulfur has the smallest number of moles, so we divide both by 1.247.
Number of moles of sulfur = 1.247 mol / 1.247 mol = 1.00 mol
Number of moles of oxygen = 3.750 mol / 1.247 mol = 3.01 mol
Now we have the mole ratio of sulfur to oxygen, which is 1.00 : 3.01. We can simplify this ratio by dividing both numbers by the smallest number (1.00).
Mole ratio of sulfur to oxygen = 1.00 : 3.01
Simplified mole ratio = 1 : 3.01
The empirical formula of the oxide of sulfur is therefore SO₃.
To know more about empirical formula, refer
https://brainly.com/question/1603500
#SPJ11
The more mass you have of a substance:
A. the greater its thermal energy
B. the slower the motion of its particles
C. the smaller its thermal energy
D. the faster the motion of its particles
Answers
how many joules of heat are absorbed when 1000g of water is heated from 18Celsius to 85celsius?
Answers
Answer + Explanations
Calculate heat absorption using the formula:
Q = mc∆T
Q means the heat absorbed, m is the mass of the substance absorbing heat, c is the specific heat capacity and ∆T is the change in temperature.
The heat absorbed is calculated by using the specific heat of water and the equation ΔH=cp×m×ΔT. 4. Water is vaporized to steam at 100oC. The heat absorbed is calculated by multiplying the moles of water by the molar heat of vaporization.
You can do this easily: just multiply the heat capacity of the substance you're heating by the mass of the substance and the change in temperature to find the heat absorbed.
To calculate the amount of heat released in a chemical reaction, use the equation Q = mc ΔT, where Q is the heat energy transferred (in joules), m is the mass of the liquid being heated (in kilograms), c is the specific heat capacity of the liquid (joule per kilogram degrees Celsius), and ΔT is the change in ...
Q = mc∆T. Q = heat energy (Joules, J) m = mass of a substance (kg) c = specific heat (units J/kg∙K) ∆ is a symbol meaning "the change in"
Precisely, water has to absorb 4,184 Joules of heat (1 calorie) for the temperature of one kilogram of water to increase 1°C. For comparison sake, it only takes 385 Joules of heat to raise 1 kilogram of copper 1°C.
A reaction that absorbs heat is endothermic. Its enthalpy will be positive, and it will cool down its surroundings. This reaction is exothermic (negative enthalpy, release of heat).
Quantitative experiments show that 4.18 Joules of heat energy are required to raise the temperature of 1g of water by 1°C. Thus, a liter (1000g) of water that increased from 24 to 25°C has absorbed 4.18 J/g°C x 1000g x 1°C or 4180 Joules of energy.
Nitric acid, HNO3(aq), can be manufacturer from ammonia using a series of three chemical reactions called the Ostwald process. The reactions involved are 4NH3(g) + 5O2(g) = 4NO(g) + 6H2O(g),
2NO(g) + O2(g) = 2NO2(g),
3NO2(g) + H2O(g) = 2HNO3(aq) + NO(g).
Determine the mass of nitric acid produced if 425 kg of ammonia reacts. Assume that plenty of oxygen is available.
Answers
Nitric acid (HNO3) is an odorless liquid that gives out yellow or red odors. Nitric acid exposure can irritate the eyes, skin, and mucous membranes.
Thus, It can also lead to dental erosion, delayed pulmonary edema, pneumonitis, and bronchitis.
The acid nitric is quite corrosive. Workers that are exposed to nitric acid risk injury. The dose, timeframe, and type of work determine the exposure level.Many industries use nitric acid.
It is employed in the production of explosives, dyes, and fertilizers. Additionally, nitric acid is utilized in the polymer sector. Many industries use nitric acid. It is employed in the production of explosives, dyes, and fertilizers.
Thus, Nitric acid (HNO3) is an odorless liquid that gives out yellow or red odors. Nitric acid exposure can irritate the eyes, skin, and mucous membranes.
Learn more about Nitric acid, refer to the link:
https://brainly.com/question/29769012
#SPJ1
Name the principal groups on the periodic table. Predict the expected charges for: 1) Na 2) Rb 3) Br 4) Fe 5) Ni
Answers
The expected charges for:Na (Sodium): +1, Rb (Rubidium): +1, Br (Bromine): -1, Fe (Iron): +2 and +3, Ni (Nickel): +2 and +3.
The major group elements or representative elements are popular names for the periodic table's key groupings. These groupings consist of:
Group 1: Alkali metals
Group 2: Alkaline earth metals
Group 13: Boron group
Group 14: Carbon group
Group 15: Nitrogen group
Group 16: Oxygen group
Group 17: Halogens
Group 18: Noble gases
The expected charges for the elements are as follows:
Na (Sodium): The alkali metals in Group 1 include sodium (Na). To obtain a stable electron configuration, alkali metals frequently lose one electron, giving them a positive charge. So, Na should anticipate to be charged at +1.
Rb (Rubidium): The alkali metals of Group 1 include rubidium (Rb). It tends to lose one electron, like other alkali metals, to create a cation with a positive charge. So, Rb should anticipate to be charged at +1.
Br (Bromine): To attain a stable electron configuration, halogens typically add one electron, giving them a -1 charge.
Fe (Iron): Depending on the particular compound or situation, iron can display several charges, most frequently +2 and +3.
Ni (Nickel): Similar to iron, nickel may display a variety of charges+2 and +3.
Learn more about charge, here:
https://brainly.com/question/14792865
#SPJ1
Why cells are called "little giants"?
Answers
Answer:
Giant cell, also called Langhans giant cell, large cell characterized by an arc of nuclei toward the outer membrane. The cell is formed by the fusion of epithelioid cells, which are derived from immune cells called macrophages. Once fused, these cells share the same cytoplasm, and their nuclei become arranged in an arc near the outer edge of the cell. Langhans giant cells typically form at the centre of granulomas (aggregates of macrophages) and are found in the tubercle, or primary focus of infection, in tuberculosis, in lesions of syphilis, leprosy, and sarcoidosis, and in fungal infections.Large cells that form by fusion in reaction to the presence in the body of foreign substances differ from Langhans giant cells in that their many nuclei are scattered throughout the cell. In giant-cell tumours of bone and tendon the cells have many nuclei crowded together. The megakaryocytes, the normal bone-marrow cells thought to be the source of the blood platelets, are also called giant cells.
Explanation:
I'M WORKING ON THIS SUBJECT TOO!!!!!
What is the wavelength (in nm) of an electron with the following kinetic energies? (a) 20.0 ev (no response) nm (b) 200 ev (no response) nm (c) 2.00 kev (no response) nm (d) 20.0 kev (no response) nm (e) 0.200 mev (no response) nm (f) 2.00 mev (no response) nm which of these energies are most suited for study of the nacl crystal structure? (select all that apply.) 20.0 ev 200 ev 2.00 kev 20.0 kev 0.200 mev 2.00 mev none of these
Answers
The wavelength of an electron can be calculated using the formula: wavelength = h / (mass of electron * velocity). Since kinetic energy is equal to the mass of the electron multiplied by the velocity squared, we can also calculate wavelength by using the formula: wavelength = h / sqrt(2mass of electron kinetic energy).
To convert the kinetic energies given in electron volts (eV) to Joules (J), you can use the formula: 1 eV = 1.6 x 10^-19 J
(a) 20.0 eV = 3.2 x 10^-18 J, wavelength = h / sqrt(2mass of electron3.2 x 10^-18 J) = 2.4 x 10^-12 m or 2.4 pm (picometers)
(b) 200 eV = 3.2 x 10^-17 J, wavelength = h / sqrt(2mass of electron3.2 x 10^-17 J) = 2.4 x 10^-11 m or 24 pm
(c) 2.00 keV = 3.2 x 10^-14 J, wavelength = h / sqrt(2mass of electron3.2 x 10^-14 J) = 2.4 x 10^-8 m or 2.4 nm
(d) 20.0 keV = 3.2 x 10^-13 J, wavelength = h / sqrt(2mass of electron3.2 x 10^-13 J) = 2.4 x 10^-7 m or 24 nm
(e) 0.200 MeV = 3.2 x 10^-11 J, wavelength = h / sqrt(2mass of electron3.2 x 10^-11 J) = 2.4 x 10^-5 m or 0.24 nm
(f) 2.00 MeV = 3.2 x 10^-10 J, wavelength = h / sqrt(2mass of electron3.2 x 10^-10 J) = 2.4 x 10^-4 m or 2.4 nm
A lower energy electron will have a longer wavelength, while a higher energy electron will have a shorter wavelength. To study the crystal structure of NaCl, you would need to use a technique such as X-ray diffraction, which typically uses X-rays with energies in the range of a few keV to a few tens of keV. Based on this, 2.00 keV and 20.0 keV energies are most suited for study of the NaCl crystal structure.
To know more about wavelength of electrons visit :
https://brainly.com/question/17295250?referrer=searchResults
#SPJ4
Calculate the number of moles of NaCl contained in 5L of a 2.2M solution.

Answers
The number of moles of NaCl contained in 5 L of a 2.2 M solution is 11 moles
How do i determine the number of moles?The molarity of a solution gives a relationship between volume and mole of substance. The mathematical relationship is given below:
Molarity = number of mole / Volume
With the above formula, we can determine the number of mole of NaCl in the solution. Details below:
Volume of solution = 5 LMolarity of solution = 2.2 MNumber of mole of NaCl =?Molarity = number of mole / Volume
Cross multiply
Number of mole = molarity × volume
Number of mole of NaCl = 2.2 × 5
Number of mole of NaCl = 11 moles
Thus, the number of moles of NaCl is 11 moles
Learn more about number of mole:
https://brainly.com/question/20522500
#SPJ1
Which mass is made up of 6.02x10^23 atoms? none of these 23 g sodium 42 g krypton 24 g carbon 78 g potassium
Answers
Answer:
23 g sodium
Explanation:
In chemistry, one mol (1mol) of a substance equals Avagadro's number or constant (6.02 × 10^23 atoms/particles/molecules).
According to this question in which the mass of different elements were given, the mass of the element that equals 6.02 × 10^23
Using the formula; mole = mass/molar mass
- Sodium = 23g/23g/mol = 1mol
- Krypton = 42g/84g/mol = 0.5mol
- Carbon = 24g/12g/mol = 2mol
- Pottasium = 78g/39g/mol = 2mol
Therefore, from the above illustration, it can be observed that only 23g of sodium contains 1 mol, hence, 23g sodium contains 6.02 × 10^23 atoms.