Answers
1. The cation in Co(NO₃)₂ is Co²⁺
2. The anion in Co(NO₃)₂ is NO₃⁻
How to determine cation and anion in Co(NO₃)₂We'll begin by defining cation and anion. This is given below:
A cation is an atom which possesses a positive charge.
An anion is an atom or group of atoms which possesses a negative charge
To obtain the cation and the anion in Co(NO₃)₂, we shall write the dissociation equation. This is illustrated below:
Co(NO₃)₂(aq) --> Co²⁺(aq) + 2NO₃⁻(aq)
From the above equation, we can conclude that the cation and the anion in Co(NO₃)₂ is:
The cation is Co²⁺The anion is NO₃⁻Learn more about cation and anion:
https://brainly.com/question/980691
#SPJ1
Related Questions
In an experiment, a student wants to increase the rate of a reaction that involves gases. Which change to the reactants would accomplish this?
Answers
The change to the reactants that would accomplish increase in reaction rate is increase in concentration of reactant.
Reaction rate is the speed at which a chemical reaction proceeds.
It is expressed in terms of either the concentration of a product that is formed in a unit of time or the concentration of a reactant that is consumed in a unit of time.
Here, a student wants to increase the rate of a reaction that involves gases.
The increase in reaction rate will be result of increase in concentration of reactant.
In case of gases, it can be done via:
Increasing the pressure and decreasing the volume.
Hence, the change to the reactants that would accomplish increase in reaction rate is increase in concentration of reactant. Increasing the pressure and decreasing the volume are ways to accomplish this.
Learn more about reaction rate click here https://brainly.com/question/8592296
#SPJ1
The density of a substance that has a mass of 7.0 g and a volume of 3.4 cm3 is
Answers
Answer:
2.05882 g/cm3
Explanation:
the formula p=m/V, or density (p) is equal to mass (m) divided by volume (V)
The density of a substance will be "2.06 g/cm³".
Mass and DensityThe proportion of substance has been defined as mass, whereas volume is the amount of space filled by that of the thing. This same density of the material is determined as the percentage among these two characteristics of the substance.
According to the question,
Mass of substance, m = 7.0 g
Volume of substance, V = cm³
As we know the relation,
→ Density = \(\frac{Mass}{Volume}\)
Or,
d = \(\frac{m}{V}\)
By substituting the values, we get
= \(\frac{7.0}{3.4}\)
= 2.06 g/cm³
Thus the response above is correct.
Find out more information about density here:
https://brainly.com/question/24445340
What is the best way to measure the pH of a natural solution while out in a forest?
Answers
The best way to measure the pH of a natural solution while out in a forest is to use a portable pH meter or pH test strips specifically designed for field use. These instruments provide accurate and reliable pH measurements and are convenient for outdoor applications.
1. Prepare the necessary equipment: Before heading out to the forest, gather the required tools. You will need a portable pH meter or pH test strips, as well as the necessary reagents or calibration solutions if using a pH meter.
2. Collect the sample: Locate the natural solution you want to measure the pH of, such as a stream, pond, or soil. Use a clean container to collect a representative sample of the solution.
3. Calibrate the pH meter (if applicable): If you are using a portable pH meter, it is essential to calibrate it before taking measurements. Follow the manufacturer's instructions to calibrate the meter using the provided calibration solutions.
4. Conduct the measurement: For pH meters, immerse the electrode into the collected sample. Allow some time for the reading to stabilize, and then record the pH value indicated on the meter's display.
5. Using pH test strips: If you are using pH test strips, dip the strip into the collected sample for the recommended amount of time. Remove the strip and compare the color change with the provided color chart. Determine the corresponding pH value from the chart.
6. Repeat for accuracy: To ensure reliability, repeat the measurement process at least once and compare the results. This step helps confirm the accuracy of your measurements.
7. Record and analyze the data: Note down the pH values obtained and any relevant observations. Analyze the data as needed for your research or monitoring purposes.
By following these steps and using the appropriate equipment, you can effectively measure the pH of a natural solution while in a forest setting.
For more such questions on measurements, click on:
https://brainly.com/question/28391278
#SPJ8
The following reaction take place in a container where CONDITIONS ARE NOT STP! Calculate the volume nitogen dioxide that will be produced when 4,86 dm3 N2O5 decompose. 2N2O5(g) → 4NO2(g) + O2(g)
Answers
9.77 litres of NO2 are generated on average.
Calculation-The balanced equation for the breakdown of N2O5 is as follows:
\(2N_2O_5(g) -- > 4NO_2(g) + O_2(g)\)
determine how many moles of N2O5 decompose:
\(V(N_2O_5) / Vm = n(N_2O_5)(N_2O_5)\)
where V(N2O5) = 4.86 dm3 is N2O5's volume and Vm(N2O5) is N2O5's molar volume under the circumstances stated in the ideal gas law:
\((R*T)/P = Vm = V/n\)
when the gas constant R is used.
the kelvin scale of temperature, T
The pressure is P.
The ideal gas law:
\(n(N_2O_5) = V(N2O5) / Vm(N_2O_5) = 4.86 dm3 / (24.46 L/mol) = 0.1982 mol\)
the number of moles of NO2 is:
\(n(NO_2 = 4/2 * n(N_2O_5) = 0.3964 mol\)
then,
\(n(NO_2 = 4/2 * n(N_2O_5) = 0.3964 mol\)
to know more about the reaction here:
brainly.com/question/28984750
#SPJ1
What is the name of the compound O7I9
Answers
Answer:
question not clear can u rewrite
How many decigrams are equal to 1.35 milligrams? 135 dg 13.5 dg 0.0135 dg 0.135 dg
Answers
Answer:
0.0135 dg
Explanation:
Answer: 0.0135 dg
Explanation:
HI + LiCH -› Lil + H0 If 3 mole: H2O are produced, (how many grams of Lil will also be produced?
Answers
HI + LiOH -> LiI + H2O
According to the equation, 1 mole of HI reacts with 1 mole of LiOH to produce 1 mole of LiI and 1 mole of water (H2O).
Given that 3 moles of water (H2O) are produced, we can say that 3 moles of LiI will also be produced. This is because the stoichiometry of the reaction tells us that 1 mole of LiI is produced for every 1 mole of water (H2O) produced.
Now, we need to calculate the mass of 3 moles of LiI.
The molar mass of LiI can be calculated as follows:
LiI = Li + I
LiI = 6.941 + 126.90
LiI = 133.84 g/mol
Therefore, the mass of 3 moles of LiI is:
Mass = 3 moles x 133.84 g/mol
Mass = 401.52 g
Hence, 401.52 grams of LiI will be produced when 3 moles of water (H2O) are produced.
The unit cell for tin (Sn) has tetragonal symmetry, with a and b lattice parameters of 0.583 and 0.318 nm, respectively. If its density, atomic weight, and atomic radius are 7.30 g/cm3, 118.69 g/mol, and 0.151 nm, respectively. Determine its atomic packing factor.
Answers
Answer:
0.1334
Explanation:
The number of atoms per unit (n) is given by:
\(n=\frac{\rho a^2bN_a}{A} \\\\where\ a=5.83*10^{-8}cm,b=a=3.18*10^{-8}cm,\rho=7.3\ g/cm^3,\\N_a=Avogadro\ number=6/022*10^{23} mol^{-1},A=atomic\ weight\\=118.68 \ g/mol\\\\n=\frac{7.3* (5.83*10^{-8})^2*(3.18*10^{-8})*6.02*10^{23}}{118.69}\\\\n=4\ atoms/unit\)
The atomic packing factor (APF) is:
\(APF=\frac{n(\frac{4\pi R^3}{3} )}{a^2b} \\\\But\ R=atomic\ radius=1.51*10^{-8}\ cm\\\\APF=\frac{4(\frac{4\pi (1.51*10^{-8})^3}{3} )}{(5.83*10^{-8})^2*3.18*10^{-8}}\\\\APF=0.1334\)
Which of these is not a sign of a chemical reaction?
1. The material dissolves
2. Heat is released
3. A gas is given off
Answers
A chemical reaction is known by;
2. Heat is released
3. A gas is given off
How do you know a chemical reaction?A change in color may indicate that a chemical reaction has occurred. For example, when iron is exposed to air and moisture, it rusts and turns from silver to reddish-brown.
If a gas is produced during a reaction, it can indicate that a chemical reaction has occurred. For example, when baking soda is mixed with vinegar, carbon dioxide gas is produced, which causes bubbles to form.
Learn more about chemical reaction:https://brainly.com/question/29039149
#SPJ1
2 examples of metal’s catalytic reaction
Answers
Answer:
Example 1
palladium(II) nitrate,
Example 2
Metal catalysts such as Fe, Ni, Mo, and Co are routinely used in the manufacture of CNMs.
Explanation
The three metals used in catalytic converters — rhodium, platinum and palladium — are part of a category known as platinum group metals, or PGMs, which are known for their catalytic properties.
Define temperature in terms of kinetic energy.
Answers
Answer: :)
The temperature of a gas is directly proportional to the average kinetic energy of the particles of the gas. But the total kinetic energy of the molecules of a gas is a measure of the internal energy or thermal energy of the gas.
Explanation:
How many moles of silver are present in a silver spoon that has a mass of 12.86 g?
You do not need to type your units into the answer box. Please report your answer with three places after the decimal, and do not use scientific notation.
Answers
The number of moles of silver present in a silver spoon that has a mass of 12.86g is 0.12 moles.
How to calculate number of moles?The number of moles of a substance can be calculated by dividing the mass of the substance by its molar mass as follows:
The mass of a substance is the quantity of matter which a body contains, irrespective of its bulk or volume.
The mole of a substance is the amount of substance of a system which contains exactly 6.02214076 × 10²³ elementary entities e.g. atoms, molecules.
moles = mass ÷ molar mass
According to this question, silver spoon has a mass of 12.86g. The number of moles can be calculated as follows:
Molar mass of silver (Ag): 107.8682g/mol
moles = 12.86g ÷ 107.8682g/mol
moles = 0.12 moles
Therefore, 0.12 moles of silver are present in the silver spoon.
Learn more about no of moles at: https://brainly.com/question/12513822
#SPJ1
what is the chemical property of fresh milk?
i want the answer now pls help
Answers
Answer:
Fresh milk has several chemical properties. It is a good source of nutrients such as carbohydrates, proteins, and fats, as well as vitamins and minerals. It is also a source of enzymes and hormones. One important enzyme found in fresh milk is lactase, which helps to digest lactose, a type of sugar found in milk. Fresh milk also contains lactic acid, which gives it a slightly sour taste. It also has a pH of around 6.5-6.7, which is slightly acidic. Milk also contains a number of antimicrobial agents, such as lactoferrin and lysozyme, which help to protect against the growth of harmful bacteria.
Explanation:BRAINLIEST PLS
In which row should the student enter “No” in the column for Law?
Answers
To determine the row in which the student should enter "No" in the column for Law, we need to have a clear understanding of the table and the data in it.The question might be related to a specific table or data set. However, without that, it's not possible to come up with a definite answer. Here are some possible ways to approach the problem:
Understand the dataThe first step in determining the row in which the student should enter "No" in the column for Law is to understand the data. To do this, we need to analyze the table to identify the various categories of data presented in it. We should look for the column headings and the row headings to understand the data that is presented in the table. We should also look for any patterns or trends in the data that might help us to answer the question.Identify the column for LawOnce we have a clear understanding of the data presented in the table, we should identify the column for Law. This will help us to identify the row in which the student should enter "No" in the column for Law. We should look for the column heading that corresponds to Law and note the data that is presented in that column.Look for the appropriate valueOnce we have identified the column for Law, we should look for the appropriate value to enter in the row for the student. If the question provides a specific condition or criterion for entering the value "No," we should use that to determine the appropriate row. Otherwise, we should use our understanding of the data presented in the table to make an informed decision about which row is appropriate for the value "No."Overall, the answer to this question depends on the specific data presented in the table, and the criteria for entering the value "No" in the column for Law.For such more question on column
https://brainly.com/question/25740584
#SPJ8
How do we measure forces?
Answers
Answer:
That depends.
Explanation:
We can measure force as mass*accelerations for physics
If the magnetic energy stored by a 0.50 H inductor is 3.6 J, what is the current through it?
A. 0.13 A
B. 0.90 A
C. 1.8 A
D. 3.8 A
E. 5.4 A
Answers
The current passing through the inductor is 3.8 A. The correct option is D. 3.8 A
Energy stored in a magnetic fieldFrom the question, we are to determine the current passing through the inductor.
From the formula, for the energy stored in a magnetic field
E = 1/2LI²
Where E is the energy
L is the inductance
and I is the current
From the given information,
E = 3.6 J
L = 0.50 H
Putting the parameters into the formula, we get
3.6 = 1/2 × 0.50 × I²
3.6 = 0.25 × I²
I² = 3.6 ÷ 0.25
I² = 14.4
I = √14.4
I = 3.7947
I ≅ 3.8 A
Hence, the current passing through the inductor is 3.8 A. The correct option is D. 3.8 A
Learn more on Energy stored in a magnetic field here: https://brainly.com/question/15244801
If a medicine ball in a gym has a mass of 4.0 Kg, what is its weight?
Answers
Answer:
Weight = 0.4Explanation:
Given Information :
Mass = 4.0kg
Acceleration due to gravity = 10 m/s
Weight= ?
\(Weight = \frac{mass}{acceleration\: due \:to \:gravity} \\\\W= \frac{4.0}{10} \\\\W= \frac{2}{5} \\\\W=0.4\)
What is the concentration (molarity) of a solution of NaCl if 350. mL of a 2.5 M NaCl solution is diluted to a total volume of 5.0 mL? (NEED HELP ASAP)
Answers
The concentration (molarity) of the final NaCl solution is 175 M.
To find the concentration (molarity) of the final NaCl solution, we can use the equation:
M1V1 = M2V2
Where M1 is the initial concentration, V1 is the initial volume, M2 is the final concentration, and V2 is the final volume.
In this case, we have an initial NaCl solution with a concentration of 2.5 M and a volume of 350 mL (0.350 L). We are diluting this solution to a total volume of 5.0 mL (0.005 L).
Plugging these values into the equation, we have:
(2.5 M) * (0.350 L) = M2 * (0.005 L)
Simplifying the equation:
0.875 = 0.005 * M2
Dividing both sides by 0.005:
M2 = 0.875 / 0.005
M2 = 175M
For such more questions on molarity
https://brainly.com/question/30704561
#SPJ8
Please help, really need this turned in!
While working in the chemistry lab, you dissolve 2.5g of sodium hydroxide chips into a beaker containing 50mL of water. As you pick up the beaker to add it to a separate solution, you notice the outside of the beaker is very cold. What explains this decrease in temperature?
Question 45 options:
Energy was absorbed when the bonds between sodium and hydroxide ions were broken
Energy was gained when the sodium and hydroxide ions formed new bonds with the water.
Energy was released when the sodium and hydroxide ions came together to form NaOH.
Energy was released when the bonds between sodium and hydroxide ions were broken.
Answers
Answer:
Energy was gained when the sodium and hydroxide ions formed new bonds with the water.
Explanation:
The other answer choices are incorrect because:
Energy was absorbed when the bonds between sodium and hydroxide ions were broken: This is incorrect because breaking bonds requires energy, so energy is absorbed rather than released.
Energy was released when the sodium and hydroxide ions came together to form NaOH: This is incorrect because the reaction being described is dissolution of NaOH in water, not formation of NaOH from its constituent ions.
Energy was released when the bonds between sodium and hydroxide ions were broken: This is incorrect for the same reason as the first option. Breaking bonds requires energy, so energy is absorbed rather than released.
Answer: this correct answer will be energy was released when the sodium and hydroxide ions formed new bonds with the water.
Explanation:
What mass is contributed by Br-79?
Answers
Answer:
Calculate the mass of BR -79? Bromine has 2 naturally occurring isotopes (Br-79 and Br-81) and has an atomic mass of 79.904 amu
Explanation:
if i get this wrong srry
Which equation shows an increase in entropy?
Hint: Look at the states of matter, g s l, of the chemicals in each equation. A C2H4(g) + H2(g) + C2H6(g) в Caco3(9) + Cao(s) - CO2(g) c Fe(s) + S (s) -+ FeS (s)

Answers
The equation C2H4(g) + H2(g) + C2H6(g) → Caco3(s) + Cao(s) + CO2(g) shows an increase in entropy due to the formation of a gas as a product. Option A
In this equation, the reactants on the left-hand side consist of gases (C2H4 and H2), while the products on the right-hand side include a solid (Caco3) and a gas (CO2).
When a reaction involves a change from gaseous to solid or liquid states, there is typically a decrease in entropy because the particles become more ordered and constrained in the solid or liquid phase.
Conversely, when a reaction involves the formation of gases, there is generally an increase in entropy because gases have higher degrees of molecular motion and greater freedom of movement compared to solids or liquids.
In the given equation, the reactants include three gaseous compounds (C2H4, H2, and C2H6), and one of the products is a gas (CO2). Therefore, the overall entropy of the system increases during this reaction.
The equation Fe(s) + S(s) → FeS(s) does not show an increase in entropy. Both the reactants (Fe and S) and the product (FeS) are solids. Since solids have lower entropy compared to gases or liquids, the entropy of the system does not increase in this reaction. Option A
For more such questions on entropy visit:
https://brainly.com/question/30481619
#SPJ8
CH4(g)
AH1 CH₂
S
+ 2Cl2(g) → CCl4(g) + 2H₂(g)
--74 8 kJ/mol
AH₁ CC
--106.7 kJ/mol
Which of these are true for the reaction above? Check all that apply.
AHxn is-31.9 kJ.
AHxn is-181.5 kJ.
The reaction is endothermic, +AH.
The reaction is exothermic, -AH.
Heat is a product.
Answers
The reaction is exothermic , it is the true reaction of the equation.
What is exothermic?
Exothermic refers to a chemical reaction that produces heat. In exothermic reactions, bonds are broken in the reactants, but bonds are broken in the products more often, releasing more energy. Exothermic reactions are characterized by a rise in the reaction mixture's temperature.
What is reaction ?
One or more chemicals, sometimes referred to as reactants, are changed into one or more new substances, referred to as products, during a chemical reaction. Chemical elements and compounds are both substances.
CH4(g) + 4Cl2(g) → CCl4(l) + 4HCl(g)
ΔH= ((−139 kJ/mol) + 4 x (−92.31 kJ/mol)) - ((−74.87 kJ/mol) + (121.0 kJ/mol))= (-508.24 kJ/mol) - 46.13 kJ/mol = -554.37 kJ/mol.
Therefore, reaction is exothermic , it is the true reaction of the equation.
Learn more about exothermic from the given link.
https://brainly.com/question/2924714
#SPJ1
What instrument do you use to determine the mass of an object?
Answers
Answer: a weighing balance
7. How does the ear process sound? Put the steps in the correct order. (You may need to conduct some research)
1. _____ a. The movement against the oval window causes motion in the fluid that fills the cochlea
2. _____ b. The impulse is then translated into sound by the brain.
3. _____ c. The inner end of the stapes moves in and out of the oval window at the same rate the eardrum is vibrating.
4. _____ d. Sound waves enter the ear and travel through the auditory canal. These waves cause vibrations in the eardrum.
5. _____ e. The vibration of the eardrum causes the bones in the middle ear to move back and forth.
6. _____ f. The movement of the fluid causes the hairs in the fluid to move. This movement stimulates the attached cell to send a tiny impulse along the auditory nerve to the brain.
Answers
Answer:
D, E,C, A,F,B
Explanation:
6. How many moles are in 8.30 x 1023 molecules of CO₂?
a.
b.
C.
d.
1.37
2.8
55.5
100
Answers
Question
In Woodward's 1952 synthesis of cholesterol, an elimination reaction was used to remove the C20 alcohol
substituent as shown below. The transformation is performed with acetic acid (hint: as opposed to a strong
base). Would you classify this as an E1 or E2 elimination?
Ola.E2
O b, E1
Answers
question in what words 1952 synthesis of cholesterol and illumination reaction was used to remove the C20 alcohol substitute as shown below the transformation is performed with acidic acid hint as opposed to a strong base would you classify this as an E1 or an E2 elimination.
Answer: I would classify this as an E2 :)
how many moles are in a 25.0 grams of carbon dioxide
Answers
answer:44.0095
Explanation:
sana makatulong
Answer:
44.0095
Explanation:
We assume you are converting between grams CO2 and mole. You can view more details on each measurement unit: molecular weight of CO2 or mol This compound is also known as Carbon Dioxide. The SI base unit for amount of substance is the mole.
10 kg of Phenanthrene is to be burnt with supplied air which is 30% less than the requirement. Find the
exit gas stream average molecular weight and the leftover Phenanthrene amount in the reactor.
Answers
The exit gas stream average molecular weight is 28.97 g/mol, and there will be 3.0 kg of leftover Phenanthrene in the reactor.
The balanced combustion equation for Phenanthrene is:
C₁₄H₁₀ + 21O₂ → 14CO₂ + 5H₂O
From the equation, we can see that 21 moles of oxygen are needed to combust 1 mole of Phenanthrene. Thus, to burn 10 kg of Phenanthrene, we need:
10,000 g / 178.24 g/mol = 56.05 moles of Phenanthreneand
21 * 56.05 = 1177.05 moles of O₂However, the supplied air is 30% less than the requirement, which means only 70% of the required O₂ will be supplied. Therefore, the actual amount of O₂ supplied will be:
1177.05 * 0.7 = 823.94 moles of O₂Assuming the air is mostly nitrogen, we can calculate the exit gas stream average molecular weight using the following formula:
Mw = (0.79 * 28.01) + (0.21 * 32) = 28.97 g/molwhere 0.79 and 0.21 are the mole fractions of nitrogen and oxygen in air, respectively, and 28.01 and 32 are the molecular weights of nitrogen and oxygen, respectively.
Finally, we can calculate the amount of leftover Phenanthrene in the reactor:
Amount of Phenanthrene consumed = 56.05 moles x 178.24 g/mol = 9999.72 gLeftover Phenanthrene = 10,000 g - 9999.72 g = 0.28 gHowever, this amount is negligible due to the large scale of the reaction. Therefore, we can round it off to:
Leftover Phenanthrene = 3.0 kg
To learn more about average molecular weight, here
https://brainly.com/question/31476705
#SPJ1
PLEASE HELPPP
UNIT TEST Chemical Reactions - Part 1 Which equation is balanced? CP+20 - PO₂ 2P+ 0; --P.₂O. 4P + 50., --2P.0. 4P - 20. - 27.0. - →
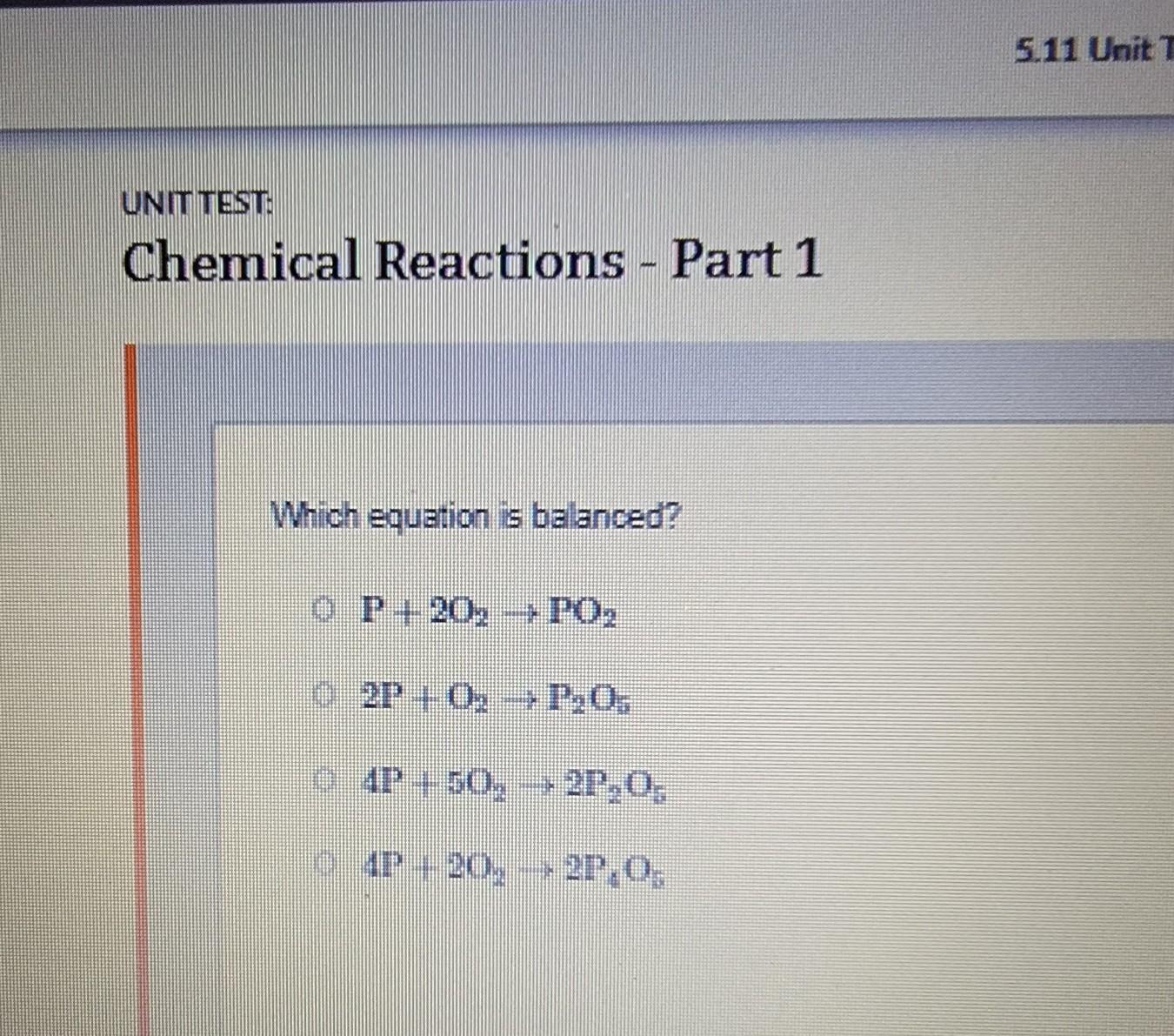
Answers
Answer:
2P+ 02 → P205Explanation:
I Thinks it B!!!
but please don't take my word i'm not really good at math.when air molecules collide with things around us, it produces _______ (2 words), which is measured with a _______.
Answers
✔ When air molecules collide with things around us, it produces pressing force , which is measured with a Pressure gauge.
